Abstracts
A transition metal/microwave irradiation (or base) free synthesis of aminopyridines has been accomplished via C-N bond forming reaction between chloropyridine and a variety of simple amides under refluxing conditions.
aminopyridine; amide; C-N bond; chloropyridine
A síntese de aminopiridinas foi realizada através de reações de formação de ligações C-N entre cloropiridina e uma variedade de amidas simples sob condições de refluxo, na ausência de metais de transição e irradiação por microondas (ou base).
ARTICLE
A simple synthesis of aminopyridines: use of amides as amine source
Arumugam KodimuthaliI, II; Anitha MungaraI; Padala Lakshmi PrasunambaII; Manojit PalI, * * e-mail: manojitpal@rediffmail.com , # # Present address: Institute of Life Sciences, University of Hyderabad Campus Gachibowli, Hyderabad 500 046, Andhra Pradesh, India.
IMatrix Laboratories Ltd, New Drugs Discovery Department, Anrich Industrial Estate, Bollaram, Jinnaram Mandal, Medak District, Andra Pradesh 502 325, India
IIDepartment of Chemistry, University College of Science, Osmania University, Hyderabad 500 007, India
ABSTRACT
A transition metal/microwave irradiation (or base) free synthesis of aminopyridines has been accomplished via C-N bond forming reaction between chloropyridine and a variety of simple amides under refluxing conditions.
Keywords: aminopyridine, amide, C-N bond, chloropyridine
RESUMO
A síntese de aminopiridinas foi realizada através de reações de formação de ligações C-N entre cloropiridina e uma variedade de amidas simples sob condições de refluxo, na ausência de metais de transição e irradiação por microondas (ou base).
Introduction
2-Aminopyridine derivatives, though well known for a long time, have attracted considerable interest recently because of their applications in various fields especially in pharmaceutical research. For example, the use of 2-aminopyridines have been reported as glucokinase activators1 or selective inhibitors of neuronal nitric oxide synthase.2 Substituted or unsubstituted aminopyridines are also useful precursors for the synthesis of a variety of heteocyclic compounds possessing a medicinal value.3-9 For example, 2-(methylamino)nicotinonitrile or 2-amino-3-nitropyridine was used as a synthetic precursor of 1H,3H-pyrido[2,3-d]pyrimidine-2,4-dione10 or pyrido[2,3-b]pyrazines11and imidazo[4,5-b]pyridines12 respectively.
While a number of methods have been reported for the synthesis of 2-aminopyridine derivatives, the most straightforward one includes the reaction of 2-halopyridines with substituted or unsubstituted amines. Essentially, these reactions are carried out either by using a transition metal catalyst e.g. palladium,13,14 or at elevated pressure.15 Alternatively, 2-aminopyridine derivatives can also be prepared via nitration of (un)substituted aminopyridines16 or oxidative amination of nitropyridines.17 Recently, microwave assisted solvent free amination of halo pyridine without transition metal catalyst have been reported.18
Introduction of N,N-dimethylamino group via the reaction of 2-chloro pyridine with DMF (N,N-dimethylformamide) in the presence of a base such as diethanolamine19 or K2CO320 has been reported in 1997 and 2004 respectively. More recently, chemoselective displacement of phenylthioether moiety of pyrazin-2(1H)-ones by N,N-dimethylamino group using DMF under microwave irradiations has been reported.21 In 2004, one of us was involved in the preparation of diethylamino derivative of pyrazolopyrimidine via the reaction of 7-chloro-1,3-dimethyl-5-phenyl-1H-pyrazolo[4,3-d]pyrimidine with DMF in the presence of (PPh3)4Pd and Na2CO3 (Scheme 1).22 Very recently, a palladium catalyzed synthesis of N,N-dimethyl[1,8]naphthyridine-2-amines by facile incorporation of NMe2 group from DMF has been reported.23 All these methods however require the use of a base (and/or a catalyst) and only limited studies have been carried out with a very few number of substrates. Due to our continuing interest24 in the use of 2-chloropyridine25 as a useful synthetic precursor we planned to study the reactions of chloropyridines with a number of commercially available simple amides. We also required a simple and scalable process for the preparation of aminopyridines in order to meet the requirement of this class of compound for our ongoing drug discovery program. Herein we report a transition metal or microwave irradiation-free practical synthesis of various aminopyridines without employing a base (Scheme 1).
