Abstracts
The main objective of this work was to evaluate the presence of residues of chlorpyrifos and thiamethoxam in potato and soil samples under different circumstances after optimization and validation of solid-liquid extraction and partitioning at low temperature technique. Recovery percentages between 93% and 105% were obtained for pesticides in the samples. The optimized and validated method was applied to potatoes from supermarkets and it was obtained chlorpyrifos residue at concentrations of 0.67 mg kg-1. Potatoes grown in the field were treated with pesticide on different days and chlorpyrifos was the only residue detected. The potatoes grown in pots were treated with chlorpyrifos and thiamethoxam only at planting. After 91 days, it was observed the concentration of thiamethoxam in the potatoes higher than the tolerable MRL. The proposed method was efficient in the determination of chlorpyrifos and thiamethoxam and it was concluded that the monitoring of pesticides in food must be performed periodically to ensure the product quality and the safety of consumer's health.
gas chromatography; chlorpyrifos; thiamethoxam; potato (Solanum tuberosum L.); soil
O principal objetivo deste trabalho foi avaliar a presença de resíduos de clorpirifós e tiametoxam em amostras de batata e solo em diferentes circunstâncias, após a otimização e validação da técnica de extração sólido-líquido e partição em baixa temperatura. Porcentagens de recuperação entre 93% e 105% foram obtidas para os pesticidas nas amostras. O método otimizado e validado foi aplicado às amostras de batata obtidas em supermercado e foi obtido resíduo de clorpirifós na concentração de 0,67 mg kg-1. Batatas cultivadas no campo foram tratadas com os pesticidas em diferentes dias e resíduo de clorpirifós foi detectado. As batatas cultivadas em vasos foram tratadas com clorpirifós e tiametoxam somente no plantio. Após 91 dias, foi observada uma concentração de tiametoxam acima do LMR permitido. O método proposto foi eficiente na determinação de clorpirifós e tiametoxam e foi concluído que o monitoramento de agrotóxicos deve ser realizado periodicamente para garantir a qualidade dos produtos e a saúde do consumidor.
ARTICLE
Determination of chlorpyrifos and thiamethoxam in potato tuber (Solanum tuberosum L.) and soil of Brazil using solid-liquid extraction with low temperature partitioning (SLE/LTP)
Leila M. B. RigueiraI, * * e-mail: drikalle@yahoo.com.br , # # Current address: Department of Chemistry, State University of Montes Claros, Menino de Deus Street, 441, 39390-000 Bocaiuva-MG, Brazil ; Kamilla de L. RibeiroI; Maria Eliana L. R. de QueirozI; Antônio A. NevesI; Laércio ZambolimII; Ricardo M. OliveiraI
IDepartment of Chemistry, Federal University of Viçosa, Vila Gianetti, Casa 24, 36570-000 Viçosa-MG, Brazil
IIDepartment of Phytopathology, Federal University of Viçosa, Av. P. H. Rolfs, s/n, 36570-000 Viçosa-MG, Brazil
ABSTRACT
The main objective of this work was to evaluate the presence of residues of chlorpyrifos and thiamethoxam in potato and soil samples under different circumstances after optimization and validation of solid-liquid extraction and partitioning at low temperature technique. Recovery percentages between 93% and 105% were obtained for pesticides in the samples. The optimized and validated method was applied to potatoes from supermarkets and it was obtained chlorpyrifos residue at concentrations of 0.67 mg kg-1. Potatoes grown in the field were treated with pesticide on different days and chlorpyrifos was the only residue detected. The potatoes grown in pots were treated with chlorpyrifos and thiamethoxam only at planting. After 91 days, it was observed the concentration of thiamethoxam in the potatoes higher than the tolerable MRL. The proposed method was efficient in the determination of chlorpyrifos and thiamethoxam and it was concluded that the monitoring of pesticides in food must be performed periodically to ensure the product quality and the safety of consumer's health.
