Abstracts
The lipase-catalyzed synthesis of thiamphenicol derivatives has been studied through complementary acylation and hydrolytic approaches, finding Candida antarctica lipase B as the most efficient biocatalyst for the selective modification of both thiamphenicol and thiamphenicol diacetate, respectively. The best results have been obtained using acylation reactions with different vinyl esters of variable length, yielding the corresponding 3'-monoesters with excellent yields and in short reaction times. The conditions have been analyzed in terms of substrate concentration, enzyme loading and type of acyl donor. The reuse of the enzyme for five-times without significant loss of the activity has also been demonstrated. Alternatively, the hydrolytic approach has allowed the preparation of some 1'-monoesters in good yields, although the reactivity and selectivity levels were lower than the ones achieved for the complementary acetylation reaction.
biocatalysis; lipases; medium engineering; regioselective processes; thiamphenicol
A síntese de derivados do tianfenicol, catalisada por lipases, foi investigada através da realização das reações de acilação e de hidrólise, como abordagens complementares, sendo a lipase Candida antarctica B o biocatalisador mais eficiente para as modificações seletivas de ambos, o tianfenicol e o correspondente diacetato do tianfenicol, respectivamente. Os melhores resultados foram obtidos nas reações de acilação com diferentes ésteres vinílicos, contendo cadeias carbônicas de comprimento variável, levando aos correspondentes 3'-monoésteres com excelentes rendimentos e em tempos reacionais curtos. As condições reacionais foram analisadas em termos de concentração de substrato, carga de enzima e tipo de doador de acila. Foi demonstrado o reuso da enzima por cinco vezes sem perda significativa de atividade. Alternativamente, a abordagem hidrolítica propiciou a preparação de alguns 1'-monoésteres em bons rendimentos, ainda que os níveis de reatividade e seletividade foram mais baixos que os obtidos na reação complementar de acetilação.
ARTICLE
Regioselective preparation of thiamphenicol esters through lipase-catalyzed processes
Marcos R. da SilvaI, II; Tasso G. C. MontenegroI, II; Marcos C. de MattosII,* * e-mail: mcdmatto@ufc.br, vgs@uniovi.es ; Maria da Conceição F. de OliveiraII; Telma L. G. de LemosII; Gonzalo de GonzaloI; Iván LavanderaI; Vicente Gotor-FernándezI; Vicente GotorI,* * e-mail: mcdmatto@ufc.br, vgs@uniovi.es
IDepartamento de Química Orgánica e Inorgánica, Instituto Universitario de Biotecnología de Asturias, Universidad de Oviedo, c/ Julián Clavería 8, 33006, Oviedo, Spain
IIDepartamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, 60451-970 Fortaleza-CE, Brasil
ABSTRACT
The lipase-catalyzed synthesis of thiamphenicol derivatives has been studied through complementary acylation and hydrolytic approaches, finding Candida antarctica lipase B as the most efficient biocatalyst for the selective modification of both thiamphenicol and thiamphenicol diacetate, respectively. The best results have been obtained using acylation reactions with different vinyl esters of variable length, yielding the corresponding 3'-monoesters with excellent yields and in short reaction times. The conditions have been analyzed in terms of substrate concentration, enzyme loading and type of acyl donor. The reuse of the enzyme for five-times without significant loss of the activity has also been demonstrated. Alternatively, the hydrolytic approach has allowed the preparation of some 1'-monoesters in good yields, although the reactivity and selectivity levels were lower than the ones achieved for the complementary acetylation reaction.
Keywords: biocatalysis, lipases, medium engineering, regioselective processes, thiamphenicol
RESUMO
A síntese de derivados do tianfenicol, catalisada por lipases, foi investigada através da realização das reações de acilação e de hidrólise, como abordagens complementares, sendo a lipase Candida antarctica B o biocatalisador mais eficiente para as modificações seletivas de ambos, o tianfenicol e o correspondente diacetato do tianfenicol, respectivamente. Os melhores resultados foram obtidos nas reações de acilação com diferentes ésteres vinílicos, contendo cadeias carbônicas de comprimento variável, levando aos correspondentes 3'-monoésteres com excelentes rendimentos e em tempos reacionais curtos. As condições reacionais foram analisadas em termos de concentração de substrato, carga de enzima e tipo de doador de acila. Foi demonstrado o reuso da enzima por cinco vezes sem perda significativa de atividade. Alternativamente, a abordagem hidrolítica propiciou a preparação de alguns 1'-monoésteres em bons rendimentos, ainda que os níveis de reatividade e seletividade foram mais baixos que os obtidos na reação complementar de acetilação.
Introduction
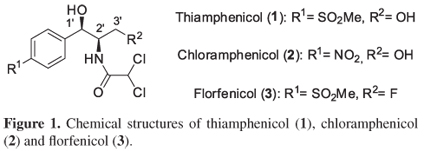
Thiamphenicol (1), also known as thiophenicol, dextrosulphenidol or 2,2-dichloro-N-{(1R,2R)-1,3-dihydroxy-1-[4-(methylsulfonyl)phenyl]propan-2-yl}acetamide, is a potent antibiotic used especially for veterinary applications against infectious diseases, and has also been successfully tested in humans.1 It possesses remarkable activity against both Gram negative and Gram positive microorganisms. Structurally, thiamphenicol is the methylsulfonyl analogue of chloramphenicol (2), displaying a similar spectrum of therapeutic activities (Figure 1). Also a fluorinated derivative of thiamphenicol (florfenicol, 3) is currently indicated for the treatment of bovine respiratory disease, among others.
