Abstract
A solid sampling electrothermal vaporization inductively coupled plasma optical emission spectrometry (ETV-ICP OES) method for determination of As, Cd, Cr, Cu, Mn, Mo, Ni, Pb, Pd, Pt, Rh, Ru and V in pharmaceuticals is proposed. Tricyclic pharmaceuticals were directly analyzed due to their difficult decomposition with acids. Pyrolysis and vaporization temperature, sample mass, and reaction gas (Freon) flow rate were evaluated. The effect of organic and inorganic compounds was evaluated for matrix matching. The limits of detection ranged from 0.04 µg g−1 (Cu) to 107 µg g−1 (As) and the relative standard deviation was lower than 10%. The investigated elements were not detected in the analyzed samples with the exception of Cr in cyclobenzaprine hydrochloride. Since there was no certified reference materials available for metals and metalloids in pharmaceuticals, the accuracy of the method was evaluated by an independent technique and by analyte recovery. Inductively coupled plasma mass spectrometry was employed for analyte determination after sample decomposition by microwave induced combustion. The agreement of the results found by both techniques was better than 87% and analyte recoveries ranged from 91 to 103%.
pharmaceuticals; metals; ETV-ICP OES
Introduction
Pharmaceuticals are recommended by both modern and traditional medicine. They are commonly present in human life to prevent and treat several diseases. According to the World Health Organization,11 World Health Organization (WHO), http://www.who.int/topics/pharmaceutical_products/en/ accessed in December 2014.
http://www.who.int/topics/pharmaceutical...
pharmaceuticals must be safe, effective and of good quality for use in life.
Some pharmaceuticals are metal-based as for instance cis-platinum and carboplatin used as therapeutic agent in anticancer therapy or barium sulfate used as X-ray contrast agent. Although in these pharmaceuticals the metals are active ingredients, in others metals and metalloids they are not desired.22 Meermann, B.; Sperling, M.; Anal. Bioanal. Chem. 2012, 403, 1501.
Despite efforts of pharmaceuticals industries, toxic elements may be present as impurities in the final product. These impurities may be originated from several sources, like manufacturing equipment, excipients or reagents used during the synthesis, mostly metal catalysts.33 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH Harmonized Tripartite Harmonized, Guideline for Elemental Impurities; ICH Q3D Guideline, Geneva, 2014.
It is known that some elements are extremely toxic for human health, even at low concentrations. The International Conference on Harmonization (ICH)33 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH Harmonized Tripartite Harmonized, Guideline for Elemental Impurities; ICH Q3D Guideline, Geneva, 2014. elaborated a guideline where elements were divided in classes according to level of safety concern. The first class includes elements that are significantly toxic (As, Cd, Hg, Pb). The second class is divided in two classes: class 2A (Co, Mo, Se, V) and class 2B (Ag, Au, Ir, Os, Pd, Pt, Rh, Ru, Tl). The latter class includes elements that are more or less toxic depending on the route of administration. The third class includes as impurities elements of relatively low safety concern (Ba, Cr, Cu, Li, Ni, Sb, Sn). The fourth class includes elements that have low inherent toxicity, for which there is not an established and permitted daily exposure (Al, B, Ca, Fe, K, Mg, Mn, Na, Zn and W). In general, most cited elements have persistent and cumulative effect in the human body. Therefore, quality control of pharmaceuticals, especially with respect to contamination with toxic elements, is necessary.
The United States Pharmacopoeia (USP),44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011. Brazilian Pharmacopoeia (FBRAS),55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. and European Pharmacopoeia (Ph.Eur.)66 European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, 2005. describe several assays for using in the quality control of pharmaceuticals. Regarding to potentially toxic elements, the limit test is usually recommended. This test is based on the visual comparison of the precipitate produced when thioacetamide is added to an aliquot of the sample solution (usually 1.0 g of sample in 25 mL) and to a standard solution of Pb (usually 2.0 mL of solution containing 10 mg L−1 of Pb). The main drawback of this method is its lack of specificity and sensitivity, since there are toxic and non-toxic elements that may be precipitated by thioacetamide. On the other hand, not all analyte elements can precipitate with thioacetamide or the color of the precipitate may be different from that in the Pb standard solution. Furthermore, the elements must be as free ions in solution. Therefore, other methods for element determination must be developed in order to have more reliable results. Methods using flame atomic absorption spectrometry (FAAS), inductively coupled plasma optical emission spectrometry (ICP OES) and inductively coupled plasma mass spectrometry (ICP-MS) have been proposed by the pharmacopoeias for element determination.44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011.
5 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010.-66 European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, 2005. In this case, samples must be in solution, preferentially decomposed to minimize interferences in measurement step.
