Abstracts
The effects of domestic cooking on proteins, organic compounds and Fe distribution in beans (Phaseolus vulgaris L.) were investigated. Sequential extraction with different extractant solutions (mixture of methanol and chloroform 1:2 v/v, water, 0.5 mol L-1 NaCl, 70% v/v ethanol and 0.5 mol L-1 NaOH) were used for extracting lipids, albumins, globulins, prolamins and glutelins, respectively. Iron determination by graphite furnace atomic absorption spectrometry (GF AAS), proteins by Bradford method and organic compounds by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) were carried out in this work. High concentration of albumins, globulins and glutelins were found in raw beans, while in the cooked beans, albumins and glutelins are main proteins types. The MALDI-TOF MS spectra of raw and cooked beans revealed that the domestic cooking altered the molecular weight of the organic compounds, since that in the cooked beans were found compounds between 2 and 3.5 kDa, which were not presented in the raw beans. Besides this, in cooked beans were also observed the presence of four compounds of high molecular weight (12-16 kDa), being that in the raw grains there is only one (ca. 15.2 kDa). In raw grains is possible to observe that Fe is mainly associated to albumins, globulins and glutelins. For cooked grains, Fe is associated to albumins and globulins.
iron; proteins; beans; GF AAS; MALDI-TOF MS
Os efeitos do cozimento doméstico na distribuição de proteínas, compostos orgânicos e Fe em feijão (Phaseolus vulgaris L.) foram investigados. Extração seqüencial com diferentes agentes extratores (mistura de metanol e clorofórmio 1:2 v/v, água, NaCl, etanol e NaOH) foi usada para extrair lipídeos, albuminas, globulinas, prolaminas e glutelinas, respectivamente. A determinação de Fe por espectrometria de absorção atômica com forno de grafite (GF AAS), de proteínas pelo método de Bradford e compostos orgânicos por espectrometria de massas por tempo de vôo acoplada à ionização dessortiva de matriz por laser (MALDI-TOF MS) foram feitas nesse trabalho. Altas concentrações de albuminas, globulinas e glutelínas foram encontradas em feijão cru, enquanto que em grãos cozidos, albuminas e glutelínas foram os principais tipos de proteínas. Os espectros de MALDI-TOF MS do feijão cru e cozido revelaram que o cozimento doméstico alterou os pesos moleculares dos compostos orgânicos, uma vez que nos grãos cozidos foram encontrados compostos entre 2 e 3,5 kDa, os quais não estavam presentes no feijão cru. Além disso, no feijão cozido foi também observada a presença de quatro compostos de alto peso molecular (12-16 kDa), sendo que em grãos crus há somente um (ca.15,2 kDa). Nos grãos crus foi possível observar que Fe está principalmente associado a albuminas, globulinas e glutelínas. Para os grãos cozidos, Fe está presente em alta concentração em albuminas e globulinas.
ARTICLE
Cooking effects on iron and proteins content of beans (Phaseolus Vulgaris L.) by GF AAS and MALDI-TOF MS
Juliana NaozukaI,** e-mail: jnaozuka@gmail.com; Pedro V. OliveiraII
IUniversidade Federal de São Paulo, Diadema-SP, Brazil
IIDepartamento de Química Fundamental, Instituto de Química, Universidade de São Paulo, São Paulo-SP, Brazil
ABSTRACT
The effects of domestic cooking on proteins, organic compounds and Fe distribution in beans (Phaseolus vulgaris L.) were investigated. Sequential extraction with different extractant solutions (mixture of methanol and chloroform 1:2 v/v, water, 0.5 mol L-1 NaCl, 70% v/v ethanol and 0.5 mol L-1 NaOH) were used for extracting lipids, albumins, globulins, prolamins and glutelins, respectively. Iron determination by graphite furnace atomic absorption spectrometry (GF AAS), proteins by Bradford method and organic compounds by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) were carried out in this work. High concentration of albumins, globulins and glutelins were found in raw beans, while in the cooked beans, albumins and glutelins are main proteins types. The MALDI-TOF MS spectra of raw and cooked beans revealed that the domestic cooking altered the molecular weight of the organic compounds, since that in the cooked beans were found compounds between 2 and 3.5 kDa, which were not presented in the raw beans. Besides this, in cooked beans were also observed the presence of four compounds of high molecular weight (12-16 kDa), being that in the raw grains there is only one (ca. 15.2 kDa). In raw grains is possible to observe that Fe is mainly associated to albumins, globulins and glutelins. For cooked grains, Fe is associated to albumins and globulins.
