Abstracts
An efficient, green and solvent-free catalysed Prins-cyclization reaction based on the simple grinding of an aldehyde and a homoallylic alcohol in the presence of catalytic amount of p-TSA on silica gel is reported. By this protocol were synthesized tetrahydropyran odorants including commercial Florol® and Clarycet®, in one and two steps respectively.
Prins-cyclisation; solvent-free catalysis; green chemistry; tetrahydropyrans; fragrances
Foi desenvolvido um procedimento eficiente e ecologicamente amigável para a promoção da ciclização de Prins em ausência de solventes orgânicos baseado na maceração de um aldeído e um álcool alílico em presença de quantidade catalítica de p-TSA disperso em sílica gel. Por esse procedimento foram sintetizadas as fragrâncias comerciais Florol® e Clarycet®, em uma e duas etapas sintéticas respectivamente.
ARTICLE
Solvent-free catalysed synthesis of tetrahydropyran odorants: the role of SiO2·p -TSA catalyst on the prins-cyclization reaction
Alexandra MacedoI; Edison P. WendlerI; Alcindo A. Dos SantosII, * * e-mail: alcindo@iq.usp.br ; Julio Zukerman-SchpectorI, * * e-mail: alcindo@iq.usp.br ; Edward R. T. TiekinkIII
IDepartamento de Química, Universidade Federal de São Carlos, Rod. Washington Luís km 235, 13565-905 São Carlos-SP, Brazil
IIInstituto de Química, Universidade de São Paulo, Av. Prof. Lineu Prestes 748, 05508-000 São Paulo-SP, Brazil
IIIDepartment of Chemistry, University of Malaya, 50603 Kuala Lumpur, Malaysia
ABSTRACT
An efficient, green and solvent-free catalysed Prins-cyclization reaction based on the simple grinding of an aldehyde and a homoallylic alcohol in the presence of catalytic amount of p-TSA on silica gel is reported. By this protocol were synthesized tetrahydropyran odorants including commercial Florol® and Clarycet®, in one and two steps respectively.
Keywords: Prins-cyclisation, solvent-free catalysis, green chemistry, tetrahydropyrans, fragrances
RESUMO
Foi desenvolvido um procedimento eficiente e ecologicamente amigável para a promoção da ciclização de Prins em ausência de solventes orgânicos baseado na maceração de um aldeído e um álcool alílico em presença de quantidade catalítica de p-TSA disperso em sílica gel. Por esse procedimento foram sintetizadas as fragrâncias comerciais Florol® e Clarycet®, em uma e duas etapas sintéticas respectivamente.
Introduction
The Prins-cyclization reaction is one of the most efficient strategies to construct the tetrahydropyran core,1 which is present in several natural products.2 Some non-natural compounds possessing this functionality were found to be of commercial interest by the perfume industry. Clarycet® (commercialized by IFF) and Florol® (commercialized by Firmenich) are floral odorants handled in the industry as a racemic diastereoisomeric mixture which can be used in a large number of fragrances and formulations conferring floral scents without changing the olfactory character of the perfume.3,4 So far as we know, there are only two works in the literature describing the synthesis of both, Clarycet®4 and Florol®. In one of these works, racemic Florol® was synthesized in 44% overall yield from geraniol/nerol mixture in 5 sequential steps. All enantioenriched stereoisomers of both odorants were prepared by an enzymatic approach in 6 sequential steps in very low yields.4
With the recent increase of environmental awareness and subsequent development of "green-chemistry" principles, several guidelines for the production of chemicals by cleaner and benign procedures are being established as crucial for the modern chemical industry processes. One of the most important requirements in this context is the development of alternative synthetic routes aimed at minimizing or substituting mineral solvents by more benign solvents such as water, acetone, ethanol, ethyl acetate, etc.5 Further, emerging solvent-free processes and the utilization of solid matrices that can act as dispersing agents or as catalysts that is attracting attention.6
In this work, a one-pot procedure for the construction of the tetrahydropyran core of the floral odorants, Clarycet®, Florol® and other two not yet commercially utilized octahydro-2H-chromen-4-ols7 using a solvent-free solid phase catalysed Prins-cyclization reaction is presented.
Results and Discussion
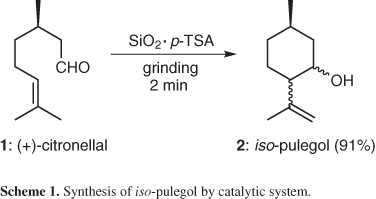
This proposition was based on previous work related to the solvent-free solid phase catalysed tetrahydropyranylation of alcohols and thiols.8 Earlier, it was observed that citronellal can be converted, in 91% isolated yield, into iso-pulegol within 2 min by an intramolecular ene-type reaction. The reaction proceeds by grinding it in a mixture of silica gel with catalytic amount of p-toluenosulfonic acid (p-TSA) according to Scheme 1.
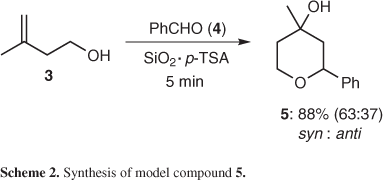
This result stimulated the application of similar reaction conditions in the preparation of tetrahydropyran derivatives by the intermolecular version of the Prins-cyclization reaction and studies related of conformational features of three tetrahydropyrans.9 A detailed stoichiometric screen showed that a physical mixture of p-TSA (0.29 mmol) in silica gel (0.5 g) was the best dispersant/catalyst system in the conversion of 3-methyl-but-3-en-1-ol (3, 1 mmol) and benzaldehyde (4, 1.1 mmol) in the corresponding Prins adduct 5, (syn:anti, 63:37) in 88% isolated yield, after 5 min grinding as presented in Scheme 2.
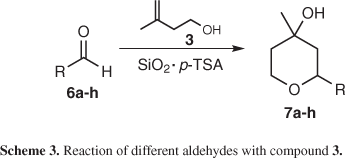
In order to investigate the role of the SiO2/p-TSA mixture, both components of this catalytic system, SiO2 and p-TSA, were investigated separately in the reaction of 3 with 4. SiO2 only did not display any catalytic behaviour even at long reaction times. On the other hand when sub-stoichiometric amount of p-TSA was put together with 3 and 4 in absence of solvents, a very vigorous and exothermic reaction took place accompanied by partial carbonization of the components of the mixture, evidenced by the formation of a black colour and the title compound was produced in low yield among many other by-products. Other solid matrices (neutral, acid and basic aluminium oxide, Celite® as well Montmorillonite K10) were also investigated as partners of p-TSA in the reaction of 3 with 4 and failed to produce 5 in good yields and synthetic useful reaction time in comparison with SiO2. These experiments allow us to conclude that SiO2 in association with p-TSA acts as catalyst and not only as a dispersant agent. In this way, were adopted the reaction conditions presented in Scheme 2, as the ideal system for the preparation of the Prins adducts by a solvent-free protocol in the reaction of the alcohol 3 with other aldehydes as presented in Scheme 3 and summarized in Table 1.