Several authors have proposed methods for element determination in pharmaceuticals and respective raw materials involving the use of FAAS,77 Scripcariu, M.; Tanase, I. G.; Fleschin, S.; Magearu, V.; Rev. Roum. Chim. 2008, 53, 149. graphite furnace atomic absorption spectrometry (GF AAS),88 Tanase, A.; Miu, A.; Rev. Roum. Chim. 2012, 57, 971. ICP OES,99 Raghuram, P.; Soma Raju, I. V.; Sriramulu, J.; Pharmazie 2010, 65, 15. total reflectance X-ray fluorescence (TXRF)1010 Antosz, F. J.; Xiang, Y.; Diaz, A. R.; Jensen, A. J.; J. Pharm. Biomed. Anal. 2012, 62, 17. and laser induced breakdown spectrometry (LIBS).1111 Carvalho, G. G. A.; Nunes, L. C.; Souza, P. F.; Krug, F. J.; Alegre, T. C.; Santos Jr., D.; J. Anal. At. Spectrom. 2010, 25, 803. Nevertheless, the ICP‑MS technique is very appropriate for element determination in pharmaceuticals1212 Lewen, N.; Mathew, S.; Schenkenberger, M.; Raglione, T.; J. Pharm. Biomed. Anal. 2004, 35, 739.,1313 Van Hoecke, K.; Catry, C.; Vanhaecke, F.; J. Anal. At. Spectrom. 2012, 27, 1909. due to its multielemental capability and excellent sensitivity (detection limits in the range of ng L−1 or ng g−1).
Excepting TXRF and LIBS, pharmaceutical samples are preferably analyzed when they are solubilized and the respective solutions analyzed. Therefore, direct solubilization of the pharmaceutical in water or another solvent is carried out. Decomposition of the pharmaceutical sample with inorganic acids is also used for reducing the organic content and ensure accurate results.1414 Mester, Z.; Sturgeon, R. In Comprehensive Analytical Chemistry - Sample Preparation for Trace Element Analysis; Barceló, D.; ed.; Elsevier: Amsterdam, 2003, p. 1338.,1515 Mitra, S. In Sample Preparation Techniques in Analytical Chemistry; Kebbekus, B. B.; ed.; John Wiley & Sons: New Jersey, 2003, ch. 5. However, pharmaceuticals such as tricyclic antidepressants are difficult to decompose by acid even at high temperature and pressure. When nitric acid is used, nitro groups can bind the benzene ring and produce very insoluble compounds.1616 Barin, J. S.; Tischer, B.; Picoloto, R. S.; Antes, F. G.; Silva, F. E. B.; Paula, F. R.; Flores, E. M. M.; J. Anal. At. Spectrom.
2014, 29, 352. Thus, the conventional acid decomposition becomes ineffective. Sample combustion in open vessel and element absorption in a solvent are not recommended, by considering the analyte loss in the first case and the organic matrix that remains in the solution in the latter. In this sense, microwave induced combustion (MIC) is useful as already demonstrated.1616 Barin, J. S.; Tischer, B.; Picoloto, R. S.; Antes, F. G.; Silva, F. E. B.; Paula, F. R.; Flores, E. M. M.; J. Anal. At. Spectrom.
2014, 29, 352.
17 Pereira, J. S. F.; Pereira, L. S. F.; Schmidt, L.; Moreira, C. M.; Barin, J. S.; Flores, E. M. M.; Microchem. J.
2013, 109, 29.-1818 Antes, F. G.; Duarte, F. A.; Mesko, M. F.; Nunes, M. A. G.; Pereira, V. A.; Müller, E. I.; Dressler, V. L.; Flores, E. M. M.; Talanta
2010, 83, 364.