Keywords: iron, proteins, beans, GF AAS, MALDI-TOF MS
RESUMO
Os efeitos do cozimento doméstico na distribuição de proteínas, compostos orgânicos e Fe em feijão (Phaseolus vulgaris L.) foram investigados. Extração seqüencial com diferentes agentes extratores (mistura de metanol e clorofórmio 1:2 v/v, água, NaCl, etanol e NaOH) foi usada para extrair lipídeos, albuminas, globulinas, prolaminas e glutelinas, respectivamente. A determinação de Fe por espectrometria de absorção atômica com forno de grafite (GF AAS), de proteínas pelo método de Bradford e compostos orgânicos por espectrometria de massas por tempo de vôo acoplada à ionização dessortiva de matriz por laser (MALDI-TOF MS) foram feitas nesse trabalho. Altas concentrações de albuminas, globulinas e glutelínas foram encontradas em feijão cru, enquanto que em grãos cozidos, albuminas e glutelínas foram os principais tipos de proteínas. Os espectros de MALDI-TOF MS do feijão cru e cozido revelaram que o cozimento doméstico alterou os pesos moleculares dos compostos orgânicos, uma vez que nos grãos cozidos foram encontrados compostos entre 2 e 3,5 kDa, os quais não estavam presentes no feijão cru. Além disso, no feijão cozido foi também observada a presença de quatro compostos de alto peso molecular (12-16 kDa), sendo que em grãos crus há somente um (ca.15,2 kDa). Nos grãos crus foi possível observar que Fe está principalmente associado a albuminas, globulinas e glutelínas. Para os grãos cozidos, Fe está presente em alta concentração em albuminas e globulinas.
Introduction
Beans (Phaseolus vulgaris L.) are a typical culture of tropical and subtropical weather. Brazil is the largest worldwide producer (2.2-2.5 million tons, in approximately 5 million hectares cultivated) and consumer (around 16 kg per capita). Beans is one of the richest foods in proteins consumed by the Brazilian population.1 Normally, countryside population is responsible for the high consumption of this food.1,2 However, consumption of beans is declining, due to the changes in traditional dietary habits of Brazilian population.2
Beans are a good source of vitamins, minerals (K, Ca, Mg, P and Fe salts), proteins (20-25%) and complex carbohydrates (50-60%).2-4 Besides their nutritional importance, beans contain certain anti-nutritional constituents, such as trypsin inhibitors, tannins, phytic acid and oligosaccharides that limit protein and carbohydrate absorption.5
The heating of beans can increase the protein and starch digestibility from 25-60% (raw grains) to 85% (cooked grains), depending on the species and cooking procedure.6 Futhermore, cooking promotes the production of desirable sensory properties in beans, such as sweet taste, cooked-bean flavor, and soft and mushy textures.2 However, cooking causes considerable changes in the composition of numerous chemical constituents, including amino acids, vitamins and minerals.3
The effects of cooking in the distribution of soluble iron were evaluated for legumes, beans, chickpeas and lentils.7 It was reported that cooking increases soluble iron contents presented in the cooking water. These studies are very important, because the heating can influence the iron bioavailability. It is generally accepted that only soluble non-heme iron can be absorbed. However, interactions with other compounds, such as anti-nutritional constituents, presented in the beans can also alter the iron bioavailability. Numerous studies have led to the conclusion that phytic acid and tannins may bind to proteins and some essential dietary minerals, making them unavailable or only partially available for absorption.8
Studies about heating effects in proteins and essential elements (e.g., Fe) are still limited, especially for cultivars commonly consumed in Brazil, where beans are the staple food. Additionally, in Brazil there are some research aiming the enrichment of beans with essential elements, such as iron. Consequently, the elaboration of methodologies that allow the evaluation of the cooking effects in the distribution of proteins and elements is imperative, since cooking is required to eat beans. Considering the nutritional importance, the aim of this work was to evaluate the effects of domestic cooking in beans on proteins, organic compounds and Fe distribution. Sequential extraction with different extracting agents (e.g., water, NaCl, ethanol and NaOH) and Fe, proteins and organic compounds determination by GF AAS, Bradford method9 and MALDI-TOF-MS, respectively, were used in this investigation.