Keywords: gas chromatography, chlorpyrifos, thiamethoxam, potato (Solanum tuberosum L.), soil
RESUMO
O principal objetivo deste trabalho foi avaliar a presença de resíduos de clorpirifós e tiametoxam em amostras de batata e solo em diferentes circunstâncias, após a otimização e validação da técnica de extração sólido-líquido e partição em baixa temperatura. Porcentagens de recuperação entre 93% e 105% foram obtidas para os pesticidas nas amostras. O método otimizado e validado foi aplicado às amostras de batata obtidas em supermercado e foi obtido resíduo de clorpirifós na concentração de 0,67 mg kg-1. Batatas cultivadas no campo foram tratadas com os pesticidas em diferentes dias e resíduo de clorpirifós foi detectado. As batatas cultivadas em vasos foram tratadas com clorpirifós e tiametoxam somente no plantio. Após 91 dias, foi observada uma concentração de tiametoxam acima do LMR permitido. O método proposto foi eficiente na determinação de clorpirifós e tiametoxam e foi concluído que o monitoramento de agrotóxicos deve ser realizado periodicamente para garantir a qualidade dos produtos e a saúde do consumidor.
Introduction
The potato (Solanum tuberosum L.) is considered a crop of great importance worldwide,1 however, one of the limiting factors of this crop is closely linked to its susceptibility to pests and diseases, which results in the use of large amounts of pesticides throughout its growing cycle.2,3
The southeastern state of Minas Gerais, Brazil, has on potato cultivation an important economic activity, being considered one of the main producing areas of the country. The association of producers in that region uses, under appropriate technical guidance, agricultural practices in order to get high productivity without severely contaminate the environment.
Among the main pesticides used in this crop, chlorpyrifos and thiamethoxam (Figure 1) are the most cited due to their high efficiency of pest control. When improperly applied, residues from these pesticides can remain in the product or soil, generating an important pathway to human exposure and environmental contamination.
Considering this, several studies have been conducted to verify the presence of pesticides residues in vegetables and soil, as well as their dissipation and adsorption in the soil.4-10
The conventional method for determining pesticide residues in fruits and vegetables is based on solvent extraction, followed by clean up steps and the chromatography analysis of the extract.11 The techniques of solid-liquid or liquid-liquid extraction with low temperature partitioning (SLE/LTP and LLE/LTP),12-14 used in this study allow that the analyte extraction and the clean up of the extracts are performed in a single step. When lowering the temperature (-20 °C), the matrix is solidified and the solvent extractor (supernatant) can be recovered for further analysis.
The main objective of this study was to evaluate the presence of pesticide residues in soil and potatoes that were purchased at supermarkets, grown in the field or in containers and treated with chlorpyrifos and thiamethoxam. For this purpose, the extraction method was optimized and validated by the SLE/LTP technique for determination of pesticides in potatoes and soils using gas chromatography electron capture detection (GC/ECD).
Experimental
Chemicals
In this work, standards of chlorpyrifos (99.0% (m/m), Chem Service) and thiamethoxam (97.0% (m/m), Syngenta) were used. The solvents acetonitrile (Mallinckrodt, HPLC grade) and ethyl acetate (Mallinckrodt) were used as extractants and anhydrous sodium sulfate (Mallinckrodt) was used to remove water from the extracts.
The stock standard solutions at concentrations of 500.0 mg L-1 of chlorpyrifos and thiamethoxam were individually prepared in acetonitrile and stored in the freezer. After dilution of the stock solutions, a working standard solution containing the pesticides chlorpyrifos and thiamethoxam at a concentration of 10.0 mg L-1 was prepared. The solutions were stored at approximately -20 °C.
Equipment
A Shimadzu (GC-17-A) gas chromatograph equipped with an electron capture detector (ECD) was used for pesticide determination. The capillary column used in the separation was a HP-5 from Agilent Technologies, with the stationary phase consisting of 5% diphenyl and 95% dimethylsiloxane, measuring 30 m long, 0.25 mm of id and 0.1 µm of film thickness, and nitrogen used as the carrier gas at a flow rate of 1.2 mL min-1. The column-heating program began at 200 °C, increasing at 10 °C per min until reaching 260 °C. The total analysis time was 6 min. The injector and detector temperatures were maintained, respectively at 280 and 300 °C. A volume of 1.0 µL from the sample was injected into the chromatograph with a split ratio of 1:5.
Samples Preparation
Potato
Pesticide free potato samples acquired from local grocery stores have been used as verified by the blank sample analysis. To study the extraction efficiency, the potato samples were crushed using a blender and 3.00 g of the sample were fortified with a standard solution containing the active ingredients, at the final concentration of 1.67 mg kg-1. The mixture was allowed to stand for 3 h to evaporate the solvent and to promote a greater interaction between the pesticides and the sample, prior to extraction.