Direct analysis of solid sample has been proposed, which simplifies the analysis and reduces sample contamination and analyte losses of volatile elements.1919 Kurfürst, U.; Solid Sample Analysis Direct and Slurry Sampling Using GF-AAS and ETV-ICP, Springer: Heidelberg, 1998. Electrothermal vaporization (ETV) associated with ICP OES or ICP-MS allows direct solid sampling analysis. Several applications dealing with biological,2020 Grindlay, G.; Mora, J.; Gras, L.; Loos-Vollebregt, M. T. C.; Anal. Chim. Acta 2009, 652, 154. environmental,2121 Masson, P.; Dauthieu, M.; Trolard, F.; Denaix, L.; Spectrochim. Acta, Part B 2007, 62, 224. geological,2222 Wu, Y.; Hu, B.; Jiang, Z.; Chen, S.; J. Anal. At. Spectrom. 2002, 17, 121. polymers,2323 Resano, M.; Aramendíaz, M.; Devos, W.; Vanhaecke, F.; J. Anal. At. Spectrom. 2006, 21, 891. refractory materials,2424 Barth, P.; Hassler, J.; Kudrik, I.; Krivan, V.; Spectrochim. Acta, Part B 2007, 62, 924. and forensic samples2525 Asfaw, A.; Wibetoe, G.; Beauchemin, D.; J. Anal. At. Spectrom. 2012, 27, 1928. analysis are reported. Nevertheless, only one work about direct element determination in solid pharmaceuticals was found.2626 Lin, M. -L.; Jiang, S. -J.; J. Anal. At. Spectrom. 2011, 9, 1813. However, in such work, a slurry of the sample under sonication was introduced in the graphite tube in the ETV system hyphenated with ICP-MS. To the best of our knowledge, determination of elemental impurities present in pharmaceuticals by electrothermal vaporization inductively coupled plasma optical emission spectrometry (ETV-ICP OES) has not been reported. Therefore, in the present work, the use of ETV-ICP OES is proposed for As, Cd, Cr, Cu, Mn, Mo, Ni, Pb, Pd, Pt, Rh, Ru and V determination in ten different types of pharmaceuticals, including matrices that are difficult to decompose by acids.
Experimental
Instrumentation
An electrothermal vaporization unit (Spectral Systems, model ETV-4000c, Germany) was used for solid sampling analysis. Pyrolytic coated graphite tubes and graphite platforms (both from Spectral Systems) were used. In this way, the diffusion of metals on graphite wall and oxidation of graphite is reduced.2727 Majidi, V.; Smith, R. G.; Xu, N.; McMahon, M. W.; Bossio, R.; Spectrochim. Acta, Part B 2000, 55, 1787. The graphite tubes are longitudinally heated and the temperature range on the ETV system can be varied from room temperature up to 3000 °C. The carrier, modifier and bypass-gas flow rates were adjusted as necessary. A polytetrafluoroethylene (PTFE) transfer line of 100 cm length and 0.6 cm i.d. was used to connect the ETV device to the ICP torch. Wet samples were dried by using a drying bank (Spectral Systems). A dual view optical spectrometer (PerkinElmer, model Optima 4300DV, USA), with a quartz torch and alumina injector tube (2.0 mm i.d.) was used. Argon with 99.996% (stored as liquid in tank) purity (White Martins, São Paulo, Brazil) was used as protection, carrier and bypass-gas on the ETV device. The same argon gas was used as plasma and auxiliary gas in the ICP OES instrument. The ETV and ICP OES parameters are summarized in Table 1.
A micro-balance (Sartorius, model M2P, Germany) with a resolution of 0.001 mg and maximum capacity of 2 g was used for sample weighing. A SpeedWave four (Berghof, Germany) equipped with twelve vessels (TFM) and operated at maximum power, temperature and pressure of 1450 W, 230 °C and 50 bar, respectively, was used to assists the sample decomposition using acid. A Multiwave 3000 (Anton Paar, Austria) equipped with eight quartz vessels and operated at maximum power, temperature and pressure of 1400 W, 280 °C and 80 bar, respectively, was used for microwave induced combustion. An ICP-MS spectrometer from PerkinElmer-SCIEX (model Elan DRC II, Canada) equipped with a baffled cyclonic spray chamber and a concentric nebulizer was used for element determination in the solutions of the acid-decomposed samples. Instrumental conditions are shown in Table 1.
Reagents, standards and samples
The water used to prepare the solutions was distillated, deionized and then purified using a Milli-Q system (Millipore, Billerica, USA, 18.2 MΩ cm). HNO3 65% m m−1 (Merck, Darmstadt, Germany) was used for sample decomposition and calibration solutions preparation. This acid was double distilled in a duoPUR 2.01E sub-boiling system (Milestone, Italy). All other reagents were of analytical grade. Calibration solutions were prepared by serial dilution of multielement stock solutions SCP33MS 10 mg L−1 (SCP Science, Canada) and CLMS-3 10 mg L−1 (Spex CertiPrep, USA) in HNO3 0.72 mol L−1. The calibration solutions used for determinations by ICP-MS ranged from 0.10 to 10.0 μg L−1. Aliquots of 0.5 to 25 μL of the solutions cotanining the investigated elements were transferred directly on the graphite platform in order to obtain the calibration curves for ETV-ICP OES.