Experimental
Instrumental
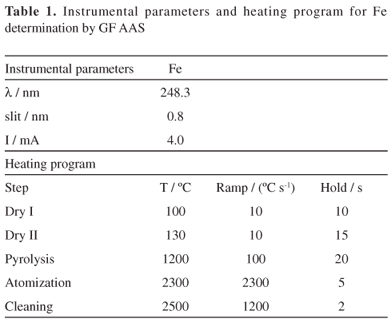
A ZEEnit 60 model atomic absorption spectrometer (AnalytikjenaAG, Jena, Germany) equipped with a transversely heated graphite atomizer, pyrolytically coated graphite tube, transversal Zeeman-effect background corrector was used for Fe determination. The spectrometer was operated using hollow cathode lamp. All measurements were based on integrated absorbance values. The instrumental conditions for the spectrometer and the heating program are shown in Table 1. Argon 99.998%, v/v (Air Liquide Brasil, São Paulo, Brazil) was used as protective and purge gas.
An Ultrospec 2100 pro spectrophotometer (Biochrom LTD, Cambridge, UK), equipped with xenon lamp and wavelength ranged to 190 from 900 nm was used for protein determination at 590 nm.
The digestion of samples and reference certified material (CRM) were carried out in a closed vessel microwave oven, model Microwave 3000 (Anton Paar, Graz, Austria), equipped with 16 fluoropolymer vessels and a ceramic vessel jacket. They support a maximum temperature and pressure of 240 ºC and 4 MPa, respectively. The internal temperature and pressure are monitored in only one controlling vessel using a sensor-protecting glass tube that enters directly into contact with digested solution. The external temperature is controlled in all vessels using an IR sensor, which measures each digestion vessel through ports in the rotor base.
An orbital shaker (Quimis, São Paulo, Brazil) was used to mix the samples and extracting agents using rotation velocity of 250 rpm for 30 min.
A vacuum filtration system of borosilicate glass, model XX15 047 00 (Millipore, USA) and a 0.45 mm Nylon® membrane filter (Millipore, USA) and a model Q222TM centrifuge (Quimis, São Paulo, Brazil) were used to separate the supernatant of the solid material after extractions.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS), model Ettan MALDI-TOF Pro MS (Amersham Biosciences, Uppsala, Sweden) was used for quantitative determination of organic species of beans after and before cooking.
Reagents and samples
All solutions were prepared with analytical reagent grade chemicals using high-purity deionized water obtained by Milli-Q® water purification system (Millipore, Bedford, USA). Nitric acid 65% (m/v) (Merck, Darmstadt, Germany) distilled in a quartz sub-boiling still (Marconi, SP, Brazil) and H2O2 30% (m/v) (Merck) were used for sample digestion. Tritisol® standard solution of 1000 mg L -1 of Fe (Merck) was used to prepare the reference analytical solutions by serial dilution in 0.14 mol L-1 HNO3.
Analytical grade chloroform, ethanol, methanol, NaCl and NaOH (Merck) were used for solid-liquid extractions.
The total protein concentration in each extractant (water, NaCl, ethanol and NaOH) was determined using Bradford method.9 The Bradford reagent was prepared using 10 mg of Coomassie Blue G-250, 5.0 mL of methanol and 10 mL of 85% (v/v) phosphoric acid (Sigma). The final volume (100 mL) was completed with deionized water. The protein standard was prepared dissolving 4.0 mg of ovoalbumin (BioAgency, São Paulo, Brazil) in 2.0 mL of deionized water, using Vortex stirring for 2 min. Afterward this solution was 10-fold diluted with deionized water.
