Abstracts
The influence of pH on the degradation of the pharmaceutical diclofenac (DCF) by the solar/photo-Fenton process was investigated using ammonium iron(III) citrate complex (FecitNH4). Although the degradation efficiency decreased as the pH increased (in the pH range 5 to 8), the DCF concentration was lower than its limit of detection after 45 min irradiation while 77% of the total organic carbon was removed after 150 min irradiation at initial pH 7 (without further adjustment). A pseudo-first-order kinetics of DCF degradation was observed, which kinetic constant increased with the increase of the concentrations of FecitNH4 and H2O2. Lower DCF degradation was observed when present in a sewage treatment plant (STP) effluent sample, mainly due to the high concentration of carbonate and bicarbonate at pH 7. However, after adjusting the pH to 5, the DCF concentration was lower than its detection limit after 75 min irradiation.
pharmaceutical; wastewater; advanced oxidation process; iron complex
A influência do pH na degradação do fármaco diclofenaco (DCF) por processo foto-Fenton/solar foi avaliada usando o complexo citrato de amônio e ferro(III) (FecitNH4). Embora a eficiência de degradação diminua com o aumento do pH (no intervalo de pH de 5 a 8), a concentração de DCF ficou abaixo do limite de detecção após 45 min de irradiação enquanto que 77% do carbono orgânico total foi removido após 150 min em pH 7 (sem ajuste posterior). Foi verificada cinética de primeira ordem para a degradação de DCF, cuja constante de velocidade aumentou com o aumento das concentrações de FecitNH4 e H2O2. Menor degradação de DCF foi observada quando presente na matriz efluente de estação de tratamento de esgoto (ETE), devido principalmente à alta concentração de carbonato e bicarbonato em pH 7. Entretanto, após ajuste de pH para 5, a concentração de DCF ficou abaixo do limite de detecção após 75 min de irradiação.
ARTICLE
Diclofenac abatement using modified solar photo-fenton process with ammonium iron(III) citrate
Alam G. Trovó# # Present address: Instituto de Química, Universidade Federal de Uberlândia, CP 593, 38400-902 Uberlândia-MG, Brazil ; Raquel F. P. Nogueira* * e-mail: nogueira@iq.unesp.br
Instituto de Química de Araraquara, Universidade Estadual Paulista, CP 355, 14801-970 Araraquara-SP, Brazil
ABSTRACT
The influence of pH on the degradation of the pharmaceutical diclofenac (DCF) by the solar/photo-Fenton process was investigated using ammonium iron(III) citrate complex (FecitNH4). Although the degradation efficiency decreased as the pH increased (in the pH range 5 to 8), the DCF concentration was lower than its limit of detection after 45 min irradiation while 77% of the total organic carbon was removed after 150 min irradiation at initial pH 7 (without further adjustment). A pseudo-first-order kinetics of DCF degradation was observed, which kinetic constant increased with the increase of the concentrations of FecitNH4 and H2O2. Lower DCF degradation was observed when present in a sewage treatment plant (STP) effluent sample, mainly due to the high concentration of carbonate and bicarbonate at pH 7. However, after adjusting the pH to 5, the DCF concentration was lower than its detection limit after 75 min irradiation.
Keywords: pharmaceutical, wastewater, advanced oxidation process, iron complex
RESUMO
A influência do pH na degradação do fármaco diclofenaco (DCF) por processo foto-Fenton/solar foi avaliada usando o complexo citrato de amônio e ferro(III) (FecitNH4). Embora a eficiência de degradação diminua com o aumento do pH (no intervalo de pH de 5 a 8), a concentração de DCF ficou abaixo do limite de detecção após 45 min de irradiação enquanto que 77% do carbono orgânico total foi removido após 150 min em pH 7 (sem ajuste posterior). Foi verificada cinética de primeira ordem para a degradação de DCF, cuja constante de velocidade aumentou com o aumento das concentrações de FecitNH4 e H2O2. Menor degradação de DCF foi observada quando presente na matriz efluente de estação de tratamento de esgoto (ETE), devido principalmente à alta concentração de carbonato e bicarbonato em pH 7. Entretanto, após ajuste de pH para 5, a concentração de DCF ficou abaixo do limite de detecção após 75 min de irradiação.