In all cases the tetrahydropyran derivatives were formed, as a diastereoisomeric mixture, in reasonable to good yields by the simple grinding of the reagents in a mixture of silica gel and catalytic amount of p-TSA. While aldehydes 6a, 6c and 6g, as well as benzaldehyde 4, were converted to the corresponding products in good yields in only 5 min of reaction (entries 1, 3, 7 and Scheme 2), the aromatic 6b, 6d, cycloalkyl 6f and alkenyl 6h aldehydes required a longer reaction time for conversion to the corresponding products (entries 2, 4, 6 and 8). Aldehyde 6e required 25 min to be converted into the product 7e in 69% yield (entry 5).
The structure of the tetrahydropyran derivative 7f (major diastereoisomer) was subsequently confirmed to have the syn relation, by a single-crystal X-ray structure determination, as shown in Figure 1.10
The major diastereoisomer of 7h was submitted to nOe experiment and the syn relation was confirmed, as show in Figure 2.
The strong 1D nOe for methyl group and carbinolic hydrogen provided unquestionable proof for the assigned syn relation. On the basis of nOe experiment acquired for compound 7h, the relation of the others tetrahydropyrans 5, 7a-g was similarly assigned.
With the reaction conditions established, this method was applied to the synthesis of Florol® (11, in a one-pot procedure 87%, 15 min) and Clarycet® (12) by reacting alcohol 3 with the appropriate aldehydes (8 and 9, respectively), and two other odorants of commercial interest5 (15 and 16) by reacting iso-pulegol 2 with aldehydes 13 and 14, respectively (Scheme 4).
Despite the low yield of 15 (49%), due the high volatility of aldehyde 13, since the reactions were conduced by grinding the reagents in an open mortar, the synthesis can be considered attractive since it can be performed in a single step and by a solvent-free procedure.
Conclusion
In conclusion, a one-pot strategy has been developed and applied to the synthesis of three important odorants: Florol® and compounds 15 and 16. The synthesis of Clarycet® was accomplished by a usual acetylation of the Prins adduct 10, with 55% overall yield. The great appeal of this procedure is found in its operational practicality, since the simple mixture of the reagents in a mortar allows the preparation of the target compounds in a few minutes. Currently, some variants of this protocol are under investigation aimed towards the total synthesis of industrially important compounds.
Experimental
General
The following includes general experimental procedures, specific details for representative reaction, and isolation and spectroscopic information for the new compounds prepared. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 400 MHz. Infrared spectra were recorded on a FT-IR Bomem-Hartman & Braun MB-102. Low resolution mass spectra were obtained on a GC-17A Shimadzu, equipped with HP-5MS column (5% phenyl / 95% dimethyl polysiloxane, 30 m × 0.25 mm × 0.25 μm), coupled with a GCMS-QP5000 Shimadzu (70 eV).
High resolution mass spectra were obtained on a LC-MS Bruker Daltonics MicroTOF Ic by direct infusion. Analytical thin-layer chromatography (TLC) was performed on glass plates coated with 0.50 mm 230-400 mesh silica gel containing a fluorescent indicator (Merck). TLC plates were visualized by exposure to ultraviolet light (254 nm) and/or by immersion in an acidic staining solution of p-anisaldehyde followed by heating on a hot plate. Organic solutions were concentrated by rotary evaporation at ca. 30-100 mmHg. Flash column chromatography was performed on Fluka silica gel 60 (220-440 mesh).10 Solvents used were of commercial grade and were previously treated according to the literature.11
General Procedure
In a mortar containg silica gel (200-400 mesh) (0.5 g, 8.33 mmol) and p-toluenesulfonic acid (0.05 g, 0.29 mmol) was added the aldehyde (1 mmol) and homoallylic alcohol (1.1 mmol). The resulting mixture was ground for the appropriate time and the reaction was monitored by thin layer chromatography. The reaction media was directly purified by column chromatography by elution with appropriate mixture of solvents.
(5R)-5-Methyl-2-(prop-1-en-2-yl)cyclohexanol (2)
The product was analysed by CG-FID and compared with commercial standard of iso-pulegol purchased from Aldrich Chemical Company (Milwaukee, Wisconsin, USA) (CAS 628693-74-3).
4-Methyl-2-phenyltetrahydro-2H-pyran-4-ol (5)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.66; colorless oil, yield 55%; 1H NMR (400 MHz, CDCl3) δ 1.45 (s, 3H), 1.66 (s, OH), 1.68 (dq, J1 12.94 Hz, J2 2.39 Hz, 1H), 1.72-1.86 (m, 2H), 1.89 (dt, J1 12.94 Hz, J2 2.39 Hz, 1H), 3.62 (td, J1 12.48 Hz, J2 2.31 Hz, 1H), 4.13 (ddd, J1 11.86 Hz, J2 5.24 Hz, J3 1.69 Hz, 1H), 4.36 (dd, J1 11.86 Hz, J2 2.31 Hz, 1H), 7.23-7.37 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 25.3, 40.1, 48.4, 66.0, 69.2, 77.7, 125.9, 127.6, 128.5, 142.1. (CAS 63500-72-1).
Anti diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.81; colorless oil, yield 33%; 1H NMR (400 MHz, CDCl3) δ 1.29 (s, 3H), 1.49-1.56 (m, 2H), 1.64 (dd, J1 13.71 Hz, J2 11.71 Hz, 1H), 1.73-1.83 (m, 2H), 3.90-4.03 (m, 2H), 4.70 (dd, J1 11.71 Hz, J2 2.31 Hz, 1H), 7.21-7.43 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 31.8, 38.5, 46.6, 64.1, 68.1, 75.2, 125.9, 127.4, 128.4, 142.8. (CAS 63500-72-1).
(E)-4-Methyl-2-styryltetrahydro-2H-pyran-4-ol (7a)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.56; colorless oil, yield 58%; IR νmax/cm-1 3431, 2969, 2935, 2851, 1713, 1599, 1494, 1449, 1377, 1250, 1174, 1104, 965, 935, 880, 818, 753, 738, 693 (film); 1H NMR (400 MHz, CDCl3) δ 1.39 (s, 3H), 1.60 (t, J 12.87 Hz, 1H), 1.64 (dq, J1 12.87 Hz, J2 2.22 Hz, 1H), 1.77 (td, J1 12.87 Hz, J2 5.08 Hz, 1H), 1.81 (dt, J1 12.87 Hz, J2 2.22 Hz, 1H), 2.05 (s, OH), 3.55 (td, J1 12.23 Hz, J2 2.38 Hz, 1H), 4.02 (ddq, J1 12.23 Hz, J2 5.88 Hz, J3 1.43 Hz, 1H), 4.06 (ddd, J1 12.23 Hz, J2 5.08 Hz, J3 2.22 Hz, 1H), 6.19 (dd, J1 16.05 Hz, J2 5.88 Hz, 1H), 6.61 (dd, J1 16.05 Hz, J2 1.43 Hz, 1H), 7.20-7.39 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 25.4, 40.3, 46.5, 65.3, 68.8, 75.8, 126.5, 127.7, 128.6, 129.7, 130.5, 136.7; MS: m/z 218 (M+, 34%), 204 (2), 200 (38), 185 (57), 171 (2), 148 (19), 147 (100), 129 (65), 115 (57), 104 (44), 91 (57), 71 (72), 55 (27); HRMS [M-Na+] Found: 241.1199. Calc. for C14H18O2: 241.1204.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.73; colorless oil, yield 33%; IR νmax/cm-1 3430, 2943, 2919, 2855, 1674, 1603, 1518, 1458, 1381, 1281, 1124, 1088, 1035, 935, 886, 818, 741, 637 (film); 1H NMR (400 MHz, CDCl3) δ 1.29 (s, 3H), 1.49 (dq, J1 12.94 Hz, J2 2.31 Hz, 1H), 1.51 (dd, J1 13.56 Hz, J2 11.51 Hz, 1H), 1.66-1.76 (m, 2H), 1.89 (s, OH), 3.89 (td, J1 11.40 Hz, J2 2.15 Hz, 1H), 3.90 (dd, J1 7.24 Hz, J2 1.85 Hz, 1H), 4.35 (ddq, J1 11.40 Hz, J2 6.01 Hz, J3 1.54 Hz, 1H), 6.17 (dd, J1 16.02 Hz, J2 6.01 Hz, 1H), 6.61 (dd, J1 16.02 Hz, J2 1.54 Hz, 1H), 7.18-7.40 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 31.7, 38.3, 44.7, 63.5, 67.7, 73.4, 126.3, 127.4, 128.4, 130.1, 130.2, 136.9; MS: m/z 218 (M+, 20%), 207 (3), 200 (24), 185 (42), 171 (3), 148 (19), 147 (100), 129 (85), 115 (60), 104 (43), 91 (63), 71 (82), 55 (49); HRMS [M-Na+] Found: 241.1200. Calc. for C14H18O2: 241.1204.