For MALDI-TOF MS analysis, the dialyzed fractions (water fraction) were adequately diluted in saturated solution of sinapinic acid (LaserBio Labs, France) in 50% (v/v) acetronitrile (Merck, USA) and 0.5% (v/v) trifluoroacetic acid (TFA, Fluka, Switzerland).
The bean, called "carioca", used in this research was purchased at local markets. Before analysis, sample was washed with deionized water several times.
Corn (SRM 8433) as certified reference material from NIST (National Institute of Standards and Technology, Gaithersburg, Maryland, USA) was used to check the entire accuracy of the analytical method.
Sample digestion
Sample mass ranging from 150 to 250 mg were digested using a diluted oxidant mixture (2 mL HNO3 + 1 mL H2O2 + 3 mL H2O).10 This mixture was submitted to digestion in a closed-vessel microwave system. The heating program was performed in three steps (Temperature/ºC; ramp/min; hold/min): 1 (140; 5; 1); 2 (180, 4, 5); and 3 (220, 4, 10). There is a fourth step for cooling down the system through forced ventilation for a period of 20 min. After the digestion, samples (beans and corn bran) and analytical blank solutions were transferred to plastic flasks and made up to 10 mL with deionized water. Samples and CRM (corn) were digested in triplicate.
Cooking procedure
After cleaned with deionized water the beans were separated in two parts. The first portion was ground in a household food grinder and dried in stove at 60 ºC until constant mass. The second portion (ca. 500 g) was cooked in stainless steel pressure cooker in 1.0 L of deionized water for 40 min. A mixture of cooked grains and water was ground and dried using the same procedure adopted for the raw beans.
Sequential extraction
The sequential extraction procedure was described by Naozuka et al.11 Masses around 5.0 g of dried raw and cooked grains were used to solid-liquid sequential extraction with 10 mL of different extractants: methanol and chloroform (1:2 v/v), deionized water, 0.5 mol L-1 NaCl, 70 % (v/v) ethanol and 0.5 mol L-1 NaOH. An orbital shaker was used for extraction for 30 min. The separation of solid phase was carried out after filtration with 0.45 µm membrane filter (methanol and chloroform phase) and centrifugation using rotation velocity of 350 rpm for 30 min (other phases). Proteins and Fe were determined in the supernatants, except in the methanol/chloroform fraction. This experiment was performed in triplicate.
Total protein determination
The total protein concentration was determined in water, NaCl, ethanol, and NaOH fractions by the Bradford method. The spectrophotometer calibration was performed using analytical reference solutions of 4, 6, 8, 10, 12, 16 and 20 µg of ovoalbumin in 1.0 mL of Bradford reagent. Water and NaCl extracts were diluted 10-fold and NaOH fraction was diluted 20-fold. After dilution, a volume of 100 µL of these solutions was used for protein determination. The ethanol portion was not diluted.
MALDI-TOF MS analyses
The aqueous supernatants of raw and cooked grains were analyzed by MALDI-TOF MS. Aqueous supernatants volumes of 5 mL were put in a cellulose tubing of 32 mm diameter (D0530, Sigma-Aldrich) for dialysis in 1.0 L of deionized water over agitation (100 rpm for 24 h). The dialyzed aqueous fractions were mixed 1:1 (v/v) (raw grains) and 1:10 (v/v) (cooked grains) with a saturated solution of sinapinic acid in 50% (v/v) acetronitrile and 0.5% (v/v) trifluoroacetic acid. This mixture was agitated and subsequently centrifuged using rotation velocity of 5000 rpm for 10 s. An aliquot of 0.5 µL was spotted onto a MALDI plate and analyzed by MALDI-TOF MS.11
The analyses were performed in the linear mode using an acceleration voltage of 20 kV and a vacuum pressure of 1.5 10-6 bar. Laser pulses were generated by a nitrogen laser (8 pulses per second). Each spot was analyzed twice, accumulating mass spectra composed of a total of approximately 200 laser shots.