The total asymmetric synthesis of (1R,2R)-thiamphenicol has received attention in recent years, considering different key steps for the introduction of chirality, such as Sharpless asymmetric epoxidation of an allylic alcohol intermediate,2 Ag(I) promoted asymmetric bromo-methoxylation of an α,β-unsaturated amide precursor,3 chemical resolution of a racemic propanediol,4 or the addition of an enantiopure sulfinimine to the lithium enolate of methyl bromoacetate.5
Enzymes have received considerable attention in recent years, as they display high selectivities under environmentally and mild reaction conditions.6 For instance, adequate thiamphenicol precursors have been obtained by means of efficient stereoselective methods using lipases, amidases and hydroxynitrile lyases, starting from an adequate amide, ester or aldehyde precursor, respectively.7 Nowadays, thiamphenicol is a commercially available product, and a challenging task is the development of selective chemical modifications for the synthesis of thiamphenicol analogs with improved therapeutical activities.
The solubility is a key issue for multiple in vivo applications, and thiamphenicol presents a negligible solubility in water. On the other hand, organic solvents such as diethyl ether, ethyl acetate or chloroform allow its complete solubility at high substrate concentrations. Preparation of thiamphenicol derivatives remains currently as an attractive area, because of the possible improved biological activities shown by them in comparison with the parent substrate. To solve this problem, biocatalysis appears as a useful tool allowing the development of regioselective processes. Thus, different enzymes, especially hydrolases, have allowed the selective modification of identical functional groups in different regions of a target molecule.8
Lipases are hydrolytic enzymes widely used for synthetic transformations.9 Advantages arise from their complementary action in hydrolytic and synthetic procedures, especially for transesterification reactions. Their versatility to act in both media offers a myriad of opportunities, especially when there is a clear interest to obtain monoesters starting from diols or diesters. This fact clearly improves traditional chemical methods, which usually require tedious sequences of protection and deprotection steps on selected functionalities.
In fact, the regioselective formation of a thiamphenicol ester from 1 was successfully demonstrated for the first time by Carrea and co-workers, obtaining in almost quantitative conversion the 3'-palmitate derivative by lipase G-catalyzed acylation reaction in acetone.10 More recently, Lin and co-workers reported the synthesis of thiamphenicol vinyl esters employing the Mucor miehei lipase and divinyl esters of variable length.11 Herein, we wish to report an effective and selective general methodology for the production of thiamphenicol esters, the action of lipases being optimized for the isolation of the corresponding target compounds in good yields by means of acylation procedures and complementary hydrolytic reactions.
Experimental
General methods
Chemical reagents were purchased from different commercial sources and used without further purification. Candida antarctica lipase type B (CAL-B, Novozyme 435, 7300 PLU g-1) and Rhizomucor miehei lipase (Lipozyme RM IM, < 15% in weight) were a gift from Novozymes (Denmark). AK lipase from Pseudomonas fluorescens (22100 U g-1), and Candida rugosa lipase (965 U mg-1 of solid using olive oil) were purchased from Sigma-Aldrich. Solvents were distilled over an adequate desiccant under nitrogen. Flash chromatographies were performed using silica gel 60 (230-240 mesh). IR spectra were recorded on using NaCl plates or KBr pellets in a Perkin-Elmer 1720-X F7.
High performance liquid chromatography (HPLC) analyses were carried out in a Hewlett Packard 1100 chromatograph UV detector at 210 nm using a Daicel CHIRALCEL IA or a CHIRALPAK AS column (25 cm × 4.6 mm i.d.). For the measurement of the conversion values, calibration curves were done for all the compounds involved in the enzymatic processes. Standard solutions at 1000 ppm substrate concentration were prepared and from these ones the 50, 100, 150, 200 and 250 ppm solutions were prepared. Analyses of individual substrates were done in the HPLC conditions indicated for each organic compound. Absorbance values were obtained and used with the Origin® program to attain graphics and equations for calibration curves. Correlation coefficients were in all cases r2 > 0.999 for curve fitting. Reaction times were as follows: (i) reactions for the synthesis of acetylated derivatives: Column Chiralpak AS with a 0.8 mL min-1 flow using 25% 2-propanol/hexane as eluent at 40 ºC: 1 (12.3 min), 5a (22.7 min), 7a (27.5 min) and 8a (13.4 min); (ii) reactions for the synthesis of propionated derivatives: Column Chiralpak IA with a 0.8 mL min-1 flow using 25% 2-propanol/hexane as eluent at 40 ºC: 1 (7.9 min), 5b (10.1 min), 7b (9.5 min) and 8b (8.8 min); (iii) reactions with vinyl decanoate: Column Chiralpak AS with a 0.8 mL min-1 flow using 25% 2-propanol/hexane as eluent at 40 ºC: 1 (12.3 min), 5c (6.5 min) and 7c (10.6 min); (iv) reactions with vinyl laurate: Column Chiralpak AS with a 0.8 mL min-1 flow using 25% 2-propanol/hexane as eluent at 40 ºC: 1 (12.3 min), 5d (5.9 min) and 7d (9.9 min); (v) reactions with vinyl palmitate: Column Chiralpak AS with a 0.8 mL min-1 flow using 20% 2-propanol/hexane as eluent at 40 ºC: 1 (12.3 min), 5e (5.6 min) and 7e (11.1 min).
General procedure for the enzymatic acylation reaction of ()-thiamphenicol with vinyl esters 4a-e
To a suspension of ()-thiamphenicol (1, 50 mg, 0.14 mmol) and CAL-B (50 mg) in dry MeCN (1.4 mL), the corresponding vinyl ester 4a-e (5 eq, 0.70 mmol) was added under nitrogen atmosphere, and the reaction shaken at 20 ºC and 250 rpm. Aliquots were regularly analyzed by HPLC and the reaction stopped when complete consumption of the starting material was observed (1-3 h, see Table 1). Finally, the enzyme was filtered off and washed with EtOAc (3 × 5 mL), the solvent evaporated under reduced pressure, and the reaction crude purified by flash chromatography on silica gel (60% EtOAc/hexane), affording the corresponding monoesters 5a (96%), 5b (98%), 5c (94%), 5d (96%) and 5e (98%).