Introduction
The human consumption of pharmaceuticals as well as veterinary practices and industrial activities including aquaculture has lead to contamination of surface and groundwater. The incomplete removal of these compounds during conventional sewage treatment contributes to contamination by pharmaceuticals what is of great environmental concern in recent years.1-3 Different classes of pharmaceuticals, such as antibiotics, hormones, anaesthetics and anti-inflammatories, have been detected at average concentrations ranging from ng L-1 to μg L-1 in sewage treatment plant (STP) effluents, as well as in surface and groundwater.4-11 These contaminants do not need to persist in the environment to cause a negative effect, since their high transformation/removal rates can be compensated by their continuous introduction into the environment. Among the pharmaceuticals, the anti-inflammatory diclofenac (DCF) has been found in most STP effluents due the its low biodegradability during conventional sewage treatment (ranging from 9 to 60%).8,11 Therefore, it is important to evaluate new procedures designed to prevent water contamination, considering the risks that residual pharmaceuticals can present to human health and to the environment.12 The application of advanced oxidation processes (AOPs), based on the generation of highly oxidizing hydroxyl radicals (·OH), can be a good alternative, due to their simplicity and the possibility of using sunlight, as in the photo-Fenton process and heterogeneous photocatalysis, which reduces energy costs.13-15
Reports of the direct photolysis of DCF in lake and pure water under sunlight and UV irradiation, as well as its degradation using different oxidation processes such as H2O2/UV and ozonation, are indicative of significant dechlorination and mineralization.16-19 Degradation using the photo-Fenton process has been demonstrated to be more efficient than heterogeneous photocatalysis, achieving total oxidation of DCF in half the time.20 However, during the photo-Fenton treatment it was observed that DCF precipitates at pH below 4 (pKa = 4.15), and high H2O2 concentrations (between 1360 and 3740 mg L-1) were necessary for complete DCF degradation.
Considering the optimum pH range (2.5-3.0) for the Fenton reaction, the use of iron complexes is a promising alternative for DCF degradation at circumneutral pH, since in the presence of organic ligands the iron can be maintained soluble over a wider pH range.
Another advantage of using iron complexes is their high absorption in the UV-Vis region, which allows use of a considerable portion of the solar spectrum, reducing the operating costs. In addition, degradation efficiency is usually higher in the presence of iron complexes, compared to iron salts.21 The high quantum yield of Fe(II) generation has been highlighted as one of the main reasons for the high efficiency of iron(III) complexes with citrate, oxalate and other carboxylates.
Various chelating agents have been evaluated for activation of hydrogen peroxide at circumneutral pH.22 However, the harmful effects of some chelating agents, such as ethylenediaminetetraacetic acid and nitrilotriacetic acid, have recently been acknowledged, and their use in environmental applications has been avoided. In contrast, citrate is a representative natural organic chelating agent that is environmentally friendly since it is readily biodegradable.23
The use in the photo-Fenton process of ferric citrate complex, prepared in situ through the addition of citrate to iron salts, has previously been shown to be viable at higher pH values.24-28 In the present work, the commercial ammonium iron(III) citrate complex (FecitNH4) was employed.
Therefore the goal of the present work was to evaluate the DCF degradation by the photo-Fenton process using FecitNH4. The effect of pH, as well as the influence of iron and H2O2 concentrations was evaluated. Considering that DCF is often present in STP effluents, its degradation was also evaluated in this matrix to verify to which extent the effluent constituents might affect the efficiency of the photo-Fenton process.