Fe determination by GF AAS
The digested samples and supernatants (water, NaCl, ethanol and NaOH) were analyzed by GF AAS. Appropriated dilution in deionized water, ranging from 2 to 50-fold, was performed, depending on the extractant. An aliquot of 10 mL was introduced into the graphite tube without chemical modifier and submitted to the heating program described in Table 1. The calibration was done using analytical reference solutions: 10-40 mg L-1 of Fe.
Chemical interferences were verified by addition and recovery test. For this, it was added an analytical solution of 40 µg L-1 Fe in all of the extractants, except in the methanol/chloroform fraction.
Results and Discussion
Cooking effects on protein concentration
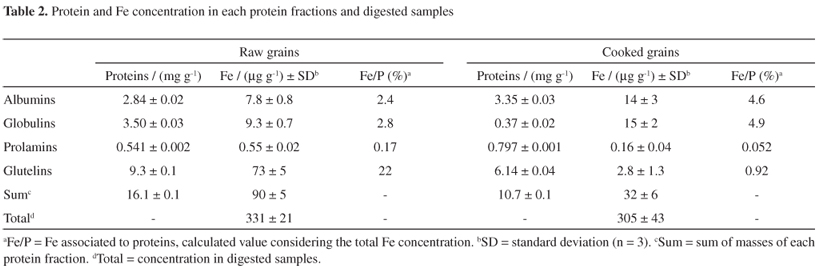
The protein determination by Bradford method9 was carried out in each extractant obtained after the sequential extraction (water, NaCl, ethanol and NaOH). The results are shown in the Table 2. It is well discussed in the literature12,13 that the extractants water, NaCl, ethanol and NaOH promoted the separation of different groups of proteins: albumins, globulins, prolamins and glutelins, respectively.12,13 The total protein concentration obtained by the masses sum of all extracts revealed that cooking promoted a sensible decrease in the protein content, mainly in the globulins fraction, when compared to the uncooked beans. Previous investigations showed that the total protein concentration ranged from 22.57 to 24.42 g per 100 g and from 23.25 to 26.29 g per 100 g for raw and cooked beans, respectively.14 Comparing these protein value with obtained protein concentration (sum of each fraction), it is possible to observe that low quantity of protein was extracted (6.6-7.0% and 4.1-4.6% for raw and cooked beans, respectively). However, it is important to point out that the domestic cooking changed the albumins, prolamins, globulins and glutelins distribution, since high concentrations of albumins, globulins and glutelins were found in raw grains, while albumins and glutelins were the main groups of proteins determined in cooked grains. Such behavior can be associated to the association-dissociation properties of proteins that are expected to be changed by heating, resulting in a decrease of the protein solubility.15
Cooking effects on organic compounds (MALDI-TOF MS)
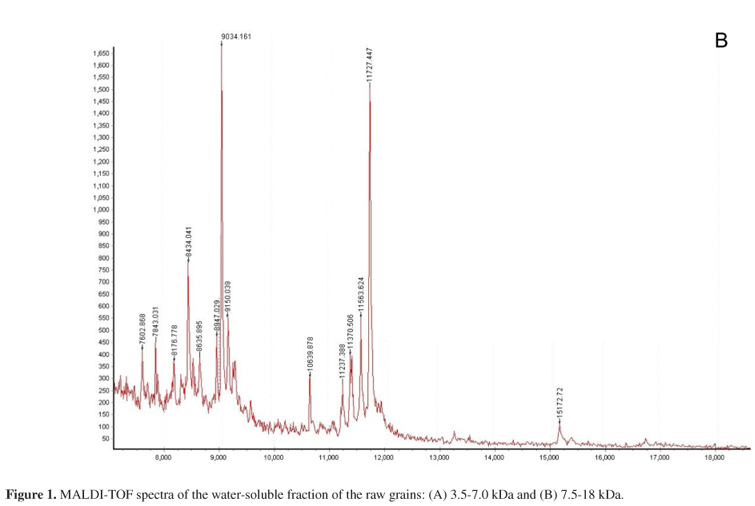
In Figures 1 and 2 are shown the MALDI-TOF MS spectra of aqueous supernatant phases of raw and cooked grains, respectively. Comparing the results, it is possible to observe that the heating procedure decreased the number of compounds of molecular weights ranged from 2 to 17 kDa (1 Da = 1.66110-24 g).16 The raw grains spectrum presented 32 compounds, while in the cooked grains results show only 22 compounds. Cooking resulted in a decrease of the molecular weights of the compounds in the beans. Compounds of molecular weight in the range of 2 and 3.5 kDa were only found in cooked grains. In the cooked beans, four high molecular weight compounds (12-16 kDa) were observed in the MALDI TOF MS spectrum (Figure 2). However, in the raw beans only one high molecular weight compound (ca. 15.2 kDa) is observed (Figure 1B). Even as the proteins, some organic compounds can be degraded with cooking procedure, such as tannins and phytate.14 Additionally, the association and dissociation of proteins can explain the presence of new compounds of low and high molecular weights after the domestic cooking. In immature seeds of flageolet bean, the cooking improved the high protein quality by destroying or inactivating heat-labile anti-nutritional factors, such as trypsin inhibitors, phytic acid, tannins and oligosaccharides.3,5
Cooking effects on Fe distribution in different extracts