General procedure for the enzymatic acylation reaction of ()-thiamphenicol with vinyl propionate (4b) at 1 g scale
To a suspension of ()-thiamphenicol (1, 1 g, 2.74 mmol) and CAL-B (1 g) in dry MeCN (28 mL), vinyl propionate (4b, 298 µL, 2.74 mmol) was added under nitrogen atmosphere, and the reaction shaken at 20 ºC and 250 rpm. Aliquots were regularly analyzed by HPLC and the reaction stopped when complete consumption of the starting material was observed after 2 h. Finally, the enzyme was filtered off and washed with EtOAc (3 × 10 mL), the solvent evaporated under reduced pressure, and the reaction crude purified by flash chromatography on silica gel (60% EtOAc/hexane), affording the corresponding monoester 5b (97% isolated yield).
General procedure for the acylation reaction of ()-thiamphenicol with vinyl decanoate (4c). Enzyme recycling studies
To a suspension of ()-thiamphenicol (1, 50 mg, 0.14 mmol) and CAL-B (50 mg) in dry MeCN (1.4 mL), vinyl decanoate (4c, 182 µL, 5 eq, 0.71 mmol) was added, and the reaction shaken at 20 ºC and 250 rpm under nitrogen atmosphere. Aliquots were regularly analyzed by HPLC and the reaction stopped when complete consumption of the starting material was observed after 1 h. Finally, the enzyme was filtered off and washed with EtOAc (3 × 10 mL), the solvent evaporated under reduced pressure, and the reaction crude purified by flash chromatography on silica gel (60% EtOAc/hexane) affording the corresponding 3'-monoester 5c (96% isolated yield). This experiment was repeated with the recycled enzyme for five times without any loss of the enzyme activity.
General procedure for the chemical synthesis of diesters 7a-e from ()-thiamphenicol (1)
4-(N, N-dimethylamino)pyridine (DMAP, 3.4 mg, 0.028 mmol), Et3N (58 µL, 0.421 mmol) and the corresponding anhydride 6a, b or acid chloride 6c-e (0.421 mmol) were added to a thiamphenicol (1, 100 mg, 0.281 mmol) solution in dry CH2Cl2 (2.8 mL) under nitrogen atmosphere. The reaction was stirred at room temperature during 1 h and after that time the solvent was evaporated in a rotary evaporator. The resulting crude was purified by flash chromatography on silica gel (30% EtOAc/hexane) to afford the desired diester 7a (37%), 7b (54%), 7c (61%), 7d (50%) and 7e (41%).
General procedure for the chemical acylation reaction of ()-thiamphenicol (1) to obtain monoesters 5a-e and 8a-e for analytical purposes
DMAP (11 mg, 0.09 mmol), Et3N (204 µL, 1.47 mmol) and the corresponding anhydride 6a, b or acid chloride 6c-e (1.47 mmol) were added to a thiamphenicol (1, 347 mg, 0.98 mmol) solution in dry CH2Cl2 (4.0 mL) under nitrogen atmosphere. The reaction was stirred at room temperature during 1 h and after that time the solvent was evaporated in a rotary evaporator. The resulting crude was purified by flash chromatography on silica gel (30% EtOAc/hexane) to afford the standards of the desired monoesters 5a-e and 8a-e.
General procedure for the enzymatic hydrolysis reaction of ()-thiamphenicol diesters 7a, b
A suspension of diester 7a, b (30 mg) and CAL-B or Candida rugosa lipase (CRL) (ratio 1:1 m/m with respect to the diester) in a mixture of phosphate buffer 100 mmol L-1 pH 7.0/MeCN (80/20 v/v), was shaken at 20 ºC and 250 rpm. The reaction was stopped after 24-48 hours, and the enzyme filtered off. The organic solvent was evaporated and the residue extracted with EtOAc (3 × 10 mL). Finally, the solvent was evaporated under reduced pressure, and the reaction crude purified by flash chromatography on silica gel (60% EtOAc/hexane).
Full characterization of novel compounds 5a-e, 7a-e, and 8a-b
(2'R,3'R)-Thiamphenicol-3'-acetate (5a): Rf (60% EtOAc/hexane): 0.18; m.p. 133-135 ºC; IR (NaCl) νmax/cm-1: 3055, 2987, 1743, 1699, 1521, 1421, 1316, 1265, 1151 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 2.08 (s, 3H), 3.00 (s, 3H), 4.17-4.40 (m, 3H), 5.04 (s, 1H), 5.80 (s, 1H), 7.03 (d, 1H, J 8.5 Hz), 7.50 (d, 2H, J 8.2 Hz), 7.74 (d, 2H J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 20.9, 44.6, 54.3, 63.1, 66.3, 70.7, 127.1, 127.4, 139.6, 147.2, 164.5, 171.4; HRMS (ESI+) calcd. for C14H17Cl2NNaO6S (M): 420.0046; found: 420.0057; [α]D20 = +0.75 (c 0.8, CHCl3).