Results and Discussion
Initially, we choose 2-chloro-3-nitropyridine (1a) to conduct our preliminary study which was reacted with a variety of formamides (2) under refluxing conditions. The results of these studies are summarized in Table 1.
As presented in Table 1 that the reaction of formamide 2a with 1a was completed within 15 h providing a good yield of desired product 3a (Entry 1, Table 1). On the other hand reaction of acetamide 2b with 1a was found to be slow (Entries 2 and 3, Table 1) and only 69% yield of product was isolated even after 70 h (Entry 3, Table 1). Similarly, the monosubstituted formamide 2c reacted faster than acetamide 2d providing a better yield of product 3b (Entry 4 vs 5 and 6, Table 1). Simple formamide 2e also provided the desired product when reacted with 1a (Entry 7, Table 1).
Having prepared a number of aminopyridines (3a-c) from 2-chloro-3-nitropyridine (1a) successfully we decided to explore the use of other chloropyridines under the reaction conditions studied. Accordingly, a variety of unsubstituted and substituted aminopyridines (3d-l) were prepared from various chloropyridine analogues (Table 2). Various functional groups such as nitro (Entries 1, 5, 8, 11 and 14, Table 2), trifluoromethyl (Entries 2, 6, 9 and 12, Table 2) and cyano (Entries 3, 7, 10 and 13, Table 2) present on the pyridine ring were well tolerated. Notably, unsubstituted chloropyridine (1e) also participated in the present amination reaction providing the desired products albeit in low yields (Entries 4 and 15, Table 2).
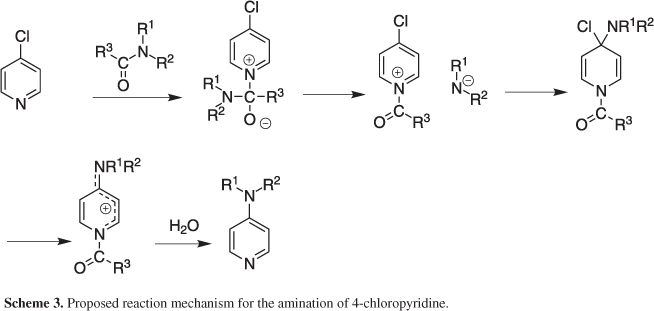
We have shown that suitably substituted chloropyridines can be converted to the corresponding amino derivatives using simple amides as a source of amine. It was evident from Table 2 that a chloro group present at the C-2 position of the pyridine ring participated well in this reaction. This can be explained by the higher reactivity of the azomethine chloro group compared to that present at C-4. Moreover, the reaction was facilitated by the presence of electron withdrawing group present on the pyridine ring perhaps favoring a nucleophilic attack on the chlorine bearing carbon. Nevertheless, the reaction seemed to proceed via the cleavage of an amidic N-C bond of the reactant amide used thereby serving as a potential source of amine under the condition employed (Schemes 2 and 3).26
Due to the requirement of a large quantity of N,N-dimethyl-5-nitropyridin-2-amine (3d) for our own research we carried out its synthesis using 50 g of 1b with DMF according to the condition mentioned above (Entry 1, Table 2). The desired compound was isolated in 80% yield thereby demonstrating the potential for scalability of this process. 1H NMR spectra of compound 3d is shown in Figure 1.
