Abstracts
Three new 8-O-4'-neolignans, fargesiphenols A-C, together with six known neolignans, were isolated from the flower buds of Magnolia fargesii. The structures were elucidated by spectroscopic methods, including extensive 1D and 2D-NMR techniques. Compounds were also tested for their anti-HIV-1 activities and cytotoxicities.
Magnolia fargesii; 8-O-4'-neolignans; anti-HIV-1 activity; cytotoxicity
Três novos derivados de 8-O-4'-neolignanas, fargesifenols A-C, juntamente com seis neolignanas conhecidas, foram isoladas de botões de flores de Magnolia fargesii. As estruturas foram elucidadas por métodos espectroscópicos, incluindo técnicas de RMN 1D e 2D. Os compostos foram também testados quanto à sua atividade anti-HIV-1 e quanto às citotoxicidades.
ARTICLE
8-O-4'-Neolignans from flower buds of Magnolia fargesii and their biological activities
Xuemei GaoI; Yanqiong ShenI; Liying YangI,II; Lidang ShuI; Ganpeng LiI,II,* * e-mail: huqiufena@yahoo.com, ganpeng_li@sina.com ; Qiu-Fen HuI,* * e-mail: huqiufena@yahoo.com, ganpeng_li@sina.com
IKey Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Yunnan University of Nationalities, Kunming 650031, P. R. China
IIKey Laboratory of Tobacco Chemistry of Yunnan Province, Yunnan Academy of Tobacco Science, Kunming 650106, P. R. China
ABSTRACT
Three new 8-O-4'-neolignans, fargesiphenols A-C, together with six known neolignans, were isolated from the flower buds of Magnolia fargesii. The structures were elucidated by spectroscopic methods, including extensive 1D and 2D-NMR techniques. Compounds were also tested for their anti-HIV-1 activities and cytotoxicities.
Keywords: Magnolia fargesii, 8-O-4'-neolignans, anti-HIV-1 activity, cytotoxicity
RESUMO
Três novos derivados de 8-O-4'-neolignanas, fargesifenols A-C, juntamente com seis neolignanas conhecidas, foram isoladas de botões de flores de Magnolia fargesii. As estruturas foram elucidadas por métodos espectroscópicos, incluindo técnicas de RMN 1D e 2D. Os compostos foram também testados quanto à sua atividade anti-HIV-1 e quanto às citotoxicidades.
Introduction
The genus of Magnolia (Magnoliaceae) has traditionally been used as herb medicine in China for a long time. Especially, Xinyi (dried flower buds of Magnolia fargesii), has been used for the treatment of inflammatory-related diseases such as nasal congestion, empyema, sinusitis, and allergic rhinitis.1,2 Previous phytochemical investigations have reported that this species contains several secondary metabolites such as lignans,3-5 neolignans,6,7 sesquiterpenes,2,8 and essential oils,9 which show various biological activities.
To search for more new bioactive compounds from this plant, we reexamined the flower buds of M. fargesii, which led to the isolation of three new 8-O-4'-neolignans, named fargesiphenols A-C (1-3), along with six known compounds (4-9). In addition, the anti-HIV-1 activities and their cytotoxicities were evaluated. Their structure elucidation and biological activities are described in this paper.