Soil
Soil classified as Oxisol Red-Yellow was collected at the campus of the University of Viçosa in a single location at depth of 0 to 20 cm after removal of vegetation cover, and used in the optimization and technical validation stages of SLE/LTP. This site was selected for being a pesticide free area. The sample was air dried and sieved through a 2 mm mesh.
For evaluation of extraction efficiency, 1.00 g of soil sample was spiked with known amounts of pesticides, homogenized and the mixture allowed to stand for 2 h before initiating the extraction procedure. The final pesticide concentration in these soil samples was 5.0 mg kg-1.
Optimization of the solid-liquid extraction technique with low-temperature partition
Potato samples
The methodology optimized and validated by Dardengo et al. (2011)15 for the extraction of chlorpyrifos, λ-cyhalothrin, cypermethrin and deltamethrin in potato samples has been adapted for the simultaneous extraction of chlorpyrifos and thiamethoxam from these samples. In this method, 3.00 g of potato pulp was placed in contact with 1.0 mL of the 0.2 mol L-1 NaH2PO4 solution, 6.5 mL of acetonitrile and 1.5 mL of ethyl acetate, followed by 45 min of mechanical stirring at 180 rpm. Subsequently, the mixture was placed in the freezer at approximately -20 °C for 12 h. After the allotted time, the extract was filtered through filter paper, previously washed with 5.0 mL of acetonitrile containing 1.5 g of anhydrous sodium sulfate. The extract was recovered in 10.0 mL volumetric flask and volume completed with acetonitrile. The solution was stored in a vial and frozen prior to chromatographic analysis.
The technique optimized and validated was used for all samples, with recovery percentages averaging 93% and 96% for chlorpyrifos and thiamethoxam, respectively.
Soil samples
Factorial design
In optimizing the SLE/LTP technique for chlorpyrifos and thiamethoxam extraction from soil samples, a 23 factorial design was used to study the concurrent behavior of three factors: (1) proportion of acetonitrile:ethyl acetate in the extraction solvent, (2) ionic strength and (3) sample agitation time. These three factors were studied at two levels (Table 1) and analysis was performed in triplicate. The levels of the three factors used in the factorial design were established based on previous experiments.
To a clear 22.0 mL vial with a cap, 1.00 g of the soil sample spiked with known amounts of chlorpyrifos and thiamethoxam was added. This was followed by the addition of either 4.0 mL of water or the NaH2PO4 0.2 mol L-1 solution, corresponding to the (-) and (+) levels, respectively. Next, 8.0 mL of the extraction solution was added as indicated in the factorial design (Table 1). This solution was maintained under mechanical agitation in a shaker (TE Tecnal - 420) for 30 or 45 min corresponding to the levels (-) and (+), respectively, at 25 °C and 180 oscillations per minute (rpm).
The samples were stored at -20 ºC for approximately 12 h. After this period, the organic liquid phase was recovered by simple filtration containing 1.5 g of anhydrous sodium sulfate (Nuclear - P.A.). The paper filter was previously rinsed with approximately 5.0 mL of cooled acetonitrile. The volume of the extracts were adjusted to 10.0 mL, transferred to vials and stored in a freezer until chromatographic analysis.
The recovery percentages obtained in each test were used to evaluate the effects of each factor and their interactions.
Ultrasound effect on the extraction efficiency of the chlorpyrifos and thiamethoxam from soil
After the 23 factorial designs using the best conditions for extraction efficiency, the effect of ultrasonic waves on pesticide extraction was evaluated.
Soil samples (1.00 g) previously spiked with pesticides were added to the ideal solvent extractor mixture, established in the factorial design. Subsequently, samples were subjected to ultrasound for 5 and 10 min. The later stages of freezing and filtering through paper with sodium sulfate were identical to those described previously.
Analytical curve
Quantification of potato and soil extracts containing the pesticides chlorpyrifos and thiamethoxam was evaluated through an external standard; analytical curves were constructed with seven concentrations ranging from 10.0 to 1000.0 µmg L-1.
Method validation
The figures of merit related to the validation process including selectivity, limit of detection (LOD), limit of quantification (LOQ), linearity response, precision and accuracy were determined as recommended by Brazilian16,17 and international regulatory agencies,18 and by related publications.19,20
SLE/LTP technique application
Potato samples purchased at supermarkets in Viçosa
The validated method was applied for determination of pesticides in potato samples randomly acquired from three supermarkets in the city of Viçosa, Minas Gerais, Brazil. Samples of 1 kg of potatoes of each supermarket were collected and stored in a freezer until analysis. These samples were purchased in November, during a warm and rainy season.