(2'R,3'R)-Thiamphenicol-3'-propionate (5b): Rf (60% EtOAc/hexane): 0.33; m.p. 111-113 ºC; IR (NaCl) νmax/cm-1: 3055, 2987, 2305, 1743, 1699, 1521, 1421, 1315, 1265, 1151, 1088 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 1.13 (t, 3H, J 7.5 Hz), 2.36 (q, 2H, J 7.5 Hz), 3.00 (s, 3H), 4.18-4.23 (m, 1H), 4.29-4.41 (m, 2H), 5.04 (s, 1H), 5.80 (s, 1H), 7.27 (d, 1H, J 8.5 Hz), 7.50 (d, 2H, J 8.2 Hz), 7.74 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 9.1, 27.6, 44.6, 54.4, 63.0, 66.6, 70.8, 127.1, 127.4, 139.6, 147.2, 164.5, 174.8; HRMS (ESI+) calcd. for C15H19Cl2NNaO6S (M): 434.0202; found: 434.0218; [α]D20 = +0.11 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-3'-decanoate (5c): Rf (60% EtOAc/hexane): 0.67; m.p. 85-87 ºC; IR (NaCl) νmax/cm-1: 3055, 2928, 2856, 2305, 1743, 1699, 1519, 1421, 1316, 1265, 1151 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.88 (t, 3H, J 7.1 Hz), 1.26 (s, 12H), 1.63 (t, 2H, J 7.2 Hz), 2.36 (t, 2H, J 7.4 Hz), 3.02 (s, 3H), 4.16-4.47 (m, 3H), 5.04 (s, 1H), 5.30 (s, 2H), 5.70 (s, 1H), 6.92 (d, 1H, J 8.8 Hz), 7.54 (d, 2H, J 8.2 Hz), 7.82 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.3, 22.8, 25.0, 29.3-29.5, 32.0, 34.3, 44.7, 54.4, 62.7, 66.3, 70.9, 127.1, 127.6, 140.1, 146.7, 164.5, 174.4; HRMS (ESI+) calcd. for C22H33Cl2NNaO6S (M): 532.1307; found: 532.1298; [α]D20 = 0.54 (c 0.8, CHCl3).
(2'R,3'R)-Thiamphenicol-3'-laurate (5d): Rf (60% EtOAc/hexane): 0.47; m.p. 94-96 ºC; IR (NaCl) νmax/cm-1: 3055, 2928, 2855, 2306, 1733, 1699, 1519, 1421, 1316, 1265, 1151 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.88 (t, 3H, J 6.6 Hz), 1.26 (s, 16H), 1.63 (t, 2H, J 7.1 Hz), 2.35 (t, 2H, J 7.6 Hz), 3.00 (s, 3H), 4.17-4.44 (m, 3H), 5.04 (s, 1H), 5.78 (s, 1H), 6.97 (d, 1H, J 8.7 Hz), 7.52 (d, 2H, J 8.2 Hz), 7.77 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.2, 22.8, 25.0, 29.3-29.8, 32.1, 34.3, 44.7, 54.4, 62.9, 66.3, 70.8, 127.1, 127.5, 139.8, 147.0, 164.5, 174.3; HRMS (ESI+) calcd. for C24H37Cl2NNaO6S (M): 560.1639; found: 560.1611; [α]D20 = +0.62 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-3'-palmitate (5e): Rf (60% EtOAc/hexane): 0.54; m.p. 108-110 ºC; IR (NaCl) νmax/cm-1: 3019, 2927, 2855, 2346, 1733, 1698, 1521, 1420, 1316, 1265, 1151 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.87 (t, 3H, J 6.7 Hz), 1.25 (s, 24H), 1.62 (t, 2H, J 7.0 Hz), 2.35 (t, 2H, J 7.6 Hz), 3.00 (s, 3H), 4.17-4.44 (m, 3H), 5.03 (s, 1H), 5.77 (s, 1H), 6.96 (d, 1H, J 8.7 Hz), 7.51 (d, 2H, J 8.2 Hz), 7.76 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.3, 22.8, 25.0, 29.3-29.6, 32.1, 34.3, 44.7, 54.4, 62.9, 66.3, 70.9, 127.1, 127.5, 139.8, 147.0, 164.4, 174.4; HRMS (ESI+) calcd. for C28H45Cl2NNaO6S (M): 616.2247; found: 616.2237; [α]D20 = +0.38 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1',3'-diacetate (7a): Rf (60% EtOAc/hexane): 0.26; m.p. 115-117 ºC; IR (NaCl) νmax/cm-1: 3055, 2987, 2412, 2305, 1750, 1703, 1523, 1421, 1316, 1265, 1153, 1049 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 2.07 (s, 3H), 2.16 (s, 3H), 3.05 (s, 3H), 4.02 (dd, 1H, J 6.0, 12.0 Hz), 4.16 (dd, 1H, J 6.0, 12.0 Hz), 4.57-4.66 (m, 1H), 5.88 (s, 1H), 6.05 (d, 1H, J 6.0 Hz), 6.89 (d, 1H, J 9.3 Hz), 7.55 (d, 2H, J 8.2 Hz), 7.93 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 20.8, 20.9, 44.5, 52.9, 62.3, 66.2, 73.1, 127.9, 128.1, 141.1, 142.6, 164.4, 169.8, 170.7; HRMS (ESI+) calcd. for C16H19Cl2NNaO7S (M): 462.0151; found: 462.0176; [α]D20 = 3.89 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1',3'-dipropionate (7b): Rf (60% EtOAc/hexane): 0.46; m.p. 105-107 ºC; IR (NaCl) νmax/cm-1: 3059, 2928, 2305, 1743, 1707, 1527, 1463, 1313, 1267, 1151, 1084 