Freon R12 (CCl2F2) was used as reaction gas. Citric acid (C6H8O7, Synth, Brazil), ascorbic acid (C6H8O6, Belga Química, Brazil), oxalic acid (C2H2O4, Nuclear, Brazil), ethylenediaminetetraacetic acid (C10H16N2O8, Reagen, Brazil) and sodium chloride (NaCl, Merck, Germany) were also evaluated for matrix matching. Ammonium nitrate (NH4NO3, SigmaAldrich) 6 mol L−1 was used as igniter for MIC. Oxygen with purity of 99.9991% (White Martins, Brazil) was used to pressurize the quartz vessels for MIC.
Ten samples of pharmaceuticals, including amitriptyline hydrochloride, carbamazepine, clozapine, cyclobenzaprine hydrochloride, imipramine hydrochloride, ketotifen fumarate, loratadine, nortriptyline hydrochloride, promethazine hydrochloride and tetracycline hydrochloride, were obtained from local drugstores. The samples were in powder or tablet form. Those in tablet form were grounded in an agate mortar to have particles size lower than 65 μm. Then, they were stored in decontaminated plastic vials. The pharmaceuticals in powder form were analyzed without any treatment.
Procedures
ETV-ICP OES
The ETV-ICP OES method was optimized in order to find conditions that would allow the analysis of all the pharmaceutical samples. A steel tweezers provided by the ETV manufacturer was used for graphite platform handling whereas a steel spatula was used to tranfer the sample to the platform. Before use, the empty graphite platform was cleaned by submiting it to the heating program of the ETV at temperature up to 2400 °C. The amitriptyline hydrochloride sample was used for method development. To this end, 2.5 mg of sample were weighed into the graphite platform and submitted to the heating program of the ETV. For better signal visualization, the sample was spiked with 5 μL of solution containing 300 ng of As, 150 ng of Mo and 30 ng of the other analytes. This solution was added onto the sample on the graphite platform and then dried using the drying bank set at 90 °C. A graphite platform containing only 5 μL of HNO3 0.72 mol L−1 was used as blank. For standard addition calibration, different volumes of calibration solutions were added onto the sample previously weighed in the graphite platform. When solution or sample spiked with the anlaytes were analyzed, they were dried before introducing the graphite platform into the furnace.
The analytes signal intensities were obtained by setting the ICP OES spectrometer for 40 readings during 0.01 s each. Peak area was used for signal and data processing, which was possible by means of a software developed (Sisvap, Brazil) in order to integrate the transient signals.
Sample preparation by MIC
Since there was no certified reference materials (CRM) available, results were validated by comparison with those obtained by ICP-MS after sample decomposition using MIC and by analyte recovery test. More details of the MIC procedure are described by Flores et al.2828 Flores, E. M. M.; Barin, J. S.; Mesko, M. F.; Knapp, G.; Spectrochim. Acta, Part B 2007, 62, 1051. Briefly, pellets of samples (125 mg) were prepared by pressing the powdered material in a manual hydraulic press (Specac, UK) set at 5 ton for 30 s. The pellets were transferred to the quartz sample holder containing a purified filter paper humidified with 50 μL of 6.0 mol L−1 NH4NO3 solution. The NH4NO3 acts as igniter. Quartz flasks were pressurized with O2 to 20 bar. A 14.4 mol L−1 HNO3 solution (6 mL) was used as absorbing solution. The microwave oven program used for MIC consisted of an irradiation step for 5 min at 1400 W, followed by a cooling step for 20 min (0 W). The absorbing solution was further diluted to 30 mL with water and the investigated elements determined in the final solution using ICP-MS.
Results and Discussion
Since carbon is the major constituent of the analyzed samples, the ETV heating program was adjusted in order to eliminate the carbon matrix before the analyte vaporization. It was observed that at temperature from 300 to 350 °C, the sample matrix was almost completely eliminated. This was verified by weight loss after the sample is submited to pyrolysis step. Thus, the furnace was heated up to 200 °C using a fast temperature ramp and then the temperature was slowly raised to 350 °C (Table 2). In this way, the organic matter was eliminated and matrix effects reduced as observed by the similarity of the analyte signal profiles in the presence and absence of sample. Additionally, as observed by Detcheva et al.2929 Detcheva, A.; Barth, P.; Hassler, J.; Anal. Bioanal. Chem. 2009, 394, 1485. and Barth et al.,2424 Barth, P.; Hassler, J.; Kudrik, I.; Krivan, V.; Spectrochim. Acta, Part B 2007, 62, 924. halogen-containing modifiers (such as CCl2F2) buffer differences related with the analyte chemical form, which may be different in samples and calibration solution. Consequently, the analyte present in the solid sample and in the dried solution has a quite similar vaporization behavior, as observed in the present work.