Potato samples grown in the field
Experiments were conducted under field conditions to determine residue levels in potatoes in comparison with pesticides recommended for pest control in this crop. Experiments were conducted in the municipality of Senador Amaral, in southern Minas Gerais which is the largest potato producing region in Brazil. The experiment consisted of the following treatments:
Treatment 1: control (free of pesticides); treatment 2: application of thiamethoxam in the furrow before planting as recommended by the manufacturer (15 kg ha-1); treatment 3: application of chlorpyrifos in the furrow before planting as recommended by the manufacturer (4.0 L ha-1) plus two sprayings of the plant tillers (5 and 8 weeks after planting) and once during drying of the foliage, using the same pesticide. Pesticides were applied using a manual backpack sprayer, coupled with conical jet nozzle and pressure of 30 psi. The volume of each solution was approximately of 600 L ha-1. A randomized block experimental design with three treatments and seven replications was used. Each replicate (experimental unit) consisted of two rows of 200 plants.
After foliage drying, fifteen potato samples were harvested (about 3.00 kg) from each replicate per treatment. The samples were packed in coolers with dry ice and taken to the laboratory. Each potato sample was crushed, subjected to the stages of extraction using the SLE/LTP technique, and analyzed by GC/ECD.
Potato samples grown in containers
In this study, seed potatoes certified by Embrapa were planted in containers (diameter: 19 cm, height: 16 cm). In the planting furrow of the three containers, 0.1 mL of the commercial suspension was applied containing chlorpyrifos as the active ingredient. This dose is in accordance with the one recommended by the manufacturer of 4 L ha-1. In three other containers, 0.4 g of the commercial product containing the active ingredient thiamethoxam was applied to the furrow, corresponding to 15 kg ha-1 as recommended by the manufacturer. Three containers were maintained as control (Blank). The containers were kept in open air at the Laboratório de Química Analítica (Analytical Chemistry Laboratory) Laqua/UFV, being irrigated every three days.
Collection of potatoes and soil samples for analysis was performed on the 13th week after planting. All soil and potato samples were collected from each container. Samples from each treatment were carefully homogenized and tests were performed in triplicate, submitted to the same extraction stages, and analyzed by GC/ECD.
Results and Discussion
Chromatographic analysis
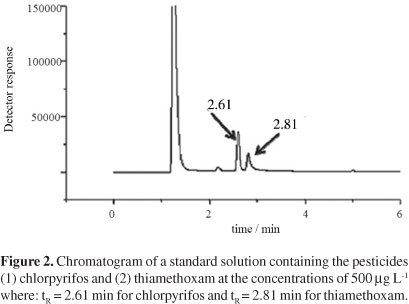
Figure 2 shows a chromatogram of the standard solution, consisting of 500 µg L-1 of the pesticides prepared in acetonitrile. Peaks with retention times (tR) equal to 2.61 min and 2.81 min correspond to chlorpyrifos and thiamethoxam, respectively.
It is observed in Figure 2 that the total analysis time was of 6 min. Despite the pesticides tR being close, the resolution was adequate, with non-overlapping peaks, thus allowing the identification and quantification of the pesticides.
Optimization of the solid-liquid extraction technique with low-temperature partitioning for soil samples
Factorial design
During optimization of the extraction technique and analysis of pesticides, effects of the three factors were evaluated simultaneously: the proportion acetonitrile to ethyl acetate in the solvent extraction, ionic strength and sample agitation time. In this evaluation, a 23 factorial design was utilized and the effects evaluated with regards to recovery percentages. The assays, conducted in duplicate, generated 16 responses allowing for estimation of the experimental errors associated with determining each average response. With these repetitions it was also possible for the statistical software Statistic® to calculate the average recovery percentages, the effects of each factor and the interactions among the factors in the extraction of each pesticide. Errors associated with each effect and their interactions were assessed by the "t" test at 95% probability. Some results of the statistical analysis are presented in Table 2.
Statistical analysis of the results contained in Table 2 shows that none of the factors have a significant effect at the probability level of 95% on the percent recovery of pesticides in the matrices.
Although at the assessed levels the factors had no significant effect on the recovery percentages of active ingredients, a tendency is noted. Table 2 shows that for chlorpyrifos extraction from the soil, factors 1 and 2, evaluated separately, showed a significant trend, i.e., when working with the level (+) of the solvent extractor mixture and level (-) in relation to ionic strength of the medium. Factor 3, agitation time, presented no significance and can be used at any level within the limits studied.