Experimental
Reagents
Potassium diclofenac (All Chemistry) was used as received for solution preparation. Sodium diclofenac standard (Sigma-Aldrich) was used to construct calibration curves for quantification. H2O2 (30% m/m) (Synth), ammonium iron(III) citrate complex (Vetec), H2SO4 and NaOH (Synth), and bovine liver catalase (Sigma-Aldrich) were used as received. Ammonium metavanadate (Vetec) solution was prepared at a concentration of 0.06 mol L-1 in 0.58 mol L-1 H2SO4. KH2PO4 (Mallinckrodt) and H3PO4 (Synth) were also used as received. All reagents were analytical grade. All solutions were prepared using distilled water (DW), except in experiments using STP effluent. Methanol for high performance liquid chromatography (HPLC grade) was used in HPLC analysis.
Sewage treatment plant effluent
In order to evaluate matrix effects on DCF degradation, a sample of effluent was collected from a STP employing activated sludge treatment and serving the population of almost 200,000 inhabitants of the city of Araraquara, Brazil. The sample was collected after complete treatment and kept refrigerated, for a maximum of one week, until the experiments were performed. The main parameters determined for the sample are shown in Table 1. The samples were spiked with DCF and filtered through 0.45 mm membranes before chemical analysis.
Photodegradation procedure
The photodegradation experiments were carried out in Araraquara, Brazil (22°S 48°W), using a solar reactor composed of a 49.5 cm long glass tube with an internal diameter of 3.6 cm, previously described by Trovó et al.14 The experiments were performed during winter, between 10 am and 2 pm.
All experiments were carried out at an initial DCF concentration of 33.4 mg L-1 (0.1 mmol L-1 potassium diclofenac, 16.8 mg of C per L of total organic carbon). Although this concentration is far above the expected levels in the environment, this concentration was chosen due to the difficulty in working at trace levels with HPLC and to permit DCF quantification during irradiation since the detection and quantification limits determined were 0.14 and 0.46 mg L-1, respectively. Taking into consideration the maximum concentration of iron allowed in wastewater according to Brazilian legislation (15 mg L-1 or 0.27 mmol L-1), the experiments were performed at three different initial concentrations of ammonium iron (III) citrate (FecitNH4) (0.05, 0.1 and 0.2 mmol L-1), which are below this limit. Based on previous work, the initial H2O2 concentration studied ranged from 34 to 170 mg L-1.15 For DCF kinetics evaluation in DW and STP effluent, 68 mg L-1 H2O2 were added at the beginning of the reaction, with new additions of H2O2 during the experiments, according to the consumption of H2O2. The initial pH value of each solution was adjusted to the desired value within the range 5-8 without a buffer solution, by adding H2SO4 or NaOH.27
The irradiated volume of the reactor was 510 mL, and a total volume of 800 mL of the pharmaceutical solution was recirculated at a flow rate of 80 mL min-1, using a peristaltic pump (Masterflex 7518-12), after the addition of FecitNH4 complex solution, adjustment of the pH and addition of H2O2. The reactor remained covered while the glass tube was being filled. Once the tube was completely filled, it was exposed to the sunlight. The solar energy dose accumulated during the exposure time, and the average irradiance, were measured using a radiometer (PMA 2100 Solar Light Co.) in the UVA region (320-400 nm), with the sensor placed at the same angle as that of the reactor. The average solar irradiance measured during the experiments was 14.8 ± 1.2 W m-2.
Chemical analysis
Before HPLC analysis, the solution pH was adjusted to between 6 and 8, and 250 mL of catalase solution (0.1 g L-1) was added to 10 mL of sample to consume residual hydrogen peroxide and consequently interrupt the degradation reaction. During irradiation the concentration of DCF was determined, after filtration through 0.45 mm membranes, by HPLC analysis using a Shimadzu LC10 AVP coupled to a diode array detector (SPD-M10A) and a Phenomenex reversed phase Luna C18 column (250 mm × 4.6 mm, 5 mm). The mobile phase used as eluent consisted of a binary mixture of 25 mmol L-1 KH2PO4 buffer (pH 3.5) and methanol (30:70), at a flow rate of 1 mL min-1. The injection volume was 20 mL and the column temperature 40 °C. Under these conditions, the retention time of DCF was 10.8 min.