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 1.12 (t, 3H, J 7.5 Hz), 1.14 (t, 3H, J 7.5 Hz), 2.34 (q, 2H, J 7.5 Hz), 2.43 (q, 2H, J 7.5 Hz), 3.04 (s, 3H), 4.02 (dd, 1H, J 6.0, 12.0 Hz), 4.17 (dd, 1H, J 6.0, 12.0 Hz), 4.60 (m, 1H), 5.88 (s, 1H), 6.04 (d, 1H, J 6.0 Hz), 6.88 (d, 1H, J 9.3 Hz), 7.54 (d, 2H, J 7.5 Hz), 7.93 (d, 2H, J 7.5 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 9.0, 9.1, 27.4, 27.6, 44.6, 53.0, 62.3, 66.2, 72.8, 127.9, 128.1, 141.1, 142.7, 164.3, 173.3, 174.1; HRMS (ESI+) calcd. for C18H23Cl2NNaO7S (M): 490.0464; found: 490.0484; [α]D20 = 3.7 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1',3'-didecanoate (7c): Rf (60% EtOAc/hexane): 0.87; m.p. 82-84 ºC; IR (NaCl) νmax/cm-1: 3054, 2928, 2856, 2305, 1743, 1703, 1522, 1421, 1317, 1265, 1153 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.88 (t, 6H, J 6.8 Hz), 1.26 (br s, 24H), 1.63 (t, 4H, J 4.6 Hz), 2.35 (t, 2H, J 7.7 Hz), 2.42 (t, 2H, J 7.7 Hz), 3.05 (s, 3H), 4.02 (dd, 1H, J 6.0, 12.0 Hz), 4.16 (dd, 1H, J 6.0, 12.0 Hz), 4.55-4.62 (m, 1H), 5.30 (s, 2H), 5.86 (s, 1H), 6.05 (d, 1H, J 6.0 Hz), 6.76 (d, 1H, J 9.3 Hz), 7.55 (d, 2H, J 8.2 Hz), 7.95 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.3, 22.8, 24.9, 25.0, 29.3-29.6, 32.0, 34.2, 34.3, 44.6, 53.1, 62.2, 66.3, 72.8, 127.9 , 128.2, 141.3, 142.7, 164.6, 172.7, 173.5; HRMS (ESI+) calcd. for C32H51Cl2NNaO7S (M): 686.2664; found: 686.2656; [α]D20 = 1.24 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1',3'-dilaurate (7d): Rf (60% EtOAc/hexane): 0.75; m.p. 48-50 ºC; IR (NaCl) νmax/cm-1: 3055, 2928, 2855, 2306, 1743, 1703, 1522, 1421, 1317, 1265, 1152 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.88 (t, 6H, J 6.6 Hz), 1.25 (br s, 32H), 1.61 (s, 4H), 2.32 (t, 2H, J 8.6 Hz), 2.40 (t, 2H, J 8.6 Hz), 3.04 (s, 3H), 4.02 (dd, 1H, J 6.0, 12.0 Hz), 4.19 (dd, 1H, J 6.0, 12.0 Hz), 4.55-4.64 (m, 1H), 5.86 (s, 1H), 6.04 (d, 1H, J 6.0 Hz), 6.76 (d, 1H, J 9.3 Hz), 7.55 (d, 2H, J 8.2 Hz), 7.95 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.2, 22.8, 24.9 , 29.2-29.6, 32.0, 34.2, 44.6, 53.1, 62.2, 66.2, 72.8, 127.9, 128.1, 141.2, 142.8, 164.2, 172.7, 173.5; HRMS (ESI+) calcd. for C36H59Cl2NNaO7S (M): 742.3294; found: 742.3282; [α]D20 = 0.64 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1',3'-dipalmitate (7e): Rf (60% EtOAc/hexane): 0.86; m.p. 47-49 ºC; IR (NaCl) νmax/cm-1: 3054, 2927, 2854, 2306, 1743, 1703, 1522, 1466, 1317, 1265, 1153 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 0.87 (t, 6H, J 6.7 Hz), 1.24 (br s, 48H), 1.61 (s, 4H), 2.32 (t, 2H, J 8.6 Hz), 2.42 (t, 2H, J 8.6 Hz), 3.04 (s, 3H), 4.02 (dd, 1H, J 6.0, 12.0 Hz), 4.19 (dd, 1H, J 6.0, 12.0 Hz), 4.55-4.64 (m, 1H), 5.86 (s, 1H), 6.04 (d, 1H, J 6.0 Hz), 6.83 (d, 1H, J 9.3 Hz), 7.55 (d, 2H, J 8.2 Hz), 7.93 (d, 2H, J 8.2 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 14.2, 22.8, 24.9, 29.2-29.6, 32.0, 34.2, 44.6, 53.1, 62.2, 66.2, 72.8, 127.9, 128.1, 141.2, 142.8, 164.2, 172.7, 173.5; HRMS (ESI+) calcd. for C44H75Cl2NNaO7S (M): 854.4555; found: 854.4534; [α]D20 = 1.6 (c 1.0, CHCl3).
(2'R,3'R)-Thiamphenicol-1'-acetate (8a): Rf (60% EtOAc/hexane): 0.15; IR (NaCl) νmax/cm-1: 3328, 3019, 2928, 1735, 1688, 1533, 1410, 1303, 1225, 1147 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 2.13 (s, 3H), 3.06 (s, 3H), 3.49 (dd, 1H, J 6.0, 12.0 Hz), 3.66 (dd, 1H, J6.0, 12.0 Hz), 4.33-4.38 (m, 1H), 5.94 (s, 1H), 6.18 (d, 1H, J 7.2 Hz), 7.10 (d, 1H, J 9.2 Hz), 7.63 (d, 2H, J 8.3 Hz), 7.93 (d, 2H, J 8.3 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 21.1, 44.6, 55.6, 61.0, 66.4, 73.4, 128.0, 128.2, 140.9, 143.4, 164.7, 170.4; HRMS (ESI+) calcd. for C14H17Cl2NNaO6S (M): 420.0083; found: 420.0046; [α]D20 = 3.6 (c 0.5, CHCl3).