2-(4-Hydroxyphenyl)-4-methyltetrahydro-2H-pyran-4-ol (7b)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.22; colorless oil, yield 13%; IR νmax/cm-1 3363, 2940, 2861, 1614, 1518, 1443, 1377, 1250, 1225, 1170, 1143, 1080, 1047, 1013, 939, 899, 506 (film); 1H NMR (400 MHz, CDCl3) δ 1.39 (s, 3H), 1.56-1.86 (m, 4H), 3.63 (td, J1 12.07 Hz, J2 2.25 Hz, 1H), 4.01 (ddd, J1 12.07 Hz, J2 5.30 Hz, J3 2.25 Hz, 1H), 4.31 (dd, J1 12.07 Hz, J2 2.25 Hz, 1H), 6.74 (dt, J1 8.59 Hz, J2 1.95 Hz, 2H), 7.16 (dt, J1 8.59 Hz, J2 1.95 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 25.2, 41.1, 49.1, 66.8, 69.5, 78.8, 116.1, 128.5, 134.6, 158.0; MS: m/z 208 (M+, 2%), 190 (8), 175 (29), 161 (1), 137 (3), 121 (70), 119 (28), 91 (32), 77 (38), 71 (75), 58 (100); HRMS [M-Na+] Found: 231.0992. Calc. for C12H16O3: 231.0997.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.49; white solid, yield 54%, m.p. 194 °C; IR νmax/cm-1 3467, 2920, 2899, 2855, 1671, 1615, 1518, 1466, 1373, 1261, 1084, 1039, 1007, 902, 830, 737 (KBr); 1H NMR (400 MHz, CDCl3) δ 1.25 (s, 3H), 1.52 (dq, J1 13.84 Hz, J2 1.69 Hz, 1H), 1.62 (dd, J1 13.70 Hz, J2 11.30 Hz, 1H), 1.65-1.74 (m, 3H), 3.86 (ddd, J1 11.30 Hz, J2 5.22 Hz, J3 1.13 Hz, 1H), 3.94 (td, J1 11.30 Hz, J2 2.40 Hz, 1H), 4.61 (dd, J1 11.30 Hz, J2 2.40 Hz, 1H), 4.86 (s, OH), 6.74 (dt, J1 8.61 Hz, J2 1.97 Hz, 2H), 7.16 (dt, J1 8.61 Hz, J2 1.97 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 31.6, 39.1, 47.1, 65.2, 68.5, 76.6, 116.1, 128.6, 134.8, 157.9; MS: m/z 208 (M+, 11%), 190 (36), 176 (12), 175 (100), 161 (2), 137 (5), 121 (81), 119 (22), 91 (19), 71 (37), 58 (43); HRMS [M-Na+] Found: 231.0991. Calc. for C12H16O3: 231.0997.
2-(4-Methoxyphenyl)-4-methyltetrahydro-2H-pyran-4-ol (7c)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (2:1), Rf 0.31; colorless oil, yield 27%; IR νmax/cm-1 3426, 2948, 2915, 2847, 1614, 1587, 1518, 1470, 1377, 1249, 1176, 1080, 1031, 942, 898, 830, 770, 737, 605 (film); 1H NMR (400 MHz, CDCl3) δ 1.43 (s, 3H), 1.66 (qd, J1 12.87 Hz, J2 2.38 Hz, 1H), 1.70-1.88 (m, 4H), 1.95 (s, OH), 3.61 (td, J1 12.87 Hz, J2 1.74 Hz, 1H), 3.79 (s, 3H), 4.10 (ddd, J1 12.08 Hz, J2 5.24 Hz, J3 1.74 Hz, 1H), 4.30 (dd, J1 12.08 Hz, J2 1.74 Hz, 1H), 6.87 (dt, J1 8.74 Hz, J2 2.06 Hz, 2H), 7.27 (dt, J1 8.74 Hz, J2 2.06 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 25.2, 40.3, 48.1, 55.2, 65.8, 69.1, 77.2, 113.7, 127.2, 134.2, 159.0; MS: m/z 222 (M+, 16%), 204 (30), 190 (13), 189 (100), 173 (5), 151 (6), 135 (95), 133 (23), 119 (18), 92 (10), 91 (26), 71 (46), 58 (31); HRMS [M-Na+] Found: 245.1152. Calc. for C13H18O3: 245.1154.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (2:1), Rf 0.46; white solid, yield 62%, m.p. 82 °C; IR νmax/cm-1 3422, 2947, 2919, 2843, 1611, 1518, 1462, 1377, 1249, 1176, 1144, 1084, 1035, 943, 898, 830, 766, 605 (film); 1H NMR (400 MHz, CDCl3) δ 1.27 (s, 3H), 1.50 (dq, J1 13.82 Hz, J2 2.22 Hz, 1H), 1.59-1.81 (m, 4H), 3.78 (s, 3H), 3.92-3.98 (m, 2H), 4.64 (dd, J1 11.44 Hz, J2 2.54 Hz, 1H), 6.86 (dt, J1 8.74 Hz, J2 2.06 Hz, 2H), 7.26 (dt, J1 8.74 Hz, J2 2.06 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 31.8, 38.4, 46.4, 55.3, 64.1, 68.1, 74.9, 113.9, 127.5, 135.0, 159.1; MS: m/z 222 (M+, 15%), 204 (29), 190 (13), 189 (100), 173 (4), 151 (5), 135 (86), 133 (23), 109 (15), 92 (9), 91 (21), 71 (37), 58 (27); HRMS [M-Na+] Found: 245.1156. Calc. for C13H18O3: 245.1154.