Mineralization of the DCF solutions (conversion to carbon dioxide and water) during the experiments was evaluated by measuring the decay of the TOC concentration using a carbon analyzer (TOC 5000A, Shimadzu). The TOC analysis was always performed immediately after the sample was withdrawn, to avoid further reaction. The TOC concentration includes the carbon content of the target compound, intermediates generated during the experiments, citrate and also organic matter (in the case of STP effluent). The effluent samples were filtered through 0.45 μm membranes prior to analysis, so that only the dissolved organic carbon (DOC) was determined.
During degradation of DCF, the residual hydrogen peroxide remaining was measured by the spectrophotometric method employing ammonium metavanadate, using a Shimadzu mini-1240 UV spectrophotometer, as described by Nogueira et al.29
Results and Discussion
Influence of pH
The low pH necessary for the photo-Fenton process (2.5-3.0) is a disadvantage for its application in effluent treatment, since neutralization steps are required before to discharge into the environment. The use of in situ generated ferric citrate complex was previously reported by Silva et al.27 for the degradation of the herbicide tebuthiuron, where although the degradation efficiency of the iron citrate complex decreased as the pH increased, tebuthiuron oxidation at pH 6 was higher than that observed in the presence of Fe(NO3)3 at pH 2.5.
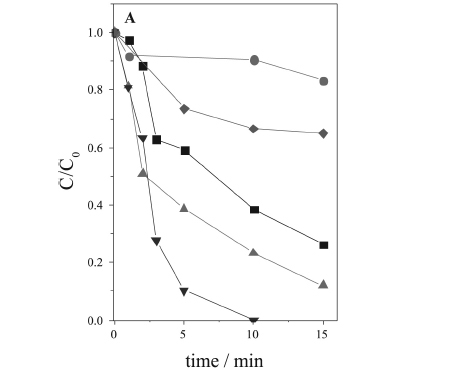
In the particular case of DCF degradation, the pH limitation is critical, because above pH 3.0 the iron precipitates, and at pH 2.8 DCF presents very low solubility (pKa = 4.15). Thus, the use of an iron complex soluble over a wide pH range, which promotes an efficient degradation, is especially interesting for the degradation of DCF. Therefore, the degradation of DCF was evaluated at four different pH values (5, 6, 7 and 8), using FecitNH4 as the source of iron for the photo-Fenton process. The experiments were carried out using 0.2 mmol L-1 FecitNH4 and 68 mg L-1 H2O2. It was observed that the initial pH strongly affected DCF oxidation and mineralization (Figure 1). Although the oxidation efficiency decreased with increase of pH, the DCF concentration was below the detection limit after only 10 min at pH 5, while after the same time, 77% and 62% of oxidation were obtained at pH 6 and 7, respectively (Figure 1A). At pH 8, the degradation was strongly hindered, achieving only 10% removal after 10 min. This result was even lower than the direct photolysis of DCF (pH 4.2), which caused 35% degradation after 15 min solar irradiation.
Although, in addition to the DCF carbon content, the TOC concentration includes intermediates generated and the carbon content of citrate, it can be estimated that most of the TOC removal was due to the mineralization of DCF and its intermediates, since only 8% of TOC was removed due to the photolysis of citrate (0.2 mmol L-1 FecitNH4 at pH 6) in a control experiment in the absence of DCF and H2O2, after 30 min solar irradiation (data not shown).
The initial pH had similar effect on the TOC removal as observed in the DCF degradation (Figure 1B). The highest mineralization was obtained at initial pH 5, achieving almost 50% after 20 min of solar irradiation, during which a plateau was achieved due to the total consumption of H2O2. At pH 6, 7 and 8, 29%, 24% and 10% of the TOC was removed, respectively (Figure 1B). As the pH increases, the predominant species is Fe(OH)(cit) -, which exhibits lower photoactivity and quantum yield of Fe(II) generation,24,26,30,31 consuming less H2O2, with a lower generation of hydroxyl radicals and consequently, a lower DCF degradation efficiency (Figure 1B).