Results and Discussion
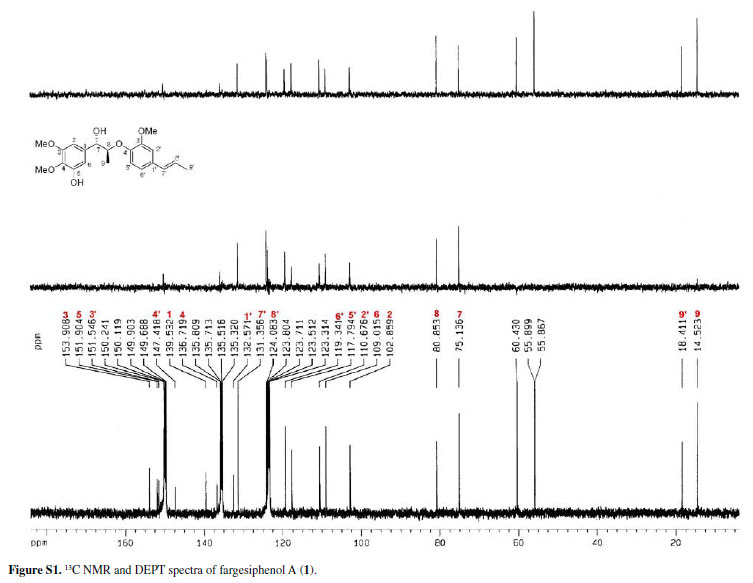
A 70% aq. acetone extract prepared from the flower buds of M. fargesii was partitioned between EtOAc and H2O. The EtOAc layer was subjected repeatedly to column chromatography on Si gel, Sephadex LH-20, RP-18 and preparative HPLC to afford compounds 1-9, including three new 8-O-4'-neolignans named fargesiphenols A-C (1-3), together with six known neolignans, polysyphorin (4),10 virolin (5),11 7S,8S-threo-4,7,9,9'tetrahydroxy-3,3'-dimethoxy-8-O-4'-neolignan (6),12 7S,8R-erythro- 4,7,9,9'-tetrahydroxy-3,3'-dimethoxy-8-O4'-neolignan (7),12 (7R,8S)-1-(3,4-dimethoxyphenyl)-2-[4(3-hydroxy-1-propenyl)-methoxy-phenoxy]-propane-1,3diol (8),13 and rhaphidecursinol A (9).10 The structures of compounds 1-9 are shown in Figure 1, and the 1H and 13C NMR data of compounds 1-3 are listed in Table 1.
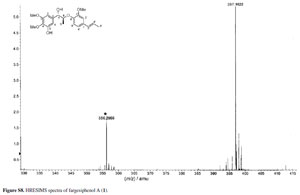
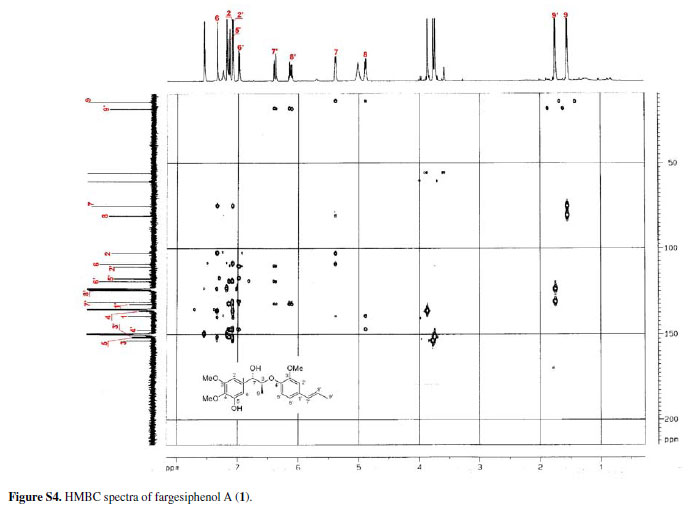
Compound 1 was obtained as pale yellow gum. Its molecular formula was determined as C21H26O6 from the HRESIMS quasi-molecular ion peak [M+Na]+ at m/z 397.1622 (calc. 397.1627). Its 1H and 13C NMR spectra showed signals of 26 hydrogens and 21 carbons, respectively, corresponding to two aromatic rings with five aromatic protons (δH 6.99, 7.34, 7.09, 7.15, 6.98), two methyl groups (δC 14.5, 18.4), three methoxyl groups (δC 55.9, 55.9, 60.4), two oxidated methine groups (δC 75.1, 80.9), one allyl group (δC 131.4, 124.1, 18.4; δH 6.38 d J 15.8, 6.10-6.17 m, 1.75 d J 6.5), and a phenolic hydroxyl group (δH 11.25). Strong absorption bands accounting for hydroxyl (3498 cm-1) and aromatic groups (1608, 1520, 1478 cm-1) could also be observed in its IR spectrum. The UV spectrum of 1 showed absorption maxima at 285 and 210 nm, which confirmed the existence of the aromatic functions. The 1H-1H COSY correlations H-7/H-8/H-9, H-7'/H-8'/H-9', together with HMBC correlations (Figure 2) of H-7 (δH 5.38) with C-8 (δC 80.9), C-9 (δC 14.5), C-1 (δC 139.5), C-2 (δC 102.9) and C-6 (δC 109.0), of H-7' (δH 6.38) with C-2' (δC 110.7), C-1' (δC 132.6) and C-6' (δC 119.3), and H-8 (δC 4.88) with C-1 (δC 139.5) and C-4' (δC 147.4), suggested that 1 is a 8-O-4'neolignan possessing three methoxyl groups and a phenolic hydroxyl group.14
Three methoxyl groups located at C-3, C-4, C-3' were assigned from the HMBC correlations of the methoxyl proton signals (δH 3.77, 3.88, 3.74) with C-3 (δC 153.9), C-4 (δC 136.7), and C-3' (δC 151.9), respectively. The presence of a phenolic hydroxyl group at C-5 was supported by the HMBC correlations of the phenolic hydroxyl proton signal (δH 11.25) with C-4 (δC 136.7), C-5 (δC 151.9), and C-6 (δC 109.0), respectively. Thus, the plain structure of 1 was established.