Conclusions
In conclusion, a simple and practical synthesis of aminopyridines has been accomplished via the reaction of chloropyridine with a variety of commercially available simple amides. The reactions were carried out in neat and under refluxing conditions to afford the expected products in good yields. The reaction is free from the use of transition metal catalysts or microwave irradiation or any organic/inorganic base. The methodology therefore may have advantages over the previously reported processes. Because of easy availability of starting materials and operational simplicity the present method is amenable for scale up. We expect that the present process would find wide application in the preparation of diversity based pyridine analogues especially for pharmaceutical research.
Experimental
General
Unless stated otherwise, reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254), visualizing with ultraviolet light or iodine spray. Flash chromatography was performed on silica gel (60-120 mesh) using distilled petroleum ether and ethyl acetate. 1H NMR spectra were determined either in CDCl3 or DMSO-d6 solution using 300 or 400 MHz spectrometers, respectively. Proton chemical shifts (δ) are relative to tetramethylsilane (TMS, δ = 0.0) as internal standard and expressed in parts per million. Spin multiplicities are given as s (singlet), d (doublet), t (triplet), and m (multiplet) as well as b (broad). Coupling constants (J) are given in hertz. Infrared spectra were recorded on a FTIR spectrometer. Melting points were determined by using thermal analysis and differential scanning calorimetry (DSC) was generated with the help of DSC-60A detector. MS spectra were obtained on a LC-MSD-Trap-SL mass spectrometer. All the reagents used are commercially available.
General procedure for the preparation of amino pyridines (3)
A solution of chloropyrdine 1 (5 mmol) in an appropriate amide 2 (10 mL) was refluxed for the time as mentioned in Tables 1 and 2. The reaction mixture was concentrated under vacuum, cooled to room temperature, quenched using ice water (25 mL) with stirring and extracted with ethyl acetate (2 × 50 mL). The organic layers were collected, combined, washed with cold water (100 mL), dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under vacuum and the residue was purified by column chromatography to yield the desired product.
N,N-Dimethyl-3-nitropyridin-2-amine27 (3a)
Colorless liquid; 1H NMR (DMSO-d6, 400 MHz) δ 8.38 (dd, 1H, J 4.4; 1.6 Hz), 8.21 (dd, 1H, J 8.0; 1.6 Hz), 6.81 (dd, 1H, J 8.0; 4.4 Hz), 2.97 (s, 6H); IR (KBr) νmax/cm-1: 2933, 1600, 1556, 1509, 1411, 1332, 1257, 962, 755; MS (CI method): 168 (M+1, 100%).
N-Methyl-3-nitropyridin-2-amine28 (3b)
Yellow solid; mp 66.5-68 ºC; 1H NMR (DMSO-d6, 400 MHz) δ 8.50 (dd, 1H, J 4.4; 1.7 Hz), 8.49 (bs, 1H), 8.40 (dd, 1H, J 8.3; 1.7 Hz ), 6.75 (dd, 1H, J 8.3; 4.4 Hz), 3.04 (d, 3H, J 4.8 Hz); IR (KBr) νmax/cm-1: 3405, 1610, 1573, 1511, 1442, 1415, 1378, 1355, 1313, 1251, 1211, 1033, 877, 759; MS (CI method): 154 (M+1, 100%).
3-Nitropyridin-2-amine29 (3c)
Pale yellow solid; mp 159-162 ºC; 1H NMR (DMSO-d6, 300 MHz) δ 8.35-8.40 (m, 2H), 7.87 (bs, 2H), 6.75 (dd, 1H, J 8.3; 4.5 Hz); IR (KBr) νmax/cm-1: 3464, 3273, 3093, 1638, 1566, 1514, 1438, 1354, 1336, 1240, 821, 758; MS (CI method): 140 (M+1, 100%).