Influence of FecitNH 4 and H 2 O 2 concentrations
The photo-Fenton process at pH 7 is advantageous for the treatment of effluents, avoiding the need for pH adjustment before either discharge into the environment, or prior to biological treatment, where necessary. Considering that reasonable degradation was achieved at this pH, the next experiments were carried out at pH 7.
DCF degradation was performed using three different FecitNH4 concentrations (0.05, 0.1 and 0.2 mmol L-1), and the initial H2O2 concentration was 68 mg L-1. As illustrated in Figure 2A, the degradation of DCF follows a pseudo-first-order kinetics for all FecitNH4 concentrations. The rate constants of DCF degradation increase linearly with the increment of FecitNH4 concentration, as shown in Table 2.
The same behaviour was observed for TOC removal. Increase of the FecitNH4 concentration improved mineralization and H2O2 consumption (Figure 2B). When 0.2 mmol L-1 FecitNH4 was employed, almost 35% TOC removal was achieved and 39 mg L-1 of hydrogen peroxide was consumed. In contrast, 22% of TOC removal and 18 mg L-1 of H2O2 consumption was obtained when 0.1 mmol L-1 of FecitNH4 was used. Only 10% mineralization and 5 mg L-1 of H2O2 consumption were observed when the FecitNH4 concentration was reduced to 0.05 mmol L-1 (Figure 2B).
The influence of the initial H2O2 concentration on DCF degradation was evaluated in the presence of 0.2 mmol L-1 FecitNH4 at pH 7. A pseudo-first-order kinetics for DCF degradation for all H2O2 concentrations was also observed (Figure 3A). The rate of DCF decay was enhanced due to the increment of H2O2 concentration from 34 to 68 mg L-1, resulting in an increase (2.6 fold) of the kinetic constants for the degradation of DCF, and a reduction of the half-life time from 21 to 8 min (Table 2). However, further increase of H2O2 concentration (68 mg L-1 to 170 mg L-1) improved only slightly the degradation, reducing the DCF half-life time from 8 to 7.5 min.
Considering TOC removal (Figure 3B), increase of the initial H2O2 concentration from 34 to 68 mg L-1 resulted in an increase of mineralization from 14% to 30% after 30 min of solar irradiation. However, no improvement was observed with further increase to 170 mg L-1, probably due to a self-decomposition reaction (equation 1) and scavenging of hydroxyl radicals by excess H2O2 (equation 2).32
H2O2 + H2O2 → 2 H2O + O2 (1)
H2O2 + HO· → H2O + HO2· (2)
Solar/photo-Fenton degradation of DCF in STP effluent
Since DCF is a commonly used anti-inflammatory that is often detected in STP effluents, at concentrations ranging from ng L-1 to mg L-1,33,34 its degradation in this type of medium was studied in order to evaluate matrix effects on the photo-Fenton process efficiency.
The experiment carried out in DW, using 0.2 mmol L-1 FecitNH4, 68 mg L-1 H2O2 (with further additions) and pH 7 (without further pH adjustment) resulted in total degradation of DCF after 45 min (Figure 4A), while a plateau around 75% of TOC removal was achieved after 90 min irradiation due to iron precipitation (Figure 4B). This is probably due to FecitNH4 degradation and the formation of hydroxo-iron species, hindering further photo-Fenton reaction. The initial pH decreased from 7 to 5.5 after 5 min. No further pH decrease was observed up to 150 min of irradiation. Although iron precipitation occurred, mineralization and H2O2 consumption was observed at a lower rate after 90 min irradiation. Therefore a new experiment, with adjustment of pH to 2.5 after 90 min irradiation, was carried out using the same concentrations of FecitNH4 and H2O2. The pH was adjusted to 2.5 only after 90 min, when the concentration of DCF was below the detection limit. As illustrated in Figure 4B, there was an improvement in TOC removal and an increase in H2O2 consumption, achieving almost total mineralization after 150 min irradiation. In the case of STP effluent, a slow decrease of TOC was observed in the first 45 min, especially at pH 7. A sharp decrease was then observed, achieving a plateau after 120 min, with residual TOC concentrations of 17 mg L-1 at pH 5 and 22 mg L-1 at pH 7.