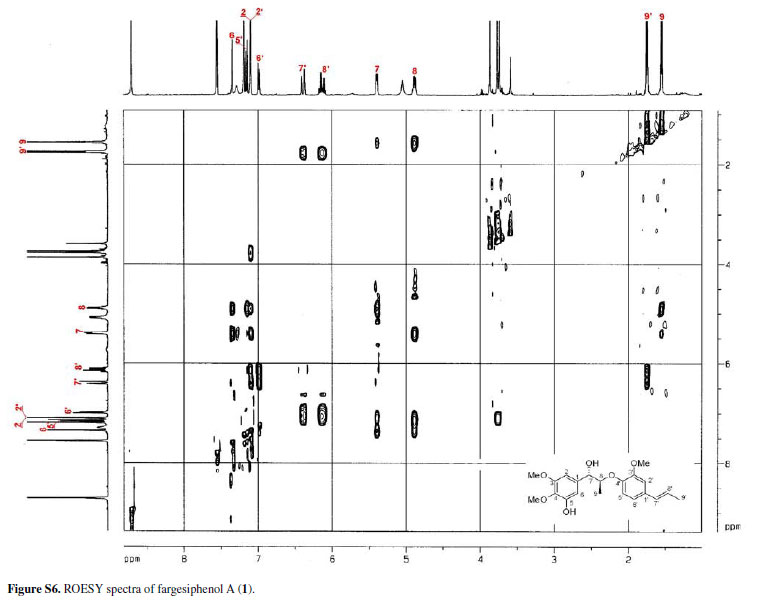
The configuration of the two chiral carbons (C-7 and C-8) was considered to be threo according to the coupling constant (J 7.2 Hz) between H-7 and H-8,15 which is distinct from that of the erythro diastereoisomers.16,17 The absolute configurations at C-7 and C-8 of 1 could be established on the basis of ROESY and CD spectroscopic evidence.18 The CD spectrum of 1 gave a positive cotton effect at 237 nm, and clear ROESY correlations observed between H-7 and H-8, H-7 and H-2, H-7 and H3-9 (Figure 3) indicated the 7S, 8S-configuration of 1, which was named fargesiphenol A.
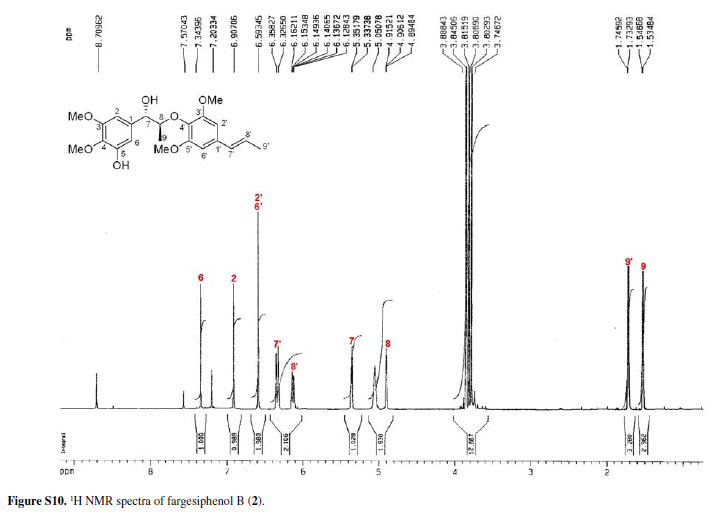
Compounds 2 and 3 (fargesiphenols B and C) were all obtained as yellow gums. HRESIMS analysis of 2 demonstrated that it has the molecular formula C22 H28O7 (m/z 427.1730, for [M+Na]+, calc. 427.1733). The NMR spectra of 2, when compared to those of 1, displayed an additional methoxy group (δH 3.85; δC 55.6), which was located at C-5' from the HMBC cross-peak between this carbon (δC 152.3) and the methoxyl protons (δC 3.85).