N,N-Dimethyl-5-nitropyridin-2-amine30 (3d)
Yellow solid; mp 155-157 °C; 1H NMR (DMSO-d6, 400 MHz) δ 9.02 (d, 1H, J 2.8 Hz), 8.26 (dd, 1H, J 9.2; 2.8 Hz), 6.80 (d, 1H, J 9.2 Hz), 3.25 (s, 6H); IR (KBr) νmax/cm-1: 2924, 2854, 1597, 1335, 1295, 1118, 810; MS (CI method): 168 (M+1, 100%).
N,N-Dimethyl-5-(trifluoromethyl)pyridine-2-amine31 (3e)
Off-white solid; mp 43-45 °C; 1H NMR (CDCl3, 400 MHz) δ 8.38 (s, 1H), 7.60 (dd, 1H, J 9.2; 2.4 Hz), 6.50 (d, 1H, J 9.2 Hz), 3.14 (s, 6H); IR (KBr) νmax/cm-1: 2925, 1619, 1562, 1535, 1409, 1330, 813; MS (CI method): 191 (M+1, 100%).
2-(Dimethylamino)nicotinonitrile32 (3f)
Colorless liquid; 1H NMR (DMSO-d6, 400 MHz) δ 8.34 (dd, 1H, J 4.8; 1.8 Hz), 7.97 (dd, 1H, J 7.6; 1.8 Hz), 6.76 (dd, 1H, J 7.6; 4.8 Hz), 3.20 (s, 6H); IR (KBr) νmax/cm-1: 2927, 2211, 1589, 1552, 1511, 1409, 1245, 960, 759; MS (CI method): 148 (M+1, 100%).
N,N-Dimethylpyridine-4-amine33 (3g)
White solid; mp 109-111 ºC; 1H NMR (DMSO-d6, 400 MHz) δ 8.10 (dd, 2H, J 5.2; 1.5 Hz), 6.58 (dd, 2H, J 5.1; 1.3 Hz), 2.94 (s, 6H); IR (KBr) νmax/cm-1: 2902, 2825, 1604, 1536, 1445, 1377, 1223, 1104, 1068, 988, 808, 749; MS (CI method): 145 (M+Na, 100%).
N-Methyl-5-nitropyridin-2-amine34 (3h)
Yellow solid; mp 180-182.3 ºC; 1H NMR (DMSO-d6, 400 MHz) δ 8.92 (s, 1H), 8.09 (m, 2H), 6.53 (d, 1H, J 9.2 Hz), 2.90 (bs, 3H); IR (KBr) νmax/cm-1: 3370, 1619, 1600, 1542, 1481, 1463, 1419, 1394, 1324, 1265, 831; MS (CI method): 154 (M+1, 100%).
N-Methyl-5-(trifluoromethyl)pyridine-2-amine35 (3i)
Off-white solid; mp 50-53 ºC; 1H NMR (DMSO-d6, 400 MHz) δ 8.35 (s, 1H), 7.66 (d, 1H, J 7.2 Hz), 7.32 (bs, 1H), 6.60 (d, 1H, J 8.8 Hz), 2.86 (d, 3H, J 4.8 Hz); IR (KBr) νmax/cm-1: 3276, 1625, 1540, 1394, 1328, 1106, 939, 825; MS (CI method): 177 (M+1, 100%).
2-(Methylamino)nicotinonitrile36 (3j)
Yellow solid; mp 86.3-88.4 ºC; 1H NMR (DMSO-d6, 300 MHz) δ 8.28 (dd, 1H, J 4.9 & 1.9 Hz), 7.87 (dd, 1H, J 7.6; 1.9 Hz), 7.06 (bs, 1H), 6.62 (dd, 1H, J 7.6; 4.9 Hz), 2.85 (d, 3H, J 4.6 Hz); IR (KBr) νmax/cm-1: 3378, 2220, 1599, 1583, 1527, 1471, 1400, 1347, 1276, 1256, 1157, 861, 760; MS (CI method): 134 (M+1, 100%).