For extraction of thiamethoxam from soil, the interaction between factors 2 and 3 shows that better results are obtained when these factors are at level (+). Factors 1 and 3, the proportion of solvent extractor and agitation time, should also be utilized at the level (+), as shown in Table 2.
The decrease in the solvent extractor polarity favored the extraction of the pesticides chlorpyrifos and thiamethoxam in soil samples. The increase in ionic strength generally results in reduced solubility of the analyte in the matrix, facilitating its extraction.12 This was observed for the extraction of thiamethoxam in soil samples. However, it was found in the present work that for the extraction of chlorpyrifos from soil, the increase in the salt concentration can hinder the formation of a single phase, thus decreasing the pesticide extraction percentage.
Effects of ultrasound on the extraction efficiency of chlorpyrifos and thiamethoxam in soil
The sample agitation stage is a relevant factor in the optimization of technique. Agitation plays a role on interaction of the solvents with the analyte of interest in this stage.
Sonication has been used as an option for agitation and to assist extraction processes.21 Soil samples spiked with chlorpyrifos and thiamethoxam were subjected to 5 and 10 min of sonication, following the other parameters optimized in the 23 factorial designs.
The use of ultrasound during the period studied did not favor extraction of the referred pesticides from soil samples, therefore mechanical agitation was the option used.
Optimization methodology
The optimized method for analysis of chlorpyrifos and thiamethoxam in soil samples using the SLE/LTD technique was as follows: 1.00 g of soil was weighed, and then 4.0 mL of water, 6.5 mL of acetonitrile and 1.5 mL of ethyl acetate were added, with subsequent mechanical agitation for 45 min at 180 rpm.
Samples were then placed in the freezer for about 12 h. After this period, the extracts were filtered through paper along with 1.5 g of anhydrous sodium sulfate, where the paper filter was previously washed with 5.0 mL of cold acetonitrile. The extract volumes were adjusted to 10.0 mL with acetonitrile, and then transferred to vial and stored in the freezer until the moment of chromatographic analysis.
It is relevant to mention that due to the complexity of the soil matrix and the low concentration levels at which pesticides are found, the sample preparation becomes an important step in the analysis to obtain reliable results. Thus, the extraction procedure used in this work is very important because it involves fewer steps and these ones are enough for cleaning of extracts, not necessitating a subsequent clean up.
Method validation
The detector response was linear in the range of the concentration studied (10.0 to 1000 µg L-1). Determination coefficients (r2) obtained from the linear regression of experimental data are in accordance with the minimum established by Inmetro (2010)17 which recommends values higher than 0.99.
Detection and quantification limits were determined by the signal to noise ratio method.22,23 The detection limit of chlorpyrifos in potato and soil samples was lower than 0.004 mg kg-1 and the quantification limit was less than 0.012 mg kg-1. As for the pesticide thiamethoxam, the detection and quantification limits in potato and soil samples were 0.006 and 0.015 mg kg-1. The LOQ values were lower than the maximum residue levels for chlorpyrifos and thiamethoxam in potato established by the Codex Alimentarius (FAO/WHO),24 which are 1.0 and 0.02 mg kg-1, respectively.
The repeatability of the SLE/LTP technique for analysis of the pesticides chlorpyrifos and thiamethoxam in potatoes and soils was determined by pesticide extraction from spiked samples in seven replicates for calculation of the coefficient of variation CV (%), as recommended by the Inmetro (2010).17 The obtained coefficients of variation were below 5% for pesticides found in potato and soil samples. These values are considerably lower than the maximum acceptable level for complex samples, which can be up to 20%.18-19
Intermediate precision of the method was evaluated using the coefficient of variation CV (%), obtained from tests performed on different days by the same analyst. The results showed a coefficient of variation lower than 5% for chlorpyrifos and thiamethoxam in the matrices studied. It was also confirmed that the technique of extraction and analysis of pesticides in potato and soil is not influenced when performed on different days in the same laboratory and by the same analyst on different days.