Modifier evaluation
It is well known that elements such as Cr, V, Mo, B and U are difficult to vaporize by electrothermal heating in graphite tube since these elements form carbides that are volatilized only at 3000 °C or higher tempertures.3030 Matousek, J. P.; Satumba, R. T.; Spectrochim. Acta, Part B
1989, 44, 1005.,3131 Schäffer, U.; Krivan, V.; Anal. Chem.
1999, 71, 849. An alternative is to produce more volatile species of these elements, making possible their determination using electrothermal vaporization. To this end, several studies have reported the use of oxinates and halogenated compounds. Ammonium fluoride, PTFE, and halogenated gases as CCl2F2 are among the most commonly used halogenated compounds.3030 Matousek, J. P.; Satumba, R. T.; Spectrochim. Acta, Part B
1989, 44, 1005. In the case of CCl2F2, it decomposes at temperature around 700 °C, producing CF2, CF3Cl, CF4, and C2F4 radicals, which promote the analyte conversion into volatile halides.3232 Ren, J. M.; Salin, E. D.; Spectrochim. Acta, Part B
1994, 49, 555. The analyte transport increasing as a consequence of cluster formation is another advantage of using halogen modifiers in ETV.3333 Kántor, T.; Maestre, S.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B
2005, 60, 1323.
34 Kántor, T.; Güçer, S.; Spectrochim. Acta, Part B
1999, 54, 763.-3535 Grégoire, D. C.; Sturgeon, R. E.; Spectrochim. Acta, Part B
1999, 54, 773. By considering the vaporization temperature, the elements investigated in the present work could be more or less classified in three groups: (i) As and Cd as volatile elements; (ii) Pd, Pt, Rh, and Ru as refractory elements and (iii) Cr, Cu, Mo, Ni and V as carbide forming elements.
Freon was used as reaction gas in the present work, whereas the gas flow rate was evaluated in the range of 0.5 to 5.0 mL min−1. As expected, the vaporization temperature of several elements decreased drastically in the presence of Freon as a consequence of the formation of halogenated compounds. The influence of the Freon flow rate on As, Cr, Pd and Pt is shown in Figure 1. For this experiment, the pyrolysis and vaporization temperatures were set at 350 °C and 1800 °C, respectively, whereas 2.5 mg of amitriptyline hydrochloride spiked with 300 ng of As, 150 ng of Mo and 30 ng of the other investigated elements were analyzed. As can be observed in Figure 1, Cr and Pd exhibit similar behavior as a function of the Freon flow rate, differently of As that is a more volatile element. Similar behavior was observed for Cd (not shown in Figure 1), that is, the maximum Cd signal intensity was observed when the Freon flow rate was around 1.0 mL min−1. It was observed that the Cr signal intensity as well as those of Cu, Mo, Ni, V, Mn and Pb decreased at lower Freon flow rate when compared to Pd, Pt, Rh and Ru (not shown in Figure 1), probably due to the generation of halogenated compounds at lower Freon flow rate. In general, 80% of the maximum signal of Cr, Cu, Mo, Ni, V, Mn and Pb was observed when 0.5 mL min−1 of Freon was added to the carrier gas. On the other hand, the Freon flow rate should be at least 1.0 mL min−1 for Pd, Pt, Rh and Ru exhibit 80% of their maximum signal. In general, the analyte signal intensity increased with the Freon flow rate increase, differently of As and Cd. The signals of these two more volatile elements decreased with the Freon flow rate increase because the added gas impairs the nucleation process, preventing the analyte transport to the ICP. Therefore, it can be stated that effect of Freon on nucleation is more pronounced for more volatile elements. However, suppression of the As and Cd signals may also have occured as a consequence of energy transfer change in the ICP caused by Freon. Thus, as a compromise condition, the Freon flow rate was fixed in 3.0 mL min−1.
Effect of CCl2F2 flow rate on the analytes signals intensities. Sample: 2.5 mg of amitriptyline hydrochloride added with 300 ng of As and 30 ng of Cr and Pd; pyrolysis and vaporization temperatures: 350 and 1800 °C, respectively. Each point is the mean and standard deviation of three measurements.
The analyte vapor transport is usually improved in the presence of some compounds,3333 Kántor, T.; Maestre, S.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B 2005, 60, 1323. which can promote the formation of clusters. Therefore, the effect of organic compounds such as citric acid, ascorbic acid, oxalic acid, ethylenediaminetetraacetic acid, and NaCl were evaluated. These substances not only increase the analyte transport, but also serve as matrix matching. In this way, calibration can be carried out with aqueous standards instead of solid standards. However, the analyte transport was only little improved when up to 100 mg of each compound above cited was added. Besides, the carrier effect was not the same for all elements, probably because Freon masked the effect caused by the added substance. Thus, only Freon was used in further experiments, which acts as reaction gas (lowering the vaporization temperature) and carrier of several elements.