(2'R,3'R)-Thiamphenicol-1'-propionate (8b): Rf (60% EtOAc/hexane): 0.25; m.p. 109-111 ºC; IR (NaCl) νmax/cm-1: 3497, 3289, 2929, 1727, 1684, 1556, 1462, 1369, 1300, 1151, 1084 cm-1; 1H NMR (CDCl3, 300.13 MHz) δ 1.14 (t, 3H, J 7.5 Hz), 2.42 (q, 2H, J 7.5 Hz), 3.05 (s, 3H), 3.49 (dd, 1H, J 6.0, 12.0 Hz), 3.66 (dd, 1H, J 6.0, 12.0 Hz), 4.31-4.38 (m, 1H), 5.93 (s, 1H), 6.19 (d, 1H, J 7.2 Hz), 7.09 (d, 1H, J 9.2 Hz), 7.63 (d, 2H, J 8.3 Hz), 7.93 (d, 2H, J 8.3 Hz); 13C NMR (CDCl3, 75.5 MHz) δ 9.1, 27.8, 44.6, 55.7, 61.0, 66.5, 73.3, 128.0, 128.2, 140.9, 143.6, 164.6, 173.8; HRMS (ESI+) calcd. for C15H19Cl2NNaO6S (M): 434.0202; found: 434.0241; [α]D20 = 4.4 (c 1.0, CHCl3).
Results and Discussion
Regioselective acylation of thiamphenicol using vinyl esters
Based on the excellent results achieved in the selective acylation of chloramphenicol (2),12 reactions were carried out in the presence of 5 eq of different vinyl esters presenting different chain length such as acetate (4a), propionate (4b), decanoate (4c), laurate (4d) and palmitate (4e). Candida antarctica lipase type B (CAL-B, ratio 1:1 m/m with respect to 1) was selected as biocatalyst, using acetonitrile as solvent (Table 1).
All acylation reactions occurred with complete regioselectivity towards the primary alcohol, obtaining monoesters 5a-e with full conversion after 24 h. Then, we decided to monitor the reactions at shorter reaction times (1-3 h). Satisfyingly, the biotransformations reached complete conversions, requiring slightly longer reaction times for those vinyl esters with a longer chain (4c-e, entries 3-5). The corresponding thiamphenicol monoesters 5a-e were isolated with excellent yields after flash chromatography purification. These results are highly interesting as the chemical acylation of thiamphenicol with anhydrides or chloride acids led preferentially to the diacylated products 7a-e (37-61%) in the presence of an excess of the acyl donor, while only a minor amount of the 3'-monoester derivatives were obtained using 1.5 eq of the corresponding acylating agents (Scheme 1).
At this point, different experiments were carried out in order to find optimum conditions for the production of the corresponding monoesters. Focusing in the economic assessment of the biotransformation, the enzyme loading was reduced five times, recovering ester 5a after 2 h and 45 min with complete conversion and 98% isolated yield. Reactions were also performed with a higher thiamphenicol concentration (250 mmol L-1), finding for vinyl propionate (4b) the formation of the final product 5b in quantitative conversion and excellent isolated yield (97%) in a similar reaction time.
Scaling-up protocols of these acylation reactions were performed using 0.5 and 1 g of the starting material at 250 mmol L-1 substrate concentration. The processes were developed at 20 ºC using 5 eq of vinyl acetate (4a), obtaining the 3'-monoester 5a with total conversion after 2 h. Remarkably, the reactions occurred also with complete conversion employing only 2 eq of 4a but requiring in this case 3 h. Similarly, the reaction with 1 eq of vinyl propionate (4b) allowed the formation of the monoester 5b in 97% isolated yield at 100 mmol L-1 thiamphenicol concentration at 20 ºC. Finally, the recycling of the biocatalyst was analyzed, taking as model reactions the synthesis of monoesters 5a and 5c, which were studied through acylation reactions of thiamphenicol using 5 eq of vinyl acetate (4a) and vinyl decanoate (4c), respectively. Having in mind the reactivity of both esters, the biotransformations were carried out for 1 h with 4a, and 3 h for 4c, at 20 ºC and 100 mmol L-1 substrate concentration. It was demonstrated that CAL-B can be reused up to five times keeping unaltered its activity and regioselectivity in the presence of vinyl esters with both short and long chain esters.
Regioselective hydrolysis of thiamphenicol esters
Lipase-catalyzed reactions offer clear advantages in terms of selectivity but also due to the complementarity of hydrolytic and synthetic reactions. For this reason, hydrolytic transformations over thiamphenicol diesters were studied in order to provide a useful route towards the 1'-monoesters. These compounds are difficult to achieve by conventional chemical methods due to the lack of selectivity but also because of the hindered accessibility towards this 1'-position. Diacetylated compound 7a was prepared using a small excess of acetic anhydride (6a) in the presence of triethylamine (Et3N) and 4-(N, N-dimethylamino)pyridine (DMAP) in dichloromethane (Scheme 1). Then, a set of hydrolases were employed for the development of the hydrolytic reactions over diester 7a. Hence, CAL-B, Candida antarctica lipase type A (CAL-A), Candida rugosa lipase (CRL), lipase AK from Pseudomonas fluorescens (AK), Rhizomucor miehei lipase (RML), Thermomyces lanuginosus lipase (TLL) and CLEA-alcalase from Bacillus subtilis esterase were tried (Table 2).