The NMR spectra of 3, when compared to those of 2, displayed the characteristic signals of an acetoxy group (δH 2.19, δC 21.1 and δC 169.9). Its 13C NMR spectrum showed downfield-shifted signals for C-4 (δC 142.1) and C-6 (δC 111.4), and an upfield-shifted signal for C-5 (δC 144.8), thus corroborating the placement of the acetoxy group at C-5. According to the observed ROESY correlations and comparison of 1H and 13C NMR data with those of 1 (Table 1), the relative configurations of 2 and 3 were established as being the same as that of 1. The configuration of the two chiral carbons (C-7 and C-8) for 2 and 3 was considered to be threo according to the coupling constant (J 7.2 Hz) between H-7 and H-8, and a positive cotton effect at 237 nm for 2, and 238 nm for 3 in their CD spectrum indicated a 7S,8S-configuration. 15
Since some neolignans are reported to possess anti-HIV activities,19,20 these have been tested for compounds 1-9. The cytotoxicity assay against C8166 cells (CC50), and antiHIV-1 activity were evaluated by the inhibition assay for the cytopathic effects of HIV-1 (EC50), using azidothymidine (AZT) as a positive control (EC50 = 0.034 µg mL-1 and CC50 = 200 µg mL-1).21 The results (Table 2) revealed significant activity for compounds 2, 6, 7, and 8, with therapeutic index (TI) values above 30. Compounds 1, 4, and 9 showed moderate activity with TI values above 10.
Some neolignans are also reported to possess cytotoxic activities,22,23 which led us to evaluate compounds 1-9 for their cytotoxicities. The cytotoxicity tests were performed in triplicate using a previously reported procedure.24 In the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay, the IC50 was defined as the concentration of the tested compound resulting in a 50% reduction of absorbance compared with untreated cells. The cytotoxic activities against HL-60, Hep-G2, KB, and MDA-MB-231 tumor cell lines by MTT-assay (with camptothecin as the positive control) are shown in Table 3. Compound 2 revealed high cytotoxic activity to HL-60 and MDA-MB-231 cells, whereas compound 8 showed high cytotoxic activity to KB cells, both with IC50 values close to those of positive control. The other compounds displayed moderate or weak cytotoxic activity.
Experimental
General experimental procedures
Optical rotations were measured with a Horiba SEPA-300 polarimeter. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer. A Tenor 27 spectrophotometer was used for scanning IR spectra with KBr pellets. CD spectra were measured on a JASCO J-810 spectropolarimeter. 1D and 2D NMR spectra were recorded on DRX-500 spectrometers with TMS as internal standard. HRESIMS was performed on an API QSTAR time-offlight spectrometer and a VG Autospec-3000 spectrometer, respectively. Preparative HPLC was performed on a Shimadzu LC-8A preparative liquid chromatograph with a ZORBAX PrepHT GF (21.2 mm x 25 cm, 7 µm) column or a Venusil MP C18 (20 mm x 25 cm, 5 µm) column. Column chromatography was performed with Si gel (200-300 mesh, Qing-dao Marine Chemical, Inc., Qingdao, China), Lichroprep RP-18 gel (40-63 µm, Merck, Darmstadt, Germany) and MCI gel (75-150 µm, Mitsubishi Chemical Corporation, Tokyo, Japan). The fractions were monitored by TLC, and spots were visualized by heating Si gel plates sprayed with 5% H2SO4 in EtOH.
Plant material
The flower buds of M. fargesii, indigenous to Nanzhao country, Henang province, were purchased from Kunming Herb Medicine Market in September 2010. A voucher specimen (YNNI-10-9-28) has been deposited in our laboratory.