Comparing the certified (14.8 ± 1.8 mg kg-1) with the obtained values (16.8 ± 3.8 mg kg-1), it is possible to assure that they are coincident at 95% of confidence interval, according to Student t test. The addition of analytical solution of Fe (40 µg L-1) in the water, NaCl, ethanol and NaOH extracts of the cooked grains showed recoveries of 89, 123, 96 and 100%, respectively, which indicate no interference during the Fe determination by GF AAS. According to NBR ISO/IEC 1702517 the recovery tolerance ranges from 70 to 120%.
Table 2 shows total Fe concentration in the digestates and in each fraction (water, NaCl, ethanol and NaOH). These results indicate that cooking did not affect Fe concentration. Deionized water used for cooking was the blank.
Comparing the total Fe concentration (digestates) with the concentration resulting from the sum of masses of Fe associated to the different protein groups (albumins, globulins, prolamins and glutelins), Table 2, it is interesting to observe that 27 and 10% of the Fe is associated to proteins (albumins, globulins, prolamins and glutelins) in the raw and cooked beans, respectively. It is evident that the domestic cooking affected the Fe distribution in these protein types. Additionally the heating also altered the Fe percentage in each fraction (Table 2), distinguishing the lost of Fe mainly in the glutelin fraction. It is possible that the heating can release Fe of the active sites of proteins, since that the transition metal ions, such as Fe2+, have the strongest coordinating interactions, thus being found in the majority of metalloproteins.16 Studies showed that cooking may affect macro and micronutrients bioavailability. The digestibility and hence absorption of micronutrients, such as Fe, is improved upon heat processing; with the resultant softening of the food matrix, protein-bound elements is released, thus facilitating its absorption. In addition, heat processing of food also alters the inherent factors that inhibit mineral absorption, such as phytate and dietary fiber. In white beans, the traditional cooking had a positive effect on the bioavailability of Ca, Zn and Fe.6,18
In raw grains, it is possible to observe that Fe is mainly associated to albumins, globulins and glutelins (Table 2). For cooked grains, Fe is presented in high concentration in albumins and globulins. The cooking procedure altered the association of Fe with glutelins. The main amino acids constituents of albumins, globulins, prolamins and glutelins are rich in sulfur and charged groups such as methionine, cysteine, glutamic acid, arginine, aspartic acid and lysine.11,19-21 The metal ions present high-affinity for these amino acids. The cooking effects in the elements distribution in these proteins types can be associated to alteration in the aminoacids content, since that heat procedure causes considerable changes in the composition of numerous chemical constituents, including amino acids, vitamins and minerals, depending on the temperature and time of thermal treatment.3 Studies with pulses showed that the long cooking time reduces the nutritive value and the levels of some essential amino acids.5
Conclusions
Cooking affected the chemical composition of beans. Total Fe concentration was constant after the heating. Total protein concentration was reduced, indicating the domestic cooking altered the protein structure and consequently their solubility in the different extractants (water, NaCl, ethanol and NaOH). Some organic compounds were changed, considering their molecular weights (< 3.5 and > 12 kDa). The cooking also affected the Fe distribution in the different protein types (albumins, globulins, prolamins and glutelins), mainly in the glutelin fraction. The heating is capable of promoting protein denaturation, association-dissociation of proteins, inactivation or destruction of anti-nutritional components and alteration of elemental bioavailability.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support and fellowship provided. P.V.O. is thankful to CNPq for the research fellowship.