In the presence of STP effluent at pH 7, only 38% DCF oxidation was achieved after 45 min (Figure 4A), corresponding to a half-life time of 67 min, 8.4 times longer than when present in distilled water (Table 2). The significant effect of STP effluent on DCF oxidation could be due to the presence of organic matter, which competes for hydroxyl radicals, as well as inorganic carbon (carbonate), a hydroxyl radical scavenger (equations 3 and 4):
HO· + HCO3- → H2O + CO3- (3)
HO· + CO32- → HO + CO3·- (4)
Decreased pharmaceutical degradation efficiency in simulated fresh water containing a high carbonate concentration has been reported in a recent study,35 where high amounts of iron were needed to improve the efficiency.
In the present study, another experiment was carried out after adjusting the STP effluent pH to 5, in order to evaluate the effect of carbonate species on the overall efficiency of the process. At this pH, the concentration of inorganic carbon decreased from 60 mg C L-1 to 3 mg C L-1 after stirring overnight. The concentration of DCF was below the detection limit in STP effluent after 75 min irradiation (Figure 4A), demonstrating the interference of carbonate species due to the scavenging of hydroxyl radicals generated during the photo-Fenton process, reducing the efficiency of the degradation.
Comparing the results obtained in the present work with previous results where ferric sulfate was employed as the iron source (at pHs 2.8, 4 and 7),20 a higher degradation rate was obtained in the presence of FecitNH4 complex, with a lower H2O2 concentration necessary for the total degradation of DCF.
Conclusions
The use of FecitNH4 complex in the photo-Fenton degradation of DCF under solar irradiation allowed the process to be carried out at pH values between 5 and 7. Although increase of the initial pH significantly decreased the degradation efficiency, the DCF concentration was below of the detection limit and 62% DCF oxidation were achieved at pH values between 5 and 7 after only 10 min irradiation. Significant mineralization demanded longer reaction times, as 77 % TOC was removed after 150 min irradiation at pH 7, using 0.2 mmol L-1 FecitNH4 and 68 mg L-1 H2O2. Further increase of the H2O2 concentration to 170 mg L-1 only slightly improved the DCF oxidation. When present in STP effluent at pH 7, the degradation of DCF was strongly affected by the high concentration of inorganic carbon, confirmed by the improvement of degradation after elimination of carbonates.
Acknowledgments
The authors thank FAPESP (05/00172-0) for the scholarship awarded to A.G. Trovó and CNPq (471283/2007-0) for the financial support of this work.
Submitted: May 19, 2010
Published online: February 10, 2011
FAPESP has sponsored the publication of this article.
- 1. Hernando, M. D.; Mezcua, M.; Fernández-Alba, A. R.; Barceló, D.; Talanta 2006, 69, 334.
- 2. Quinn, B.; Gagné, F.; Blaise, C. ; Sci. Total Environ 2008, 389, 306.
- 3. Conkle, J. L.; White, J. R.; Metcalfe, C. D.; Chemosphere 2008, 73, 1741.
- 4. Ternes, T. A.; Water Res. 1998, 32, 3245.
- 5. Stumpf, M.; Ternes, T. A.; Wilken, R. D.; Rodrigues, S. V.; Baumann, W.; Sci. Total Environ. 1999, 225, 135.
- 6. Gros, M.; Petrovic, M.; Barceló, D.; Talanta 2006, 70, 678.
- 7. Zuccato, E.; Castiglioni, S.; Fanelli, R.; J. Hazard. Mat. 2005, 122, 205.
- 8. Lindqvist, N.; Tuhkanen, T.; Kronberg, L.; Water Res. 2005, 39, 2219.
- 9. http://biq.iqm.unicamp.br/arquivos/teses/vtls000398476.pdf accessed in December 2008.
» link - 10. Lishman, L.; Smyth, S. A.; Sarafin, K.; Kleywegt, S.; Toito, J.; Peart, T.; Lee, B.; Servos, M.; Beland, M.; Seto, P.; Sci. Total Environ. 2006, 367, 554.