Extraction and isolation
The air-dried and powdered flower buds of Magnolia fargesii (4.5 kg) were extracted four times with 70% aqueous acetone (4 x 3 L) at room temperature, filtered, and the filtrate evaporated under reduced pressure and partitioned with EtOAc (4 x 3 L). The EtOAc phase (212 g) was applied to silica gel (200-300 mesh) column chromatography, eluting with a CHCl3-MeOH gradient system (20:1, 9:1, 8:2, 7:3, 6:4, 5:5), to give six fractions A-F. Further separation of fraction B (35.8 g) by silica gel column chromatography, eluted with CHCl3-acetone (9:1-2:1), yielded mixtures B1-B6. Fraction B2 (8:2, 4.8 g) was subjected to silica gel column chromatography using petroleum ether-acetone, and semi-preparative HPLC (55% MeOH-H2O, flow rate 12 mL min-1) to give 3 (22.6 mg), 4 (18.9 mg), 5 (22.7 mg) and 9 (28.5 mg). Fraction B3 (7:3, 5.47 g) was subjected to silica gel column chromatography using CHCl3-acetone, and semi-preparative HPLC (48% MeOH-H2O, flow rate 12 mL min-1) to give 1 (11.6 mg) and 2 (28.9 mg). Fraction B4 (7:3, 3.28 g) was subjected to silica gel column chromatography using CHCl3-acetone, and semi-preparative HPLC (40% MeOH-H2O, flow rate 12 mL min-1) to give 3 (14.2 mg), 6 (22.5 mg), and 7 (18.2 mg).
Anti-HIV-1 assay
The cytotoxicity assay against C8166 cells (CC50) was assessed using the MTT method, and anti-HIV-1 activity was evaluated by the inhibition assay for the cytopathic effects of HIV-1 (EC50).21
Cytotoxicity assay
The cytotoxicity tests for these compounds were performed against HL-60, Hep-G2, KB, and MDA-MB-231 tumor cell lines by the MTT-assay using camptothecin as positive control.24
Fargesiphenol A (1)
Pale yellow gum;  +38.5 (c 0.20, MeOH); CD (c 0.05, MeOH):
+38.5 (c 0.20, MeOH); CD (c 0.05, MeOH):  220 nm +0.36,
220 nm +0.36,  237 nm +7.18,
237 nm +7.18,  280 nm -0.97,
280 nm -0.97,  320 nm -0.21; UV (MeOH)
320 nm -0.21; UV (MeOH)  (log
(log  ) 320 (2.82), 285 (3.78), 210 (4.27) nm; IR (KBr) νmax/cm-1: 3498, 2957, 2874, 1608, 1520, 1478, 1440, 1265, 958; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 397 [M+Na]+; HRESIMS m/z 397.1622 [M+Na]+ (calc. C21H26NaO6 for 397.1627).
) 320 (2.82), 285 (3.78), 210 (4.27) nm; IR (KBr) νmax/cm-1: 3498, 2957, 2874, 1608, 1520, 1478, 1440, 1265, 958; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 397 [M+Na]+; HRESIMS m/z 397.1622 [M+Na]+ (calc. C21H26NaO6 for 397.1627).
Fargesiphenol B (2)
Yellow gum;  +41.2 (c 0.22, MeOH); CD (c 0.05, MeOH):
+41.2 (c 0.22, MeOH); CD (c 0.05, MeOH):  220 nm +1.28,
220 nm +1.28,  237 nm +14.6,
237 nm +14.6,  280 nm -2.18,
280 nm -2.18,  320 nm -0.96; UV (MeOH)
320 nm -0.96; UV (MeOH)  (log
(log  ) 320 (2.88), 282 (3.82), 210 (4.23) nm; IR (KBr) νmax/cm-1: 3495, 2959, 2873, 1608, 1524, 1486, 1437, 1263, 954; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 427 [M+Na]+; HRESIMS m/z 427.1730 [M+Na]+ (calc. C22H28NaO7 for 427.1733).
) 320 (2.88), 282 (3.82), 210 (4.23) nm; IR (KBr) νmax/cm-1: 3495, 2959, 2873, 1608, 1524, 1486, 1437, 1263, 954; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 427 [M+Na]+; HRESIMS m/z 427.1730 [M+Na]+ (calc. C22H28NaO7 for 427.1733).