5-Nitropyridin-2-amine37 (3k)
Pale yellow solid; mp 182-185 ºC; 1H NMR (DMSO-d6, 300 MHz) δ 8.83 (d, 1H, J 2.8 Hz), 8.11 (dd, 1H, J 9.2; 2.8 Hz), 7.51 (bs, 2H), 6.49 (d, 1H, J 9.2 Hz); IR (KBr) νmax/cm-1: 3495, 3366, 1648, 1633, 1593, 1571, 1495, 1473, 1334, 1315, 1298, 1285, 1130, 842; MS (CI method): 140 (M+1, 100%).
Pyridine-4-amine38 (3l)
White solid; mp 154-156 ºC; 1H NMR (DMSO-d6, 400 MHz) δ 7.95 (dd, 2H, J 5.1; 1.4 Hz), 6.44 (dd, 2H, J 5.1; 1.4 Hz), 5.96 (bs, 2H); IR (KBr) νmax/cm-1: 3436, 3301, 1648, 1601, 1557, 1507, 1435, 1333, 1269, 1217, 991, 822; MS (CI method): 117 (M+Na, 100%).
Acknowledgments
A. K. thanks the management of Matrix Laboratories Ltd for continuous support and encouragement.
References
1. Aicher, T. D.; Boyd, S. A.; Chicarelli, M. J.; Condroski, K. R.; Hinklin, R. J.; Singh, A.; US Pat. 20090156603 2007.
2. Lawton, G. R.; Ranaivo, H. R.; Chico, L. K.; Ji, H.; Xue, F.; Martásek, P.; Roman, L. J.; Watterson, D. M.; Silverman, R. B.; Bioorg. Med. Chem. 2009, 17, 2371.
3. For anticancer agents, see: Temple, C. Jr.; Rose, J. D.; Comber, R. N.; Rener, G. A.; J. Med. Chem. 1987, 30, 1746; Temple, C., Jr.; Smith, B. H.; Elliott, R. D.; Montgomery, J. A.; J. Med. Chem. 1973, 16, 292.
4. For kinase inhibitors, see: Audrey, D.; Michelle, L.; Paul, L.; Peter, M.; Bin, W.; Tao, W.; Dingwei, Y.; World pat. WO 2006/082392A1 2006.
5. For selective antihistamine (H1) agents, see: Janssens, F.; Torremans, J.; Janssen, M.; Stokbroekx, R. A.; Luyckx, M.; Janssen, P. A. J.; J. Med. Chem. 1985, 28, 1943.
6. For anti-inflammatory agents, see: Gipf-Oberfrick, G. B.; Bennwil, J. D. K.; Hoelstein, J. F.; Hegenheim, R. H.; Arlesheim, P. W. M.; Efringen-Kirchen, B. W.; Biel-Benken, J. M. W.; US Pat. 2006/0217388A1 2006.
7. For Angiotensin II receptor antagonists, see: Mantlo, N. B.; Chakravarty, P. K.; Ondeyka, D. L.; Siegl, P. K. S.; Chang, R. S.; Lotti, V. J.; Faust, K. A.; Schorn, T. W.; Chen, T. B.; J. Med. Chem. 1991,34, 2919.
8. For anti-diabetic agents, see: Oguchi, M.; Wada, K.; Honma, H.; Tanaka, A.; Kaneko, T.; Sakakibara, S.; Ohsumi, J.; Serizawa, N.; Fujiwara, T.; Horikoshi, H.; Fujita, T.; J. Med. Chem. 2000, 43, 3052.
9. For thromboxane A2 receptor antagonists, see: Nicolai, E.; Claude, S.; Teulon, J. M.; J. Heterocycl. Chem. 1994, 31, 73.
10. Brunel, S.; Montginoul, C.; Torreilles, E.; Giral, L.; J. Heterocycl. Chem. 1980, 17, 235.
11. Blache, Y.; Gueiffier, A.; Chavignon, O.; Teulade, J. C.; Milhavet, J. C.; Viols, H.; Chapat, J. P.; Daupin, G.; J. Heterocycl. Chem. 1994, 31, 161.