The accuracy was calculated according to the Inmetro (2010)17 recommendations. The substances of interest were added in concentrations close to 1, 2 and 10 times the limit of quantification. Recovery percentages obtained in this study are between 93 and 105%, noting a greater dispersion of results for samples spiked with lower concentrations. Because the sample concentration is very close to the LOQ, CV (%) values of up to 20% are acceptable.18,19
SLE/LTP technique application
Potato samples purchased at supermarkets in Viçosa
The optimized and validated SLE/LTP technique was applied to potato samples collected in supermarkets located in Viçosa, MG (Brazil). Of all samples analyzed, only one sample from supermarkets showed chlorpyrifos residues at concentrations equal to 0.67 mg kg-1, which is below the maximum residue limit (MRL) established for potatoes (1.0 mg kg-1).
Figure 3 shows the chromatogram of the potato sample with a high concentration of chlorpyrifos.
Samples of potatoes grown in fields
In this study, the optimized and validated SLE/LTP technique was used to determine residues of chlorpyrifos and thiamethoxam in potato samples treated with these commercial products.
In samples of treatment 1 (control) and 2 (thiamethoxam applied in the planting furrow at the recommended dose) there were no signs of residues from the active ingredients studied.
Application of thiamethoxam in the planting furrow indicated that the use of commercial products according to manufacturer instructions results in samples that are reliable and suitable for consumption.
The treatment with chlorpyrifos (treatment 3) showed that residues were found at levels lower than the MRL established for this crop (1.0 mg kg-1). The average concentration of chlorpyrifos determined in samples of potato was 0.084 mg kg-1.
Samples of potatoes grown in containers
The optimized and validated SLE/LTP technique was applied in potato and soil samples obtained from the container in which the potatoes were grown to determine the presence of chlorpyrifos and thiamethoxam residues.
According to the results obtained, the amount of chlorpyrifos residue in potatoes and soil at 91 days after planting was 0.19 mg kg-1 and 5.0 mg kg-1, respectively. This pesticide concentration in the potato is below MRL allowed by law. Chlorpyrifos concentration in the soil was determined to be approximately 25 times higher than in potato samples. This is relevant since this active ingredient in soil can be leached or remain in the soil until its complete degradation, with impacts on the environment. This fact can be explained by considering that chlorpyrifos is a non-systemic pesticide, thus, it is not absorbed by the roots and transferred to all parts of the plants, tending to remain close to where it is applied (soil) forming a deposit on the potato surface (skin).25
Regarding thiamethoxam, concentrations of 0.12 and 0.19 mg kg-1 were determined in the potato and soil samples, respectively. According to the results obtained, thiamethoxam concentration in potato is higher than the allowed MRL (0.02 mg kg-1). The levels of this pesticide residue found in soil were similar to those found in the potatoes, a different behavior from that one observed for chlorpyrifos. Because of its systemic properties (rapid penetration and distribution within the plant), thiamethoxam can be found in similar concentrations both on the soil and in the potato.
This experiment was conducted in containers in order to maintain better control over environmental factors such as: rainfall, soil volume and quantities of the active ingredients used and their residue in both soil and potatoes, therefore, this experiment simulates a real situation of planting, so the results were evaluated only as a tendency.
Conclusions
The optimized and validated solid-liquid extraction with low temperature partitioning technique was efficient in the determination of chlorpyrifos and thiamethoxam in potato and soil samples, with recovery percentages greater than 93% and good precision and accuracy.
Potatoes samples purchased from supermarkets in Viçosa, MG (Brazil) were analyzed and the pesticide chlorpyrifos was found at a concentration of 0.67 mg kg-1. Although, this value is lower than the MRL permitted, potatoes samples should be routinely evaluated to verify the reliability of these vegetables.
The concentrations of chlorpyrifos and thiamethoxam were lower than the MRL allowed in potato samples analyzed after planting. This shows that, by following the recommendations of the manufacturer, it is possible to obtain potatoes with low pesticide concentrations.
According to the results obtained for soil and potato samples and for the potatoes grown in containers, it was observed that chlorpyrifos tends to remain at high levels in the soil, with little transfer to the potato. For thiamethoxam, similar concentrations were found in the soil and potato, both above the MRL allowed for potato samples.
Acknowledgements
We greatly appreciate the assistance of the Associação dos Bataticultores do Estado de Minas Gerais (ABASMIG) represented by Dr. José Daniel Rodrigues Ribeiro for providing the potato seedling and potato planting area, and Emater-MG, especially Dr. Raul Cassia Maria for his guidance in conducting the field work.
Submitted: May 16, 2013
Published online: October 23, 2013
-
1Zohair, A.; Food Chem. Toxicol 2001, 39, 751.