Fargesiphenol C (3)
Yellow gum;  +42.5 (c 0.22, MeOH); CD (c 0.05, MeOH):
+42.5 (c 0.22, MeOH); CD (c 0.05, MeOH):  220 nm +0.47,
220 nm +0.47,  238 nm +6.52,
238 nm +6.52,  280 nm -0.83,
280 nm -0.83,  320 nm -0.49; UV (MeOH)
320 nm -0.49; UV (MeOH)  (log
(log  ) 318 (2.89), 280 (3.86), 210 (4.20) nm; IR (KBr) νmax/cm-1: 3493, 2950, 2876, 1614, 1529, 1482, 1442, 1260, 959; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 469 [M+Na]+; HRESIMS m/z 469.1833 [M+Na]+ (calc. C24H30NaO8 for 469.1838).
) 318 (2.89), 280 (3.86), 210 (4.20) nm; IR (KBr) νmax/cm-1: 3493, 2950, 2876, 1614, 1529, 1482, 1442, 1260, 959; 1H NMR (500 MHz) and 13C NMR (125 MHz) data, Table 1; positive ESIMS m/z 469 [M+Na]+; HRESIMS m/z 469.1833 [M+Na]+ (calc. C24H30NaO8 for 469.1838).
Supplementary Information
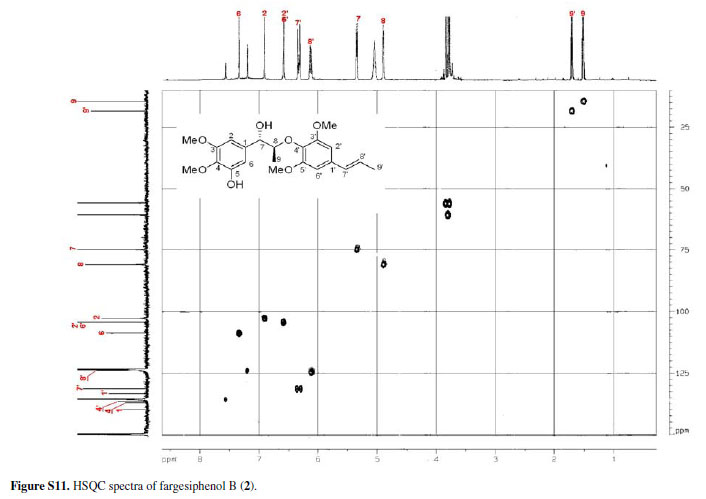
13C NMR, DEPT, 11H-1H COSY, ROESY, CD, and HRESIMS spectra of fargesiphenol A; 13C NMR, DEPT, 1H NMR, HSQC, HMBC, and CD spectra of fargesiphenol B and C, are available free of charge at http://jbcs.sbq.org.br as PDF file.
Acknowledgments
This project was supported financially by the Excellent Scientific and Technological Team of Yunnan High School (2010CI08), and Green Chemistry and Functional Materials Research for Yunnan Innovation Team (2011HC008).
Submitted: February 12, 2012
Published online: June 19, 2012
Supplementary Information
Figure S1 - Click to enlarge
Figure S2 - Click to enlarge
Figure S3 - Click to enlarge
Figure S4 - Click to enlarge
Figure S5 - Click to enlarge
Figure S6 - Click to enlarge
Figure S7 - Click to enlarge
Figure S8 - Click to enlarge
Figure S9 - Click to enlarge
Figure S10 - Click to enlarge
Figure S11 - Click to enlarge
Figure S12 - Click to enlarge
Figure S13 - Click to enlarge
Figure S14 - Click to enlarge
Figure S15 - Click to enlarge
Figure S16 - Click to enlarge
Figure S17 - Click to enlarge
Figure S18 - Click to enlarge
- 1. Miyazawa, M.; Kasahara, H.; Kameoka, H.; Phytochemistry 1992, 31, 3666.
- 2. Lee, Y. J.; Lee, Y. M.; Lee, C. K.; Jung, J. K.; Han, S. B.; Hong, J. T.; Pharmacol. Ther. 2011, 130, 157.