4-Methyl-2-(4-nitrophenyl)tetrahydro-2H-pyran-4-ol (7d)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.42; colorless oil, yield 38%; IR νmax/cm-1 3418, 2972, 2939, 2859, 1604, 1519, 1347, 1252, 1093, 1045, 1014, 940, 809, 738, 697, 595 (film); 1H NMR (400 MHz, CDCl3) δ 1.47 (s, 3H), 1.63 (t, J1 12.23 Hz, 1H), 1.71 (dq, J1 12.87 Hz, J2 2.22 Hz, 1H), 1.82 (s, OH), 1.85 (td, J1 12.39 Hz, J2 5.24 Hz, 1H), 1.91 (dt, J1 12.87 Hz, J2 2.22 Hz, 1H), 3.64 (td, J1 12.87 Hz, J2 2.22 Hz, 1H), 4.17 (ddd, J1 12.39 Hz, J2 5.24 Hz, J3 2.22 Hz, 1H), 4.47 (dd, J1 12.23 Hz, J2 2.22 Hz, 1H), 7.51 (dt, J1 8.90 Hz, J2 2.22 Hz, 2H), 8.19 (dt, J1 8.90 Hz, J2 2.22 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 25.3, 40.1, 48.4, 65.9, 68.9, 76.5, 123.7, 126.5, 147.3, 149.6; MS: m/z 219 (M+-18, 30%), 204 (82), 188 (4), 174 (15), 152 (34), 135 (3), 120 (21), 107 (35), 103 (16), 91 (22), 71 (55), 58 (100); HRMS [M-Na+] Found: 260.0894. Calc. for C12H15NO4: 260.0899.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (1:1), Rf 0.60; colorless oil, yield 32%; IR νmax/cm-1 3474, 2968, 2923, 2875, 1605, 1530, 1349, 1257, 1144, 1013, 985, 906, 803, 749, 697, 596 (film); 1H NMR (400 MHz, CDCl3) δ 1.32 (s, 3H), 1.54 (dd, J1 13.51 Hz, J2 11.76 Hz, 1H), 1.58 (dq, J1 13.51 Hz, J2 2.22 Hz, 1H), 1.74 (s, OH), 1.80 (td, J1 13.66 Hz, J2 6.51 Hz, 1H), 1.83 (dt, J1 13.66 Hz, J2 2.22 Hz, 1H), 3.99 (td, J1 11.76 Hz, J2 2.22 Hz, 1H), 4.03 (ddd, J1 11.76 Hz, J2 6.51 Hz, J3 2.22 Hz, 1H), 4.84 (dd, J1 11.76 Hz, J2 2.22 Hz, 1H), 7.52 (dt, J1 8.90 Hz, J2 2.22 Hz, 2H), 8.18 (dt, J1 8.90 Hz, J2 2.22 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 31.7, 38.2, 46.7, 64.0, 67.9, 74.2, 123.5, 126.4, 147.0, 150.5; MS: m/z 219 (M+-18, 36%), 204 (100), 202 (3), 188 (2), 174 (3), 152 (30), 144 (1), 120 (4), 103 (8), 91 (8), 71 (29), 58 (51); HRMS [M-Na+] Found: 260.0901. Calc. for C12H15NO4: 260.0899.
2-(3-Bromophenyl)-tetrahydro-4-methyl-2H-pyran-4-ol (7e)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.22; colorless oil, yield 41%; IR νmax/cm-1 3422, 2964, 2939, 2855, 1716, 1595, 1569, 1474, 1337, 1252, 1092, 1045, 996, 942, 868, 822, 784, 747, 693, 598; 1H NMR (400 MHz, CDCl3) δ 1.41 (s, 3H), 1.62 (d, J1 12.33 Hz, 1H), 1.65 (dq, J1 12.79 Hz, J2 2.46 Hz, 1H), 1.80 (td, J1 12.79 Hz, J2 5.24 Hz, 1H), 1.84 (dt, J1 12.79, J2 2.46 Hz, 1H), 1.23 (s, OH), 3.58 (td, J1 12.33 Hz, J2 2.31 Hz, 1H), 4.10 (ddd, J1 12.02 Hz, J2 5.24 Hz, J3 1.54 Hz, 1H), 4.31 (dd, J1 12.02 Hz, J2 1.54 Hz, 1H), 7.18 (t, J1 7.70 Hz, 1H), 7.24 (dt, J1 7.70 Hz, J2 1.54 Hz, 1H), 7.38 (dt, J1 7.70 Hz, J2 1.54 Hz, 1H), 7.50 (t, J1 1.54 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 25.2, 40.1, 48.3, 65.8, 69.0, 76.8, 122.5, 124.4, 129.0, 129.9, 130.6, 144.4; MS: m/z 272 (M++2, 2%), 254 (22), 237 (49), 227 (2), 209 (1), 199 (1), 185 (35), 173 (6), 157 (13), 145 (6), 128 (7), 115 (5), 103 (37), 78 (16), 71 (90), 58 (100); HRMS [M-Na+] Found: 293.0146. Calc. for C12H15BrO2: 293.0153.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.28; colorless oil, yield 28%; IR νmax/cm-1 3467, 3067, 2969, 2919, 2873, 1713, 1596, 1568, 1477, 1361, 1261, 1044, 984, 925, 864, 816, 783, 739, 692 (film); 1H NMR (400 MHz, CDCl3) δ 1.26 (s, 3H), 1.49 (dq, J1 13.84 Hz, J2 1.29 Hz, 1H), 1.55 (dd, J1 13.84 Hz, J2 11.80 Hz, 1H), 1.68-1.78 (m, 2H), 1.84 (s, OH), 3.58 (td, J1 11.53 Hz, J2 2.14 Hz, 1H), 4.66 (ddd, J1 11.53 Hz, J2 6.24 Hz, J3 1.29 Hz, 1H), 4.31 (dd, J1 11.53 Hz, J2 2.14 Hz, 1H), 7.16 (t, J1 7.76 Hz, 1H), 7.23 (d, J1 7.76 Hz, 1H), 7.36 (dt, J1 7.76 Hz, J2 1.54 Hz, 1H), 7.51 (t, J1 1.54 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 31.6, 38.3, 46.4, 64.0, 67.8, 74.4, 122.4, 124.5, 128.9, 129.8, 130.3, 145.0; MS: m/z 272 (M++2, 2%), 237 (66), 209 (1), 200 (3), 185 (41), 173(7), 157 (15), 145 (6), 128 (6), 115 (5), 103 (31), 78 (16), 71 (82), 58 (100); HRMS [M-Na+] Found: 293.0152. Calc. for C12H15BrO2: 293.0153.