12. Khanna, I. K.; Weier, R. M.; Lentz, K. T.; Swenton, L.; Lakin, D. C.; J. Org. Chem.1995, 60, 960.
13. Kim, Y. H.; Kim, Y. J.; Chang, S-Y.; Kim, B. T.; Heo, J-N.; Bull. Korean Chem. Soc. 2007, 28, 777.
14. Munson, P. M.; Thomson, W. J.; Synth. Commun. 2004, 34, 759.
15. Lurthy, N. G.; Bergstrom, F. W.; Mosher, H. S.; J. Am. Chem. Soc. 1949, 71, 1109.
16. Pino, L. N.; Zehrung, W. S.; J. Am. Chem. Soc. 1955, 77, 3154.
17. Bakke, J. M.; Svensen, H.; Tetrahedron Lett. 2001, 42, 4393.
18. Narayan, S.; Seelhammer, T.; Gawley, R. E.; Tetrahedron Lett. 2004, 45, 757; see also Tapia, R. A.; Cantuarias, L.; Cuellar, M.; Villena, J.; J. Braz. Chem. Soc. 2009, 20, 999; Bueno, M. A.; Silva, L. R. S. P.; Correa, A. G.; J. Braz. Chem. Soc. 2008, 19, 1264.
19. Cho, Y. H.; Park, J. C.; Tetrahedron. Lett. 1997, 38, 8331.
20. Agarwal, A.; Chauhan, P. M. S.; Synth. Commun. 2004, 34, 2925.
21. Sharma, A.; Mehta, V. P.; der Eycken, E. V.; Tetrahedron 2008, 64, 2605.
22. Pal, M.; Rao, Y. K.; Khanna, I.; Swamy, N. K.; Subramanian, V.; Batchu, V. R.; Iqbal, J.; Pillarisetti, S.; US pat. 2006/0128729 A1 2006.
23. Goswami, S.; Das, N. K.; J. Heterocycl. Chem. 2009, 46, 324.
24. Kalleda, S.; Padakanti, S.; Kumar Swamy, N.; Yeleswarapu, K.R.; Alexander, C. W.; Khanna, I. K.; Iqbal, J.; Pillarisetti, S.; Pal, M.; Barange, D.; World Pat. WO 2006/034473 A2 2006.
25. Pal, M.; Batchu, V. R.; Dager, I.; Swamy, N. K.; Padakanti, S.; J. Org. Chem. 2005, 70, 2376.
26. For a review on DMF, see: Muzart, J.; Tetrahedron 2009, 65, 8313.
27. Smalley, R. K.; J. Chem. Soc. Sec C 1966, 80.
28. Patriciu, O.-I.; Finaru, A.-L.; Massip, S.; Leger, J.-M.; Jarry, C.; Guillaumet, G.; Eur. J. Org. Chem. 2009, 22, 3753.
29. Aakerö y, C. B.; Beatty, A. M.; Nieuwenhuyzen, M.; Zou, M.; J. Mater. Chem. 1998, 8, 1385.
30. Heindel, N. D.; Kannewell, P. D.; Chem. Commun. 1969, 38.
31. Matsumoto, K; Toda, M.; Hashimoto, S.; Chem. Lett. 1991, 20, 1283.
32. Lamazzi, C.; Dreau, A.; Bufferne, C.; Flouzat, C.; Carlier, P.; ter Halle, R.; Besson, T.; Tetrahedron Lett. 2009, 50, 4502.
33. Katritzky, A. R.; Randall, E. W.; Sutton, L. E.; J. Chem. Soc. 1957, 1769.
34. Talik, T.; Talik, Z.; J. Prakt. Chem. 1988, 330, 84.
35. Santilli, A. A.; Scotese, A. C.; Bauer, R. F.; Bell, S. C.; J. Med. Chem. 1987, 30, 2270.
36. Chichibabin, A. E.; J. Russ. Phys.-Chem. Soc. 1920, 50, 492.
37. den Hertog, H. J.; Overhoff, J.; Rec. Trav. Chim. 1950, 69, 468.