- 3. Huang, Y. L.; Chen, C. C.; Chen, Y. P.; Hsu, H. Y.; Kuo, Y. H.; Planta Med. 1990, 56, 237.
- 4. Miyazawa, M.; Ishikawa, Y.; Kasahara, H.; Yamanaka, J.; Kameoka, H.; Phytochemistry 1994, 35, 611.
- 5. Lee, J.; Lee, D.; Jang, D. S.; Nam, J. W.; Kim, J. P.; Park, K. H.; Yang, M. S.; Seo, E. K.; Chem. Pharm. Bull 2007, 55, 137.
- 6. Lee, J.; Seo, E. K.; Jang, D. S.; Ha, T. J.; Kim, J. P.; Nam, J. W.; Bae, G.; Kim, J. S.; Chem. Pharm. Bull 2009, 57, 298.
- 7. Kelm, M. A.; Nair, M. G.; Stud. Nat. Prod. Chem 2000, 24, 845.
- 8. Jung, K. Y.; Kim, D. S.; Park, S. H.; Lee, I. S.; Oh, S. R.; Lee, J. J.; Kim, E. H.; Lee, H. K.; Phytochemistry 1998, 48, 1383.
- 9. Fang, J. Y.; Tsai, T. H.; Hung, C. F.; Wong, W. W.; J. Pharm. Pharmacol. 2004, 56, 1493.
- 10. Le, T. X.; Le, M. H.; Nguyen. M. C.; Do, T. T.; Soejarto, D. D.; Fong, H. H.; Pezzuto, J. M.; J. Nat. Prod 2001, 64, 772.
- 11. Barata, L. E.; Santos, L. S.; Ferri, P. H.; Phillipson, J. D.; Paine, A.; Croft, S. L.; Phytochemistry 2000, 55, 589.
- 12. Matsuda, N.; Kikuchi, M.; Chem. Pharm. Bull 1996, 44, 1676.
- 13. Lee, J.; Seo, E. K.; Jang, D. S.; Ha, T. J.; Kim, J. P.; Nam, J. W.; Bae, G.; Lee, Y. M.; Yang, M. S.; Kim, J. S.; Chem. Pharm. Bull 2009, 57, 298.
- 14. Barata, L. E.; Baker, P. M.; Gottlieb, O. R.; Rùveda, E. A.; Phytochemistry 1978, 17, 783.
- 15. Cavalcante, S. H.; Yoshida, M.; Gottlieb, O. R.; Phytochemistry 1985, 24, 1051.
- 16. Fukagawa, N.; Ishizu, A.; J. Wood Chem. Technol. 1991, 11, 263.
- 17. Yoshikawa, M.; Morikawa, T.; Xu, F.; Ando, S.; Matsuda, H.; Heterocycles 2003, 60, 1787.
- 18. Matsuda, N.; Kikuchi, M.; Chem. Pharm. Bull 1996, 44, 1676.
- 19. Zhang, X. J.; Yang, G. Y.; Wang, R. R.; Pu, J. X.; Sun, H. D.; Xiao, W. L.; Zheng, Y. T.; Chem. Biodiversity 2010, 7, 2692.
- 20. Pu, J. X.; Yang, L. M.; Xiao, W. L.; Li, R. T.; Lei, C.; Gao, X. M.; Huang, S. X.; Li, S. H.; Zheng, Y. T.; Huang, H.; Sun, H. D.; Phytochemistry 2008, 69, 1266.
- 21. Darke, P. L.; Huff, J. R.; Adv. Pharmacol 1994, 25, 399.
- 22. Hahm, J. C.; Lee, I. K.; Kang, W. K.; Kim, S. U.; Ahn, Y. J.; Planta Med. 2005, 71, 464.
- 23. Amblard, F.; Govindarajan, B.; Lefkove, B.; Rapp, K. L.; Detorio, M.; Arbiser, J. L.; Schinazi, R. F.; Bioorg. Med. Chem. Lett. 2007, 17, 4428.
- 24. Mosmann, T.; J. Immunol. Methods 1983, 65, 55.
Publication Dates
-
Publication in this collection
06 Aug 2012 -
Date of issue
July 2012
History
-
Received
12 Feb 2012 -
Accepted
19 June 2012