2-Cyclohexyl-4-methyltetrahydro-2H-pyran-4-ol (7f)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.62; white solid, yield 44%, m.p. 90 °C; IR νmax/cm-1 3414, 2920, 2855, 1708, 1450, 1381, 1333, 1216, 1172, 1146, 939, 862, 826, 741, 649 (KBr); 1H NMR (400 MHz, CDCl3) δ 0.72-2.04 (m, 15H), 1.31 (s, 3H), 2.47 (s, OH), 3.03 (ddd, J1 11.27 Hz, J2 6.18 Hz, J3 2.09 Hz, 1H), 3.40 (td, J1 11.86 Hz, J2 2.89 Hz, 1H), 3.96 (ddd, J1 11.86 Hz, J2 6.18 Hz, J3 2.09 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 25.5, 26.2, 26.6, 28.7, 28.9, 29.0, 40.8, 43.0, 43.5, 65.5, 69.3, 79.8; MS: m/z 180 (M+-18, 2%), 135 (1), 115 (46), 95 (10), 81 (7), 71 (100), 55 (22); HRMS [M-Na+] Found: 221.1519. Calc. for C12H22O2: 221.1517.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.40; colorless oil, yield 27%; IR νmax/cm-1 3402, 2919, 2843, 1703, 1478, 1450, 1398, 1096, 1007, 863, 826, 733, 645 (film); 1H NMR (400 MHz, CDCl3) δ 0.91-1.98 (m, 15H), 1.26 (s, 3H), 3.12 (s, OH), 3.36 (ddd, J1 11.40 Hz, J2 6.31 Hz, J3 1.54 Hz, 1H), 3.73 (td, J1 11.40 Hz, J2 1.54 Hz, 1H), 3.84 (dd, J1 11.40 Hz, J2 6.31 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 26.1, 26.2, 26.5, 28.5, 28.9, 31.9, 38.8, 41.4, 42.4, 63.7, 67.9, 77.1; MS: m/z 180 (M+-18, 11%), 165 (3), 135 (2), 125 (6), 115 (78), 95 (28), 83 (17), 69 (100), 55 (41); HRMS [M-Na+] Found: 221.1513. Calc. for C12H22O2: 221.1517.
(E)-4-Methyl-2-(prop-1-enyl)tetrahydro-2H-pyran-4-ol (7g)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (2:1), Rf 0.60; colorless oil, yield 25%; 1H NMR (400 MHz, CDCl3) δ 1.34 (s, 3H), 1.43-1.62 (m, 5H), 1.70 (dq, J1 6.55 Hz, J2 0.80 Hz, 3H), 3.48 (td, J1 12.19 Hz, J2 2.46 Hz, 1H), 3.78 (ddq, J1 11.33 Hz, J2 6.38 Hz, J3 1.06 Hz, 1H), 3.99 (ddd, J1 11.95 Hz, J2 5.11 Hz, J3 1.99 Hz, 1H), 5.49 (ddq, J1 15.38 Hz, J2 6.43 Hz, J3 1.63 Hz, 1H), 5.72 (ddq, J1 15.38 Hz, J2 6.49 Hz, J3 1.05 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 17.9, 27.7, 39.7, 46.4, 58.4, 73.2, 75.1, 128.0, 133.9; (CAS 69359-03-1).
Anti diastereoisomer: Eluent: hexane/ethyl acetate (2:1), Rf 0.73; colorless oil, yield 37%; 1H NMR (400 MHz, CDCl3) δ 1.27 (s, 3H), 1.40-1.60 (m, 5H), 1.69 (dq, J1 6.46 Hz, J2 0.84 Hz, 3H), 3.82 (td, J1 11.50 Hz, J2 2.28 Hz, 1H), 3.85 (dd, J1 6.35 Hz, J2 1.46 Hz, 1H), 4.10 (ddq, J1 11.50 Hz, J2 6.46 Hz, J3 0.84 Hz, 1H), 5.46 (ddq, J1 15.35 Hz, J2 6.56 Hz, J3 1.46 Hz, 1H), 5.72 (ddq, J1 15.35 Hz, J2 6.48 Hz, J3 1.09 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 17.8, 31.8, 38.4, 44.7, 63.5, 67.8, 73.5, 127.2, 131.9; (CAS 69359-03-1).
(E)-2-(But-1-enyl)-4-methyltetrahydro-2H-pyran-4-ol (7h)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.28; colorless oil, yield 41%; IR νmax/cm-1 3418, 2964, 2931, 2859, 1675, 1462, 1381, 1337, 1253, 1081, 971, 830 (film); 1H NMR (400 MHz, CDCl3) δ 1.00 (t, J1 7.49 Hz, 3H), 1.35 (s, 3H), 1.51 (t, J1 11.83 Hz, 1H), 1.60 (dq, J1 12.89 Hz, J2 2.23 Hz, 1H), 1.70 (dt, J1 12.76 Hz, J2 2.36 Hz, 2H), 2.05 (qt, J1 7.36 Hz, 2H), 3.49 (td, J1 12.10 Hz, J2 2.49 Hz, 1H), 3.80 (ddq, J1 11.18 Hz, J2 6.31 Hz, J3 1.18 Hz, 1H), 4.00 (ddd, J1 11.83 Hz, J2 5.13 Hz, J3 1.84 Hz, 1H), 5.46 (ddt, J1 15.52 Hz, J2 6.31 Hz, J3 1.57 Hz, 1H), 5.75 (dtd, J1 15.52 Hz, J2 6.18 Hz, J3 1.05 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.3, 25.3, 25.4, 40.4, 46.6, 65.3, 68.9, 76.0, 129.3, 134.4; MS: m/z 170 (M+, 1%), 155 (4), 141 (25), 123 (3), 107 (2), 99 (4), 83 (10), 71 (100), 55 (18); HRMS [M-Na+] Found: 193.1204. Calc. for C10H18O2: 193.1204.
Anti diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.20; colorless oil, yield 17%; IR νmax/cm-1 3434, 2964, 2927, 2875, 1623, 1466, 1381, 1264, 1080, 971, 908, 735, 650 (film); 1H NMR (400 MHz, CDCl3) δ 0.99 (t, J1 7.39 Hz, 3H), 1.27 (s, 3H), 1.45 (dd, J1 13.71 Hz, J2 11.40 Hz, 1H), 1.59 (dt, J1 13.71 Hz, J2 2.46 Hz, 2H), 1.68 (dq, J1 13.87 Hz, J2 6.16 Hz, 1H), 2.05 (qt, J1 7.24 Hz, 2H), 3.83 (td, J1 11.56 Hz, J2 2.31, 1H), 3.84-3.90 (m, 1H), 4.12 (ddd, J1 10.48 Hz, J2 6.62 Hz, J3 1.38 Hz, 1H), 5.44 (ddt, J1 15.41 Hz, J2 6.47 Hz, J3 1.54 Hz, 1H), 5.76 (dtd, J1 15.56 Hz, J2 6.31 Hz, J3 1.07 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.3, 25.3, 31.8, 38.4, 44.8, 63.5, 67.9, 73.6, 129.6, 134.1; MS: m/z 170 (M+, 2%), 152 (3), 141 (22), 123 (6), 113 (2), 99 (3), 83 (13), 71 (100), 55 (21); HRMS [M-Na+] Found: 193.1202. Calc. for C10H18O2: 193.1204.
4-Methyl-2-propyltetrahydro-2H-pyran-4-ol (10)
Syn diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.28; colorless oil, yield 21%; 1H NMR (400 MHz, CDCl3) δ 0.91 (t, J1 6.88 Hz, 3H), 1.32 (s, 3H), 1.34-1.88 (m, 9H), 3.21-3.34 (m, 1H), 3.42 (td, J1 11.96 Hz, J2 2.99 Hz, 1H), 3.96 (ddd, J1 11.96 Hz, J2 4.98 Hz, J3 2.99 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 14.0, 18.7, 25.4, 38.4, 40.7, 46.6, 65.4, 68.9, 75.2; (CAS 723340-91-8).