Heating program of the ETV system
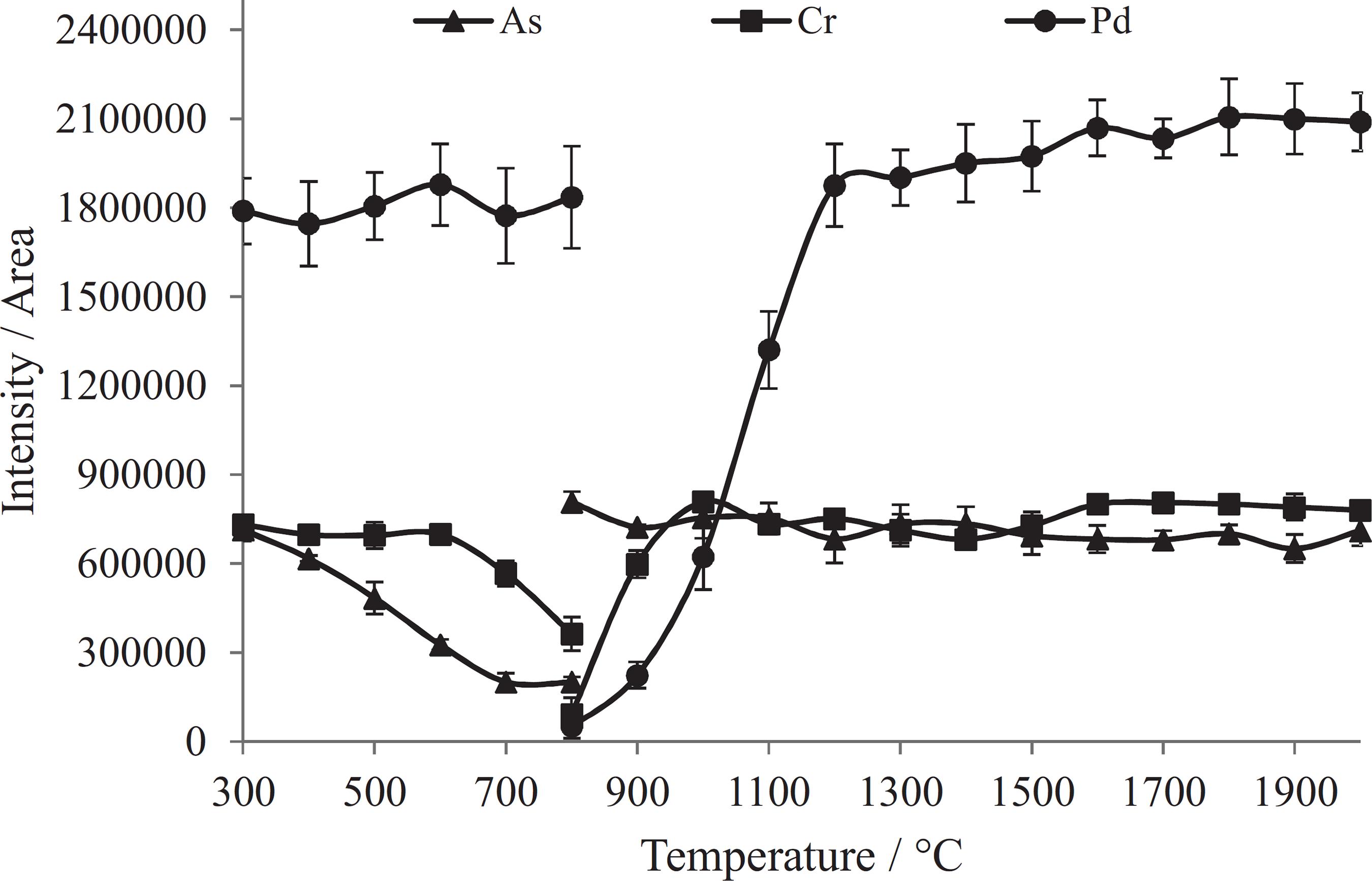
The pyrolysis (300 to 800 °C) and vaporization (800 to 2000 °C) temperatures as well as the time of each step were evaluated in order to obtain a compromise condition for the determination of all elements simultaneously at a Freon flow rate of 3.0 mL min−1. Experiments using standard solutions and sample spiked with standards were carried out. In these experiments, 300 ng of As, 150 ng of Mo and 30 ng of each of the other elements were directly analyzed or added to 2.5 mg of amitriptyline hydrochloride on the graphite platform. The ETV program evaluated and set consisted of four heating steps: (i) fast heating (5 s) to reach 200 °C and holding for 10 s; (ii) heating (about 60 s) up to the pyrolysis temperature and holding for 15 s, the temperature was slowly increased in this step with the aim to eliminate the sample matrix slowly in order to prevent the plasma extiction due to the matrix overloading; (iii) vaporization at maximum power (3000 °C s−1) and holding for 20 s and (iv) cleaning at 2400 °C for 5 s. The ETV program established is given in Table 2. When the pyrolysis temperature was evaluated that of the vaporization was fixed at 2000 °C; when the vaporization temperature was evaluated that of pyrolysis was set at 350 °C. Figure 2 shows the pyrolysis and vaporization temperature curves of As, Cr and Pd, where one can observe that the behavior of the elements is different with respect to the pyrolysis temperature. Arsenic is lost at lower temperature than Cr and Pd. Similar behavior was observed for Cr, Cu, Mn, Ni, Mo and Pb, which were lost at pyrolysis temperature higher than 500 °C. With respect to the other investigated elements, no losses were observed up to 800 °C. These findings agree with the results reported by Matousek and Satumba3030 Matousek, J. P.; Satumba, R. T.; Spectrochim. Acta, Part B 1989, 44, 1005. and Ren and Salin.3232 Ren, J. M.; Salin, E. D.; Spectrochim. Acta, Part B 1994, 49, 555.