38. Balicki, R.; Maciejewski, G.; Synth. Commun. 2002, 32, 1681.
Received: December 21, 2009
Web Release Date: March 25, 2010
- 1. Aicher, T. D.; Boyd, S. A.; Chicarelli, M. J.; Condroski, K. R.; Hinklin, R. J.; Singh, A.; US Pat. 20090156603 2007
- 2. Lawton, G. R.; Ranaivo, H. R.; Chico, L. K.; Ji, H.; Xue, F.; Martásek, P.; Roman, L. J.; Watterson, D. M.; Silverman, R. B.; Bioorg. Med. Chem. 2009, 17, 2371.
- 3. For anticancer agents, see: Temple, C. Jr.; Rose, J. D.; Comber, R. N.; Rener, G. A.; J. Med. Chem. 1987, 30, 1746;
- Temple, C., Jr.; Smith, B. H.; Elliott, R. D.; Montgomery, J. A.; J. Med. Chem. 1973, 16, 292.
- 4. For kinase inhibitors, see: Audrey, D.; Michelle, L.; Paul, L.; Peter, M.; Bin, W.; Tao, W.; Dingwei, Y.; World pat. WO 2006/082392A1 2006
- 5. For selective antihistamine (H1) agents, see: Janssens, F.; Torremans, J.; Janssen, M.; Stokbroekx, R. A.; Luyckx, M.; Janssen, P. A. J.; J. Med. Chem 1985, 28, 1943.
- 6. For anti-inflammatory agents, see: Gipf-Oberfrick, G. B.; Bennwil, J. D. K.; Hoelstein, J. F.; Hegenheim, R. H.; Arlesheim, P. W. M.; Efringen-Kirchen, B. W.; Biel-Benken, J. M. W.; US Pat. 2006/0217388A1 2006
- 7. For Angiotensin II receptor antagonists, see: Mantlo, N. B.; Chakravarty, P. K.; Ondeyka, D. L.; Siegl, P. K. S.; Chang, R. S.; Lotti, V. J.; Faust, K. A.; Schorn, T. W.; Chen, T. B.; J. Med. Chem. 1991,34, 2919.
- 8. For anti-diabetic agents, see: Oguchi, M.; Wada, K.; Honma, H.; Tanaka, A.; Kaneko, T.; Sakakibara, S.; Ohsumi, J.; Serizawa, N.; Fujiwara, T.; Horikoshi, H.; Fujita, T.; J. Med. Chem 2000, 43, 3052.
- 9. For thromboxane A2 receptor antagonists, see: Nicolai, E.; Claude, S.; Teulon, J. M.; J. Heterocycl. Chem. 1994, 31, 73.
- 10. Brunel, S.; Montginoul, C.; Torreilles, E.; Giral, L.; J. Heterocycl. Chem. 1980, 17, 235.
- 11. Blache, Y.; Gueiffier, A.; Chavignon, O.; Teulade, J. C.; Milhavet, J. C.; Viols, H.; Chapat, J. P.; Daupin, G.; J. Heterocycl. Chem. 1994, 31, 161.
- 12. Khanna, I. K.; Weier, R. M.; Lentz, K. T.; Swenton, L.; Lakin, D. C.; J. Org. Chem.1995, 60, 960.
- 13. Kim, Y. H.; Kim, Y. J.; Chang, S-Y.; Kim, B. T.; Heo, J-N.; Bull. Korean Chem. Soc. 2007, 28, 777.
- 14. Munson, P. M.; Thomson, W. J.; Synth. Commun. 2004, 34, 759.