Anti diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.46; colorless oil, yield 40%; 1H NMR (400 MHz, CDCl3) δ 0.91 (t, J1 6.98 Hz, 3H), 1.25 (s, 3H), 1.27-1.76 (m, 8H), 1.95 (s, OH), 3.53-3.89 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 14.0, 18.5, 31.7, 38.2, 38.6, 44.6, 63.5, 67.7, 72.6; (CAS 723340-91-8).
2-Isobutyl-4-methyltetrahydro-2H-pyran-4-ol (11)-Florol ®
Syn diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.48; colorless oil, yield 35%; 1H NMR (400 MHz, CDCl3) δ 0.90 (d, J1 6.54 Hz, 6H), 1.12 (ddd, J1 13.66 Hz, J2 8.39 Hz, J3 4.55, 1H), 1.25 (s, 3H), 1.28 (dd, J1 13.66 Hz, J2 11.52 Hz, 1H), 1.40-1.50 (m, 3H), 1.53 (dtl, J1 13.66 Hz, J2 2.13 Hz, 1H), 1.63 (td, J1 13.66 Hz, J2 5.83, 1H), 1.78 (hept, J1 6.54 Hz, 1H), 3.19 (sl, OH), 3.65-3.84 (m, 2H), 3.76 (td, J1 12.09 Hz, J2 2.13 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 22.4, 23.3, 24.3, 31.7, 38.7, 45.1, 45.4, 63.6, 67.8, 71.2; (CAS 723440-93-0).
Anti diastereoisomer: Eluent: hexane/ethyl acetate (3:1), Rf 0.22; colorless oil, yield 52%; 1H NMR (400 MHz, CDCl3) δ 0.90 (d, J1 6.54 Hz, 6H), 1.17 (ddd, J1 12.66 Hz, J2 8.25 Hz, J3 4.55 Hz, 1H), 1.32 (s, 3H), 1.37 (t, J1 12.24 Hz, 1H), 1.49 (ddd, J1 12.66 Hz, J2 8.25 Hz, J3 5.97 Hz, 1H), 1.58 (dq, J1 12.66 Hz, J2 2.13 Hz, 1H), 1.63 (dt, J1 12.66 Hz, J2 2.13 Hz, 1H), 1.71 (td, J1 12.66 Hz, J2 5.12 Hz, 1H), 1.77 (hept, J1 6.54 Hz, J2 1.56 Hz, 1H), 2.96 (s, OH), 3.32-3.37 (m, 1H), 3.41 (td, J1 12.24 Hz, J2 2.13 Hz, 1H), 3.95 (ddd, J1 12.66 Hz, J2 5.12 Hz, J3 1.56 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 22.3, 23.1, 24.3, 25.3, 40.5, 45.4, 46.9, 65.3, 68.8, 73.6; (CAS 723440-93-0).
4-Methyl-2-propyltetrahydro-2H-pyran-4-yl acetate (12)-Clarycet ®
To a stirred solution of 4-methyl-2-propyltetrahydro-2H-pyran-4-ol (10) (500 mg, 3.16 mmol) in pyridine (3 mL) are added acetic anhydride (322 mg, 3.16 mmol, 1.0 equiv). The mixture is magnetically stirred at room temperature for 7 h. After completion of acetylation, the solution is neutralized with solid NaHCO3, filtered and solvent evaporated. Purification by flash column chromatography on silica gel, using a mixture of hexane/ethyl acetate (10:1) as eluent, afforded 180 mg (90%) of the title compound as colourless oil.
Syn diastereoisomer: Colorless oil, yield 90%; 1H NMR (400 MHz, CDCl3) δ 0.91 (t, J1 7.20 Hz, 3H), 1.54 (ddq, J1 12.60 Hz, J2 11.50 Hz, J3 0.90 Hz, 1H), 1.30-1.55 (m, 4H), 1.62 (t, J1 0.90 Hz, 3H), 1.86 (dddq, J1 12.80 Hz, J2 12.60 Hz, J3 5.30 Hz, J4 0.90 Hz, 1H), 1.97 (s, 3H), 2.03 (dddd, J1 12.80 Hz, J2 2.30 Hz, J3 2.20 Hz, J4 1.90 Hz, 1H), 2.10 (dt, J1 12.60 Hz, J2 2.20 Hz, 1H), 3.33 (m, 1H), 3.46 (ddd, J1 12.60 Hz, J2 12.10 Hz, J3 2.30 Hz, 1H), 3.93 (ddd, J1 12.10 Hz, J2 5.30 Hz, J3 1.90 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 14.4, 19.1, 21.4, 24.4, 36.3, 37.8, 42.1, 58.5, 66.7, 73.9, 170.2; (CAS 723340-91-8).
Anti diastereoisomer: Colorless oil, yield 90%; 1H NMR (400 MHz, CDCl3) δ 0.89 (t, J1 7.20 Hz, 3H), 1.02 (dd, J1 13.80 Hz, J2 11.30 Hz, 1H), 1.28 (ddd, J1 14.10 Hz, J2 12.60 Hz, J3 5.30 Hz, 1H), 1.25-1.60 (m, 4H), 1.68 (s, 3H), 2.05 (dddd, J1 14.10 Hz, J2 2.30 Hz, J3 2.10 Hz, J4 1.60 Hz, 1H), 2.20 (dt, J1 13.80 Hz, J2 2.30 Hz, 1H), 3.52 (dddd, J1 11.40 Hz, J2 7.40 Hz, J3 4.60 Hz, J4 2.30 Hz, 1H), 3.57 (ddd, J1 12.60 Hz, J2 11.60 Hz, J3 2.10 Hz, 1H), 3.71 (ddd, J1 11.60 Hz, J2 5.30 Hz, J3 1.60 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 14.1, 18.7, 21.7, 22.5, 30.4, 37.8, 38.4, 43.5, 64.6, 74.3, 80.1, 170.3; (CAS 723340-91-8).
2-Ethyl-4,7-dimethyloctahydro-2H-chromen-4-ol (15)
Diastereoisomeric mixture: Eluent: hexane/ethyl acetate (4:1); colorless oil, yield 49%; 1H NMR (400 MHz, CDCl3) δ 0.85-1.25 (m, 15H), 1.35-1.50 (m, 3H), 1.51-1.63 (m, 2H), 1.68-1.82 (m, 5H), 1.87-1.99 (m, 3H), 3.07 (td, J1 10.44 Hz, J2 4.24 Hz, 1H), 3.24-3.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 9.9, 21.4, 22.2, 23.1, 29.1, 31.5, 34.5, 41.6, 48.0, 52.4, 70.8, 75.9, 76.9. (CAS 134869-45-7).