Submitted: October 25, 2010
Published online: November 8, 2011
FAPESP has sponsored the publication of this article.
- 1. Brigide, P.; Canniatti-Brazaca, S. G.; Food Chem. 2006, 98, 85.
- 2. Ranilla, L. G.; Genovese, M. I.; Lajolo, F. M.; J. Agric. Food Chem. 2009, 57, 5734.
- 3. Słupski, J.; Food Chem. 2010, 121, 1171.
- 4. Saha, S; Singh, G.; Mahajan, V.; Gupta, H. S.; Plant Foods Hum. Nutr. 2009, 64, 174.
- 5. Wang, N.; Hatcher, D. W.; Tyler, R. T.; Toews, R.; Gawalko, E. J.; Food Res. Int. 2010, 43, 589.
- 6. Bressani, R.; Food Rev. Int. 1993, 9, 237.
- 7. Quinteros, A.; Farre, R.; Lagarda, M. J.; Food Chem. 2001, 75, 365.
- 8. Ramírez-Cárdenas, L.; Leonel, A. J.; Costa, N. M. B.; Reis, F. P.; Food Res. Int. 2010, 43, 573.
- 9. Bradford, M. M.; Anal. Biochem. 1976, 72, 248
- 10. Naozuka, J.; Vieira, E. C.; Nascimento, A. N.; Oliveira, P. V.; Food Chem. 2011, 124, 1667.
- 11. Naozuka, J.; Marana, S. R.; Oliveira, P. V.; J. Food Compos. Anal. 2010, 23, 78.
- 12. Chunhieng, T.; Pétritis, K.; Elfakir, C.; Brochier, J.; Goli, T.; Montet, D.; J. Agric. Food Chem. 2004, 52, 4318.
- 13. Naozuka, J.; Oliveira, P. V.; J. Braz. Chem. Soc. 2007, 18, 1547.
- 14. Ramírez-Cárdenas; Leonel, A. J.; Costa, N. M. B.; Cienc. Tecnol. Aliment. 2008, 28, 200.
- 15. Carbonaro, M.; Vecchini, P.; Carnovale, E.; J. Agric. Food Chem. 1993, 41, 1169.
- 16. Garcia, J.S.; Magalhães, C. S.; Arruda, M. A. Z.; Talanta 2006, 69, 1.
- 17. Burin, R.; Burin, V. M.;Taha, P.; Bordignon-Luiz, M. T.; Ciênc. Tecnol. Aliment. 2008, 28, 973.
- 18. Lombardi-Boccia, G.; Santis, N.; Lullo, G. D.; Carnovale, E.; Food Chem. 1995, 53, 191.
- 19. Garcia, R. M.; Arocena, R. V.; Laurena, A. C.; Tecson-Mendoza, E. M.; J. Agric. Food Chem. 2005, 53, 1734.
- 20. Moreno, F. J.; Jenkins, J. A.; Mellon, F. A.; Rigby, N. M.; Robertson, J. A.; Wellner, N.; Mills, E. N. C.; Biochim. Biophys. Acta 2004, 1698, 175
- 21. Sun, S. S. M.; Leung, F. W.; Tomic, J. C.; J. Agric. Food Chem. 1987, 35, 232.
Publication Dates
-
Publication in this collection
02 Feb 2012 -
Date of issue
Jan 2012
History
-
Received
25 Aug 2010 -
Accepted
08 Nov 2011