-
2Malvoni, M. Q.; Silva, E. C.; de Mendonça, R. S.; Maciel, G. M.; Ocorrência e Entomofauna no Cultivo de Batata na Região de Alfenas-MG, http://www.abbabatatabrasileira.com.br/revista10_020.htm accessed in April 2013.
-
3Knežević, Z.; Serdar, M.; Food Control 2009, 20, 419.
-
4Lakshmi, C. V.; Kumar, M.; Khanna, S.; Int. Biodeterior. Biodegrad. 2008, 62, 204.
-
5Randhawa, M. A.; Anjum, F. M.; Ahmed, A.; Randhawa, M. S.; Food Chem 2007, 103, 1016.
-
6Obana, H.; Okinashi, M.; Akutsu, K.; Kitagawa, Y.; Hori, S.; J. Agric. Food Chem 2003, 51, 2501.
-
7Mohan, S. V.; Sirisha, K.; Rao, N. C.; Sarma, P. N.; Reddy, S. J.; J. Hazard. Mater. 2004, 116, 39.
-
8de Urzedo, A. P. F. M.; Rigitano, R. L. O.; Guerreiro, M. C.; Castro, N. R. A.; Pesticidas: R. Ecotox. Meio Ambiente 2006, 16, 31.
-
9Nault, B. A.; Taylor, A. G.; Urwiler, M.; Rabaey, T.; Hutchison, W. D.; Crop. Prot. 2004, 23, 147.
-
10Diez-Rodríguez, G. I.; de Baptista, G. C.; Trevizan, L. R. P.; Haddad, M. L.; Nava, D. E.; Neotrop. Entomol. 2006, 35, 257.
-
11Sharif, Z.; Man, Y. B. C.; Hamid, N. S. A.; Keat, C. C.; J. Chromatogr. A 2006, 1127, 254.
-
12Vieira, H. P.; Neves, A. A.; de Queiroz, M. E. L. R.; Quím. Nova 2007, 30, 535.
-
13Goulart, S. M.; de Queiroz, M. E. L. R.; Neves, A. A.; de Queiroz, J. H.; Talanta 2008, 75, 1320.
-
14de Pinho, G. P.; Neves, A. A.; de Queiroz, M. E. L. R.; Silvério, F. O.; Food Chem. 2010, 121, 251.
-
15Dardengo, R. P.; Gulart, S.; Neves, A. A.; Reis, C.; Zambolim, L.; de Queiroz, M. E. L. R.; BrJAC 2011, 3, 136.
-
16Agência Nacional de Vigilância Sanitária (ANVISA); Guia para Validação de Métodos Analíticos e Bioanalíticos, Resolução-RE No. 899, Brasil, 2003.
-
17Instituto Nacional de Metrologia, Normalização e Qualidade Industrial (Inmetro); Orientações sobre Validação de Métodos de Ensaios Químicos, DOQ-CGCRE-008, Inmetro: Brasil, 2010.
-
18EURACHEM/CITAC. Determinando a incerteza na medida analítica, QUAM: 2002 - Versão Brasileira, Brasil, 2002.
-
19Ribani, M.; Bottoli, C. B. G.; Collins, C. H.; Jardim, I. C. S. F.; Melo, L. F. C.; Quím. Nova 2004, 27, 771.
-
20Lanças, F. M.; Limite de Detecção e Quantificação, 1a. ed.; Ed. Rima: São Carlos, SP, Brasil, 1990; ch. 3.
-
21Korn, M.; Pereira, M. G.; Borges, S. S.; Boletim da Sociedade Portuguesa de Química 2005, 96, 51.
-
22Collins, C. H.; Braga, G. L.; Bonato, P. S.; Fundamentos de Cromatografia; Ed. Unicamp: Campinas, SP, Brasil, 2006.
-
23Su, Y-S.; Jen, J-F.; J. Chromatogr. A 2010, 1217, 5043.
-
24Comisión del Codex Alimentarius. Programa conjunto FAO/OMS sobre normas alimentarias, Comote del Codex sobre resíduos de Plaguicidas, USA, 1996.
-
25Barbosa, L. C. A. Os grupos químicos dos agrotóxicos, 2a. ed.; Ed. UFV: Viçosa, MG, Brasil, 2004, ch. 2.
Publication Dates
-
Publication in this collection
09 Dec 2013 -
Date of issue
Dec 2013
History
-
Received
16 May 2013 -
Accepted
23 Oct 2013