2-Isopropyl-4,7-dimethyloctahydro-2H-chromen-4-ol (16)
Diastereoisomeric mixture: Eluent: hexane/ethyl acetate (7:1); colorless oil, yield 66%; 1H NMR (400 MHz, CDCl3) δ 0.84-1.09 (m, 14H), 1.12-1.21 (m, 4H), 1.35-1.50 (m, 2H), 1.61-1.75 (m, 5H), 1.85-1.99 (m, 3H), 3.00-3.10 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 18.3 (2C), 21.0, 25.8, 26.2, 31.5, 32.5, 34.6, 36.5, 40.7, 42.3, 76.0, 78.3, 80.9. (CAS 134869-70-8)
Acknowledgments
Fapesp (05/59572-7), CNPq, CAPES for financial support and Luciana Vizotto (LNMR/Universidade Federal de São Carlos) to perform the NMR and nOe experiments.
References
1. Olier, C.; Kaafarani, M.; Gastaldi, S.; Bertrand, M. P.; Tetrahedron 2010, 66, 413; Vascondellos, M. L. A. A.; Miranda, L. S. M. A.; Quim. Nova 2006, 29, 834; Crosby, S. R.; Harding, J. R.; King, C. D.; Parker, G. D.; Willis, C. L.; Org. Lett. 2002, 4, 577; Hiebel, M. A.; Pelotier, B.; Piva, O.; Tetrahedron 2007, 63, 7874; Yadav, J. S.; Reddy, B. V. S.; Maity, T.; Kumar, G. G. K. S.; Tetrahedron Lett. 2007, 48, 8874; Chan, K. P.; Seow, A. H.; Loh, T. P.; Tetrahedron Lett. 2007, 48, 37; Yadav, J. S.; Reddy, B. V. S.; Maity, T.; Kumar, G. G. K. S. N.; Tetrahedron Lett. 2007, 48, 7155; Yadav, J. S.; Reddy, B. V. S.; Kumar, G. G. K. S. N.; Swamy, T.; Tetrahedron Lett. 2007, 48, 2205; Yadav, J. S.; Reddy, B. V. S.; Kumar, G. M.; Murthy, C. V. S. R.; Tetrahedron Lett. 2001, 42, 89.
2. Reddy, U. C.; Bondalapati, S.; Saikia, A. K.; J. Org. Chem. 2009, 74, 2605; Perron, F.; Albizati, K. F.; J. Org. Chem. 1987, 52, 4130; Clarke, P. A.; Santos, S.; Eur. J. Org. Chem. 2006, 2045, and references cited therein; Class, Y. J.; DeShong, P.; Chem. Rev. 1995, 95, 1843; Kopecky, D. J.; Rychnovsky, S. D.; J. Am. Chem. Soc. 2001, 123, 8420; Wang, Y.; Janjic, J.; Kozmin, S. A.; J. Am. Chem. Soc. 2002, 124, 13670; Tian, X. T.; Jaber, J. J.; Rychnovsky, S. D.; J. Org. Chem. 2006, 71, 3176; Hu, Y.; Skalitzky, D. J.; Rychnovsky, S. D.; Tetrahedron Lett. 1996, 37, 8679; Arundale, E.; Mikeska, L. A.; Chem. Rev. 1952, 51, 505; Miles, R. B.; Davis, C. E.; Coates, R. M.; J. Org. Chem. 2006, 71, 1493; Yadav, J. S.; Reddy, B. V. S.; Kumar, G. G. K. S. N.; Reddy, G. M.; Tetrahedron Lett. 2007, 48, 4903.
3. Gupta, P.; Sethi, V. K.; Taneja, S. C.; Shah, B. A.; Andotra, S. S.; Koul, S.; Chimni, S. S.; Oazi, G. N.; Helv. Chim. Acta 2007, 90, 196.
4. Abate, A.; Brenna, E.; Fronza, G.; Fuganti, C.; Gatti, F. G.; Serra, S.; Zardoni, E.; Helv. Chim. Acta 2004, 87, 765.
5. Alfonsi, K.; Colberg, J.; Dunn, P. J.; Fevig, T.; Jennings, S.; Johnson, T. A.; Kleine, H. P.; Knight, C.; Nagy, M. A.; Perry, D. A.; Stefaniak, M.; Green Chem. 2008, 10, 31; Abaee, M. S.; Mojtahedi, M. M.; Forghani, S.; Ghandchi, N. M.; Forouzani, M.; Sharifi, R.; Chaharnazm, B.; J. Braz. Chem. Soc. 2009, 20, 1895; Lenardão, E. J.; Trecha, D. O.; Ferreira, P. C.; Jacob, R. G.; Perin, G.; J. Braz. Chem. Soc. 2009, 20, 93; Bueno, M. A.; Silva, L. R. S. P.; Corrêa, A. G.; J. Braz. Chem. Soc. 2008, 19, 1264; Almeida, Q. A. R.; Pereira, M. L. O.; Coelho, R. B.; Carvalho, E. M.; Kaiser, C. R.; Jones Jr., J.; Silva, F. M.; J. Braz. Chem. Soc. 2008, 19, 894.
6. Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P.; J. Molec. Catal. A: Chem. 2006, 237, 93; Bartoli, G.; Bartolacci, M.; Bosco, M.; Foglia, G.; Giuliani, A.; Marcantoni, E.; Sambri, L.; Torregiani, E.; J. Org. Chem. 2003, 68, 4594; Bartoli, G.; Bartolacci, M.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Torregiani, E.; J. Org. Chem. 2005, 70, 169; Bartoli, G.; Bosco, M.; Giuli, S.; Giuliani, A.; Lucarelli, L.; Marcantoni, E.; Sambri, L.; Torregiani, E.; J. Org. Chem. 2005, 70, 1941; Bartoli, G.; Fernández-Bolaños, J. G.; Di Antonio, G.; Foglia, G.; Giuli, S.; Gunnella, R.; Mancinelli, M.; Marcantoni, E.; Paoletti, M.; J. Org. Chem. 2007, 72, 6029; Chen, J.-X.; Liu, M.-C.; Yang, X.-L.; Ding, J.-C.; Wu, H.-Y.; J. Braz. Chem. Soc. 2008, 19, 877; Costa, J. S.; Pisoni, D. S.; Silva, C. B.; Petzhold, C. L.; Russowsky, D.; Ceschi, M. A.; J. Braz. Chem. Soc. 2009, 20, 1448.
7. Sprecker, M. A.; Belko, R. P.; Hanna, M. R.; Beck, C. E. J.; Brucato, S. M.; US pat. 4,999,439 1991. (pp. 48).
8. Dos Santos, A. A.; Brito Jr., G. A.; Archilha, M. V. L.; Bele, T. G. A.; Dos Santos, G. P.; De Mello, M. B. M.; J. Braz. Chem. Soc. 2009, 20, 42.
9. Zukerman-Schpector, J.; Dos Santos, A. A.; Macedo, A.; Wendler, E. P.; Brito Jr., G. A.; Tiekink, E. R. T.; Z. Kristallogr. 2008, 223, 471.
10. The crystals of 7f are orthorhombic, space group P212121, with a = 5.5714(10) Å, b = 11.0182(12) Å, c = 18.753(3) Å, V = 1151.2(3) Å3, Dx = 1.144 g cm-3, and Z = 4. The structure was solved by direct-methods and refined by full-matrix least-squares to final R = 0.050. Tiekink, E. R. T.; Macedo, A.; Wendler, E. P.; Dos Santos, A. A.; Zukerman-Schpector, J.; Acta Cryst. 2010, E66, o1233.