- 11. Gómez, M. J.; Bueno, M. J. M.; Lacorte, S.; Fernández-Alba, A. R.; Agüera, A.; Chemosphere 2007, 66, 993.
- 12. Seiler, J. P.; Toxicol. Lett. 2002, 131, 105.
- 13. Nogueira, R. F. P.; Trovó, A. G.; Silva, M. R. A.; Villa, R. D.; Quim. Nova 2007, 30, 400.
- 14. Trovó, A. G.; Melo, S. A.; Nogueira, R. F. P.; J. Photochem. Photobiol. A 2008, 198, 215.
- 15. Trovó, A. G.; Nogueira, R. F. P.; Aguera, A.; Fernandez-Alba, A. R.; Sirtori, C.; Malato, S.; Water Res. 2009, 43, 3922.
- 16. Buser, H-R.; Poiger, T.; Müller, M. D.; Environ. Sci. Technol. 1998, 32, 3449.
- 17. Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; d'Ischia, M.; Water Res. 2004, 38, 414.
- 18. Kim, I.; Tanaka, H.; Environ. Int. 2009, 35, 793.
- 19. Coelho, A. D.; Sans, C.; Aguera, A.; Gómez, M. J.; Esplugas, S.; Dezotti, M.; Sci. Total Environ. 2009, 407, 3572.
- 20. Pérez-Estrada, L. A.; Maldonado, M. I.; Gernjak, W.; Agüera, A.; Fernández-Alba, A. R.; Ballesteros, M. M.; Malato, S.; Catal. Today 2005, 101, 219.
- 21. Nogueira, R. F. P.; Silva, M. R. A.; Trovó, A. G.; Sol. Energy 2005, 79, 384.
- 22. Sun, Y.; Pignatello, J. J.; J. Agric. Food Chem. 1992, 40, 322.
- 23. Sillanpaa, M.; Pirkanniemi, K.; Environ. Tecnhol. 2001, 22, 791.
- 24. Abrahamson, H. B.; Rezvani, A. B.; Brushmiller, J. G.; Inorg. Chim. Acta 1994, 226, 117.
- 25. Zepp, R. G.; Faust, B. C.; Hoigné, J. ; Environ. Sci. Technol. 1992, 26, 313.
- 26. Nansheng, D.; Feng, W.; Fan, L.; Mei, X.; Chemosphere 1998, 36, 3101.
- 27. Silva, M. R. A.; Trovó, A. G.; Nogueira, R. F. P.; J. Photochem. Photobiol., A 2007, 191, 187.
- 28. Sun, Y.; Pignatello, J. J.; J. Agric. Food Chem. 1993, 41, 308.
- 29. Nogueira, R. F. P.; Oliveira, M. C.; Paterlini, W. C.; Talanta 2005, 66, 86.
- 30. Hug, S. J.; Canonica, L.; Wegelin, M.; Gechter, D.; Guntn, U. V.; Environ. Sci. Technol. 2001, 35, 2114.
- 31. Faust, B. C.; Zepp, R. G.; Environ. Sci. Technol. 1993, 27, 2517.
- 32. Parra, S.; Sarria, V.; Malato, S.; Péringer, P.; Pulgarin, C.; Appl. Catal., B 2000, 27, 153.
- 33. Kim, S. D.; Cho, J.; Kim, I. S.; Vanderford, B. J.; Snyder, S. A.; Water Res. 2007, 41, 1013.
- 34. Stulten, D.; Zühlke, S.; Lamshöft, M.; Spiteller, M.; Sci. Total Environ. 2008, 405, 310.
- 35. Klamerth, N.; Miranda, N.; Malato, S.; Agüera, A.; Fernández-Alba, A. R.; Maldonado, M. I.; Coronado, J. M.; Catal. Today 2009, 144, 124.
Publication Dates
-
Publication in this collection
10 June 2011 -
Date of issue
June 2011
History
-
Accepted
10 Feb 2011 -
Received
19 May 2010