Pyrolysis and vaporization temperatures. Sample: 2.5 mg of amitriptyline hydrochloride spiked with 300 ng of As and 30 ng of Cr and Pd; CCl2F2 flow rate: 3 mL min−1; pyrolysis and vaporization temperature: 350 and 2000 °C, respectively. Each point is the mean and standard deviation of three measurements.
Sample mass evaluation
The evaluation of the effect of sample mass was carried out using amitriptyline hydrochloride spiked with the analytes, whereas the sample mass ranged from 0.1 to 7.5 mg. The minimum sample mass was limited by the difficulty in weighing sample mass lower than 0.1 mg, while the maximum sample mass was by the graphite platform capacity (about 10 mg for this kind of sample). In addition, higher sample mass would lead to plasma instability or even its extinction, mainly when the whole matrix could not be eliminated at the pyrolysis step (Table 2). The effect of the sample mass was not the same for all investigated elements and according to the results obtained in this step, they could be grouped in distinct groups: (i) the As, Cd, V and Mo signal intensity was suppressed when the amount of sample was higher than 5.0 mg; (ii) the Cr, Cu, Mn, Ni and Pb signal intensity increased gradually with the sample mass increasing; (iii) the Pd, Rh and Ru signal intensity increased with the sample mas increasing up to 0.5 mg and then remained almost constant; (iv) the maximum signal of Pt was observed for 0.5 mg of sample, which decreased for higher sample mass. The effect of sample mass on the As, Cr, Pd signals can be seen in Figure 3. As a compromise condition for multielement determination, in the present work, the sample mass was fixed at 2.5 mg.
Effect of the sample mass on the analyte signal intensity. Sample: spiked with 300 ng of As and 30 ng of Cr and Pd; CCl2F2 flow rate of 3 mL min−1; pyrolysis and vaporization temperature: 300 °C and 1600 °C, respectively. Each point is the mean and standard deviation of three measurements.
The sample constituents can increase the analyte vapor transport to the ICP, increasing the analyte signal as a consequence.3636 Kántor, T.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B 2003, 58, 1901. On the other hand, too much amount of sample overload the plasma where there is no more sufficient energy for analyte excitation. Some authors3636 Kántor, T.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B 2003, 58, 1901. stated that signal increasing and/or suppression is related to the element volatility. However, with the exception of As, in the present work, different behavior was observed for elements with similar volatility. Therefore, additional investigations are necessary by considering also the energy of the emission line involved. The effect of Freon in the ICP must also be considered because a mixed gas plasma is generated. In addition, the excitation conditions in the ICP changes in the presence of carbon as observed by Lu and Jiang,3737 Lu, H. H.; Jiang, S. J.; Anal. Chim. Acta 2001, 429, 247. Ho and Jiang3838 Ho, C. Y.; Jiang, S. J.; J. Anal. At. Spectrom. 2002, 17, 688. and Li et al.3939 Li, Y. C.; Jiang, S. J.; Chen, S. F.; Anal. Chim. Acta 1998, 372, 365.