After enzymatic screening, hydrolytic activity was only observed with CAL-B and CRL, which hydrolyzed preferentially the ester at the 3'-position at 20 ºC when the reactions were carried out in a system composed of MeCN and phosphate buffer pH 7.0 (entries 1 and 2). The best results were obtained with CAL-B, which afforded 8a in a good yield after 24 h at 20 ºC (81%, entry 1), finding the diol as a minor product (4%). As previously mentioned, due to the complementarity of lipases, the same reaction center reacted in comparison with the CAL-B catalyzed acetylation of thiamphenicol. Longer reaction times favored the formation of diol 1. On the other hand, CRL also showed activity yielding 33% of 8a, although most of the diacetate remained unreacted (64%) after 24 h (entry 2). Unfortunately, longer reaction times (48 h) led to a dramatic loss in the selectivity of the process favoring the formation of the diol at prolonged reaction times (entry 3). A great improvement was achieved when doubling the CRL loading, yielding a 61% of 8a (entry 4).
In order to improve the selectivity of this enzyme towards the formation of 1'-monoester in the hydrolysis of 7a, other cosolvents such as 1,4-dioxane, ethanol, isopropanol (IPA), isoamyl alcohol (IAA), tetrahydrofuran and 2-methyltetrahydrofuran were considered, but in all cases lower activities and selectivities were observed (entries 5-10). A similar trend was witnessed when using higher temperatures in acetonitrile (entries 11 and 12). Finally, we decided to expand this approach to another diester containing a longer chain such as the dipropionate derivative 7b, but a decrease in the selectivity was observed in the reaction catalyzed by CAL-B, obtaining a complex mixture of thiamphenicol (4%), monoester 8b (69%), monoester 5b (18%) and diester 7b (9%). Based on previous results with the chloramphenicol derivatives,12 diesters 7c-e with longer acyl chains were not studied due to the lack of CAL-B reactivity.
Conclusions
In summary, an efficient lipase-catalyzed strategy has been developed for the selective 3'-acylation of thiamphenicol with different vinyl esters of variable length, obtaining the final derivatives in excellent isolated yields (> 93%). Candida antarctica lipase B allows the formation of monoacylated derivatives in short reaction times with complete selectivities. The scaling of these processes has being optimized for 1 g scale. Hydrolytic experiments have been attempted in order to obtain the complementary monoacylated esters at 1'-position. In these experiments only CAL-B and CRL have shown remarkable activity, finding CAL-B as a more suitable enzyme for the production of the 1'-monoacetylated derivative, while the use of the dipropionated thiamphenicol derivative led to a decrease in the selectivity of the process. This is another example where the use of enzymes for regioselective modifications can overcome the limitations of traditional synthetic methods for the selective transformation of polyhydroxylated compounds.
Supplementary Information
Supplementary information (Figures S1-S36) is available free of charge at http://jbcs.sbq.org.br as PDF file.
Acknowledgments
We thank Novozymes for the generous gift of CAL-B (Novozyme 435) and Rhizomucor miehei lipase. The authors thank the Brazilian and Spanish agencies CAPES-DGU (Process: 149/07), CNPq, FUNCAP, PRONEX, Ministerio de Ciencia e Innovación (MICINN, Projects CTQ2007-61126 and CTQ2011-24237) for fellowships and financial support. I. L. (Ramón y Cajal program) thanks MICINN for a personal grant.
References
1. Cutler, R. A.; Stenger, R. J.; Suter, C. M.; J. Am. Chem. Soc. 1952, 74, 5475; Balg, C.; De Mieri, M.; Huot, J. L.; Blais, S. P.; Lapointe, J.; Chênevert, R.; Bioorg. Med. Chem. 2010, 18, 7868.
2. Bhaskar, G.; Kumar, V. S.; Rao, B. V.; Tetrahedron: Asymmetry 2004, 15, 1279; George, S.; Narina, A. V.; Sudalai, A.; Tetrahedron 2006, 62, 10202.
3. Hajra, S.; Karmakar, A.; Maji, T.; Medda, A. K.; Tetrahedron 2006, 62, 8959.
4. Giordano, C.; Cavicchioli, S.; Levi, S.; Villa, M.; J. Org. Chem. 1991, 56, 6114.
5. Davis, F. A.; Zhou, P.; Tetrahedron Lett. 1994, 35, 7525.
6. Clouthier, C. M.; Pelletier, J. N.; Chem. Soc. Rev. 2012, 41, 1585; Reetz, M. T.; J. Am. Chem. Soc. 2013, 135, 12480.
7. Kaptein, B.; van Doren, T. J. G. M.; Boesten, W. H. J.; Sonke, T.; Duchateau, A. L. L.; Broxterman, Q. B.; Kamphuis, J.; Org. Process Res. Dev. 1998, 2, 10; Lu, W.; Chen, P.; Lin, G.; Tetrahedron 2008, 64, 7822.
8. Yang, R. L.; Li, N.; Li, R. F.; Smith, T. J.; Zong, M.-H.; Bioresour. Technol. 2010, 101, 1; Magrone, P.; Cavallo, F.; Panzeri, W.; Passarella, D.; Riva, S.; Org. Biomol. Chem. 2010, 8, 5583; Villo, L.; Kreen, M.; Kudryashova, M.; Metsala, A.; Tamp, S.; Lille, Ü.; Pehk, T.; Parve, O.; J. Mol. Catal. B: Enzym. 2011, 68, 44; Zhao, H.; Zhang, Y.; Lu, F.; Bie, X.; Lu, Z.; Ning, H.; J. Mol. Catal. B: Enzym. 2011, 69, 107; Purchartová, K.; Marhol, P.; Gaak; R.; Monti, D.; Riva, S.; Kuzma, M.; Kren, V.; J. Mol. Catal. B: Enzym. 2011, 71, 119; Martínez-Montero, S.; Fernández, S.; Sanghvi, Y. S.; Gotor, V.; Ferrero, M.; Org. Biomol. Chem. 2011, 9, 5960.