- 15. Lurthy, N. G.; Bergstrom, F. W.; Mosher, H. S.; J. Am. Chem. Soc 1949, 71, 1109.
- 16. Pino, L. N.; Zehrung, W. S.; J. Am. Chem. Soc 1955, 77, 3154.
- 17. Bakke, J. M.; Svensen, H.; Tetrahedron Lett 2001, 42, 4393.
- 18. Narayan, S.; Seelhammer, T.; Gawley, R. E.; Tetrahedron Lett 2004, 45, 757;
- see also Tapia, R. A.; Cantuarias, L.; Cuellar, M.; Villena, J.; J. Braz. Chem. Soc. 2009, 20, 999;
- Bueno, M. A.; Silva, L. R. S. P.; Correa, A. G.; J. Braz. Chem. Soc. 2008, 19, 1264.
- 19. Cho, Y. H.; Park, J. C.; Tetrahedron. Lett 1997, 38, 8331.
- 20. Agarwal, A.; Chauhan, P. M. S.; Synth. Commun. 2004, 34, 2925.
- 21. Sharma, A.; Mehta, V. P.; der Eycken, E. V.; Tetrahedron 2008, 64, 2605.
- 22. Pal, M.; Rao, Y. K.; Khanna, I.; Swamy, N. K.; Subramanian, V.; Batchu, V. R.; Iqbal, J.; Pillarisetti, S.; US pat. 2006/0128729 A1 2006
- 23. Goswami, S.; Das, N. K.; J. Heterocycl. Chem 2009, 46, 324.
- 24. Kalleda, S.; Padakanti, S.; Kumar Swamy, N.; Yeleswarapu, K.R.; Alexander, C. W.; Khanna, I. K.; Iqbal, J.; Pillarisetti, S.; Pal, M.; Barange, D.; World Pat. WO 2006/034473 A2 2006
- 25. Pal, M.; Batchu, V. R.; Dager, I.; Swamy, N. K.; Padakanti, S.; J. Org. Chem. 2005, 70, 2376.
- 26. For a review on DMF, see: Muzart, J.; Tetrahedron 2009, 65, 8313.
- 27. Smalley, R. K.; J. Chem. Soc. Sec C 1966, 80.
- 28. Patriciu, O.-I.; Finaru, A.-L.; Massip, S.; Leger, J.-M.; Jarry, C.; Guillaumet, G.; Eur. J. Org. Chem. 2009, 22, 3753.
- 29. Aakerö y, C. B.; Beatty, A. M.; Nieuwenhuyzen, M.; Zou, M.; J. Mater. Chem 1998, 8, 1385.
- 30. Heindel, N. D.; Kannewell, P. D.; Chem. Commun 1969, 38.
- 31. Matsumoto, K; Toda, M.; Hashimoto, S.; Chem. Lett. 1991, 20, 1283.
- 32. Lamazzi, C.; Dreau, A.; Bufferne, C.; Flouzat, C.; Carlier, P.; ter Halle, R.; Besson, T.; Tetrahedron Lett 2009, 50, 4502.
- 33. Katritzky, A. R.; Randall, E. W.; Sutton, L. E.; J. Chem. Soc. 1957, 1769.
- 34. Talik, T.; Talik, Z.; J. Prakt. Chem. 1988, 330, 84.
- 35. Santilli, A. A.; Scotese, A. C.; Bauer, R. F.; Bell, S. C.; J. Med. Chem. 1987, 30, 2270.
- 36. Chichibabin, A. E.; J. Russ. Phys.-Chem. Soc. 1920, 50, 492.
- 37. den Hertog, H. J.; Overhoff, J.; Rec. Trav. Chim. 1950, 69, 468.
- 38. Balicki, R.; Maciejewski, G.; Synth. Commun 2002, 32, 1681.
Publication Dates
-
Publication in this collection
14 Oct 2011 -
Date of issue
2010
History
-
Received
21 Dec 2009