Received: January 22, 2010
Web Release Date: May 4, 2010
FAPESP has sponsored the publication of this article.
- 1. Olier, C.; Kaafarani, M.; Gastaldi, S.; Bertrand, M. P.; Tetrahedron 2010, 66, 413;
- Vascondellos, M. L. A. A.; Miranda, L. S. M. A.; Quim. Nova 2006, 29, 834;
- Crosby, S. R.; Harding, J. R.; King, C. D.; Parker, G. D.; Willis, C. L.; Org. Lett. 2002, 4, 577;
- Hiebel, M. A.; Pelotier, B.; Piva, O.; Tetrahedron 2007, 63, 7874;
- Yadav, J. S.; Reddy, B. V. S.; Maity, T.; Kumar, G. G. K. S.; Tetrahedron Lett. 2007, 48, 8874;
- Chan, K. P.; Seow, A. H.; Loh, T. P.; Tetrahedron Lett. 2007, 48, 37;
- Yadav, J. S.; Reddy, B. V. S.; Maity, T.; Kumar, G. G. K. S. N.; Tetrahedron Lett. 2007, 48, 7155;
- Yadav, J. S.; Reddy, B. V. S.; Kumar, G. G. K. S. N.; Swamy, T.; Tetrahedron Lett. 2007, 48, 2205;
- Yadav, J. S.; Reddy, B. V. S.; Kumar, G. M.; Murthy, C. V. S. R.; Tetrahedron Lett. 2001, 42, 89.
- 2. Reddy, U. C.; Bondalapati, S.; Saikia, A. K.; J. Org. Chem 2009, 74, 2605;
- Perron, F.; Albizati, K. F.; J. Org. Chem. 1987, 52, 4130;
- Clarke, P. A.; Santos, S.; Eur. J. Org. Chem. 2006, 2045,
- and references cited therein; Class, Y. J.; DeShong, P.; Chem. Rev. 1995, 95, 1843;
- Kopecky, D. J.; Rychnovsky, S. D.; J. Am. Chem. Soc. 2001, 123, 8420;
- Wang, Y.; Janjic, J.; Kozmin, S. A.; J. Am. Chem. Soc. 2002, 124, 13670;
- Tian, X. T.; Jaber, J. J.; Rychnovsky, S. D.; J. Org. Chem. 2006, 71, 3176;
- Hu, Y.; Skalitzky, D. J.; Rychnovsky, S. D.; Tetrahedron Lett. 1996, 37, 8679;
- Arundale, E.; Mikeska, L. A.; Chem. Rev 1952, 51, 505;
- Miles, R. B.; Davis, C. E.; Coates, R. M.; J. Org. Chem. 2006, 71, 1493;
- Yadav, J. S.; Reddy, B. V. S.; Kumar, G. G. K. S. N.; Reddy, G. M.; Tetrahedron Lett. 2007, 48, 4903.
- 3. Gupta, P.; Sethi, V. K.; Taneja, S. C.; Shah, B. A.; Andotra, S. S.; Koul, S.; Chimni, S. S.; Oazi, G. N.; Helv. Chim. Acta 2007, 90, 196.
- 4. Abate, A.; Brenna, E.; Fronza, G.; Fuganti, C.; Gatti, F. G.; Serra, S.; Zardoni, E.; Helv. Chim. Acta 2004, 87, 765.
- 5. Alfonsi, K.; Colberg, J.; Dunn, P. J.; Fevig, T.; Jennings, S.; Johnson, T. A.; Kleine, H. P.; Knight, C.; Nagy, M. A.; Perry, D. A.; Stefaniak, M.; Green Chem. 2008, 10, 31;
- Abaee, M. S.; Mojtahedi, M. M.; Forghani, S.; Ghandchi, N. M.; Forouzani, M.; Sharifi, R.; Chaharnazm, B.; J. Braz. Chem. Soc. 2009, 20, 1895;
- Lenardão, E. J.; Trecha, D. O.; Ferreira, P. C.; Jacob, R. G.; Perin, G.; J. Braz. Chem. Soc. 2009, 20, 93;
- Bueno, M. A.; Silva, L. R. S. P.; Corrêa, A. G.; J. Braz. Chem. Soc. 2008, 19, 1264;
- Almeida, Q. A. R.; Pereira, M. L. O.; Coelho, R. B.; Carvalho, E. M.; Kaiser, C. R.; Jones Jr., J.; Silva, F. M.; J. Braz. Chem. Soc. 2008, 19, 894.
- 6. Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P.; J. Molec. Catal. A: Chem. 2006, 237, 93;
- Bartoli, G.; Bartolacci, M.; Bosco, M.; Foglia, G.; Giuliani, A.; Marcantoni, E.; Sambri, L.; Torregiani, E.; J. Org. Chem. 2003, 68, 4594;
- Bartoli, G.; Bartolacci, M.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Torregiani, E.; J. Org. Chem. 2005, 70, 169;
- Bartoli, G.; Bosco, M.; Giuli, S.; Giuliani, A.; Lucarelli, L.; Marcantoni, E.; Sambri, L.; Torregiani, E.; J. Org. Chem. 2005, 70, 1941;
- Bartoli, G.; Fernández-Bolaños, J. G.; Di Antonio, G.; Foglia, G.; Giuli, S.; Gunnella, R.; Mancinelli, M.; Marcantoni, E.; Paoletti, M.; J. Org. Chem. 2007, 72, 6029;
- Chen, J.-X.; Liu, M.-C.; Yang, X.-L.; Ding, J.-C.; Wu, H.-Y.; J. Braz. Chem. Soc. 2008, 19, 877;
- Costa, J. S.; Pisoni, D. S.; Silva, C. B.; Petzhold, C. L.; Russowsky, D.; Ceschi, M. A.; J. Braz. Chem. Soc. 2009, 20, 1448.
- 7. Sprecker, M. A.; Belko, R. P.; Hanna, M. R.; Beck, C. E. J.; Brucato, S. M.; US pat. 4,999,439 1991 (pp. 48).
- 8. Dos Santos, A. A.; Brito Jr., G. A.; Archilha, M. V. L.; Bele, T. G. A.; Dos Santos, G. P.; De Mello, M. B. M.; J. Braz. Chem. Soc. 2009, 20, 42.
- 9. Zukerman-Schpector, J.; Dos Santos, A. A.; Macedo, A.; Wendler, E. P.; Brito Jr., G. A.; Tiekink, E. R. T.; Z. Kristallogr. 2008, 223, 471.
- 10. The crystals of 7f are orthorhombic, space group P212121, with a = 5.5714(10) Å, b = 11.0182(12) Å, c = 18.753(3) Å, V = 1151.2(3) Å3, Dx = 1.144 g cm-3, and Z = 4. The structure was solved by direct-methods and refined by full-matrix least-squares to final R = 0.050. Tiekink, E. R. T.; Macedo, A.; Wendler, E. P.; Dos Santos, A. A.; Zukerman-Schpector, J.; Acta Cryst. 2010, E66, o1233.
Publication Dates
-
Publication in this collection
14 Oct 2011 -
Date of issue
2010
History
-
Received
22 Jan 2010 -
Accepted
04 May 2010