9. Bornscheuer, U. T.; Kazlauskas, R. J.; Hydrolases in Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, 2005; Gotor-Fernández, V.; Brieva, R.; Gotor, V.; J. Mol. Catal. B: Enzym. 2006, 40, 111; Paravidino, M.; Boehm, P.; Gröger, H.; Hanefeld, U. In Enzyme Catalysis in Organic Synthesis, 3rd ed. ; Drauz, K.; Gröger, H.; May, O., eds.; Wiley-VCH: Weinheim, 2012, pp. 251.
10. Ottolina, G.; Carrea, G.; Riva, S.; J. Org. Chem. 1990, 55, 2366.
11. Shi, Y. Z.; Chen, Z. C.; Wang, N.; Wu, Q.; Lin, X. F.; Chin. Chem. Lett. 2005, 16, 45.
12. Bizerra, A. M. C.; Montenegro, T. G. C.; Lemos, T. L. G.; de Oliveira, M. C. F.; de Mattos, M. C.; Lavandera, I.; Gotor-Fernández, V.; de Gonzalo, G.; Gotor, V.; Tetrahedron 2011, 67, 2858.
Submitted: January 24, 2014
Published online: April 8, 2014
Supplementary Data
The supplementary data is available in pdf: [Supplementary material]
- 1. Cutler, R. A.; Stenger, R. J.; Suter, C. M.; J. Am. Chem. Soc. 1952, 74, 5475;
- Balg, C.; De Mieri, M.; Huot, J. L.; Blais, S. P.; Lapointe, J.; Chênevert, R.; Bioorg. Med. Chem. 2010, 18, 7868.
- 2. Bhaskar, G.; Kumar, V. S.; Rao, B. V.; Tetrahedron: Asymmetry 2004, 15, 1279;
- George, S.; Narina, A. V.; Sudalai, A.; Tetrahedron 2006, 62, 10202.
- 3. Hajra, S.; Karmakar, A.; Maji, T.; Medda, A. K.; Tetrahedron 2006, 62, 8959.
- 4. Giordano, C.; Cavicchioli, S.; Levi, S.; Villa, M.; J. Org. Chem. 1991, 56, 6114.
- 5. Davis, F. A.; Zhou, P.; Tetrahedron Lett. 1994, 35, 7525.
- 6. Clouthier, C. M.; Pelletier, J. N.; Chem. Soc. Rev. 2012, 41, 1585;
- Reetz, M. T.; J. Am. Chem. Soc. 2013, 135, 12480.
- 7. Kaptein, B.; van Doren, T. J. G. M.; Boesten, W. H. J.; Sonke, T.; Duchateau, A. L. L.; Broxterman, Q. B.; Kamphuis, J.; Org. Process Res. Dev. 1998, 2, 10;
- Lu, W.; Chen, P.; Lin, G.; Tetrahedron 2008, 64, 7822.
- 8. Yang, R. L.; Li, N.; Li, R. F.; Smith, T. J.; Zong, M.-H.; Bioresour. Technol. 2010, 101, 1;
- Magrone, P.; Cavallo, F.; Panzeri, W.; Passarella, D.; Riva, S.; Org. Biomol. Chem. 2010, 8, 5583;
- Villo, L.; Kreen, M.; Kudryashova, M.; Metsala, A.; Tamp, S.; Lille, Ü.; Pehk, T.; Parve, O.; J. Mol. Catal. B: Enzym. 2011, 68, 44;
- Zhao, H.; Zhang, Y.; Lu, F.; Bie, X.; Lu, Z.; Ning, H.; J. Mol. Catal. B: Enzym. 2011, 69, 107;
- Purchartová, K.; Marhol, P.; Gaak; R.; Monti, D.; Riva, S.; Kuzma, M.; Kren, V.; J. Mol. Catal. B: Enzym. 2011, 71, 119;
- Martínez-Montero, S.; Fernández, S.; Sanghvi, Y. S.; Gotor, V.; Ferrero, M.; Org. Biomol. Chem. 2011, 9, 5960.
- 9. Bornscheuer, U. T.; Kazlauskas, R. J.; Hydrolases in Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, 2005;
- Gotor-Fernández, V.; Brieva, R.; Gotor, V.; J. Mol. Catal. B: Enzym. 2006, 40, 111;
- Paravidino, M.; Boehm, P.; Gröger, H.; Hanefeld, U. In Enzyme Catalysis in Organic Synthesis, 3rd ed.
- ; Drauz, K.; Gröger, H.; May, O., eds.; Wiley-VCH: Weinheim, 2012, pp. 251.
- 10. Ottolina, G.; Carrea, G.; Riva, S.; J. Org. Chem. 1990, 55, 2366.
- 11. Shi, Y. Z.; Chen, Z. C.; Wang, N.; Wu, Q.; Lin, X. F.; Chin. Chem. Lett. 2005, 16, 45.
- 12. Bizerra, A. M. C.; Montenegro, T. G. C.; Lemos, T. L. G.; de Oliveira, M. C. F.; de Mattos, M. C.; Lavandera, I.; Gotor-Fernández, V.; de Gonzalo, G.; Gotor, V.; Tetrahedron 2011, 67, 2858.
Publication Dates
-
Publication in this collection
01 July 2014 -
Date of issue
June 2014
History
-
Received
24 Jan 2014 -
Accepted
08 Apr 2014