Calibration, limits of detection and precision
The external calibration (a) and standard addition (b) methods were evaluated using a multielement standard solution where the analyte concentration was in accordance with its sensitivity. Increasing volumes of this solution were transferred to the graphite platforms, with or without sample, and dried on the drying bank. Blank replicates consinting of 5 μL of 0.72 mol L−1 HNO3 were also analyzed. The mass range used for both calibration methods, the coefficient of determination (r2) and the linear regression equation of calibration curves are summarized in Table 3. Better coefficient of determination was observed (r2 ≥ 0.99) for the external calibration. Although Freon acts as reaction and carrier gas, minimizing differences for the analyte in aqueous solution and in the presence of sample, the standard addition method was still necessary in view of the different sensitivity observed for the analyte in prescence and absence of the sample matrix. With respect to precision, the relative standard deviation (RSD) was typically lower than 10%.
Parameter of the calibration curves obtained by external calibration and standard addition methods by using ETV-ICP OES
The limits of detection were calculated using the 3σ criterion. The standard deviation (σ) was obtained from 10 measurements of the empty graphite platforms. The limits of detection (LODs) of the proposed ETV-ICP OES are given in Table 4, where one can compare with those of the MIC ICP-MS method and the limits recommended by USP44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011. and FBRAS.55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. As can be observed in Table 4, the proposed method attends the limits recommended by USP44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011. for Cd, Cu, Ni, Rh and Ru and those of Cd, Cr, Cu, Mn, Ni, Pd, Pt, Rh, Ru and V recommended by FBRAS.55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010.
Limits of detection of the ETV-ICP OES and MIC ICP-MS methods and limits established by the USP and FBRAS pharmacopeias
Samples analysis
For quantification of the investigated elements in the pharmaceuticals samples, 2.5 mg of each sample were weighed directly into the graphite platforms, transferred to ETV and then vaporized by applying the heating program given in Table 2 under Freon at 3.0 mL min−1. Ten samples were analyzed, whereas chromium was the unique element detected (concentration: 5.7 ± 0.4 μg g−1) and only in cyclobenzaprine hydrochloride. The concentration of Cr was lower than the maximum limit recommended by FBRAS (25 μg g−1).55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. The concentrations of all other elements were lower than the respective LODs.
Considering the 16 elements recommended by the Pharmacopoeias,44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011.,55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. only Ir, Hg and Os were not included in the present method. Osmiun is very volatile and the difficult determination of Os is well documented.4040 Houk, R. S.; Jarvis, K. E.; Gray, A. L.; Handbook of Inductively Coupled Plasma Mass Spectrometry, Blackie & Son Ltd.: Glasgow, 1992. Mercury was not included in view of its higher volatility compared with the other investigated elements. Furthermore, halogenated compounds decrease the Hg transport to the ICP.3535 Grégoire, D. C.; Sturgeon, R. E.; Spectrochim. Acta, Part B 1999, 54, 773. Iridium was not included in the method due to the low sensitivity and low precision observed.
Accuracy
The accuracy of the results obtained using ETV‑ICP OES were checked using an independent method. The Pharmacopoeias44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011.
5 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010.-66 European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, 2005. recommend sample decomposition using microwave assisted digestion for water insoluble samples and adequate techniques for element detection. However, it was not possible to decompose all analyzed samples by using the recommended sample decomposition method. The obtained sample solutions had elevated residual carbon content (RCC) and precipitate, likely products of nitration reactions.1616 Barin, J. S.; Tischer, B.; Picoloto, R. S.; Antes, F. G.; Silva, F. E. B.; Paula, F. R.; Flores, E. M. M.; J. Anal. At. Spectrom.
2014, 29, 352. The FBRAS55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. recommends the use of MIC for samples of difficult decomposition, instead of microwave assisted digestion. MIC has already been used for pharmaceuticals decomposition whereas the RCC content in the final sample solution was very low.1616 Barin, J. S.; Tischer, B.; Picoloto, R. S.; Antes, F. G.; Silva, F. E. B.; Paula, F. R.; Flores, E. M. M.; J. Anal. At. Spectrom.
2014, 29, 352. It is worth citing that low RCC content is very important in ICP-MS determination because carbon promotes spectral and non-spectral interferences. Therefore, the analytes determination in the pharmaceuticals samples was carried using MIC for sample decomposition. External calibration was used for element quantification using ICP-MS. The Cr concentration found in cyclobenzaprine hydrochloride was 6.12 ± 0.12 μg g−1, which is not different of that found using ETV-ICP OES at 95% of confidence level (t-test). The other investigated elements were also not detected by using MIC and ICP-MS. In addition, analyte recovery test was also done. Analyte solutions were added to 2.5 mg of sample mass in order to achieve concentration values about five-fold higher than the LOD of each element. Recoveries in the range of 91 to 103% were observed. Therefore, these results attest the good accuracy of the proposed ETV-ICP OES method.
Conclusions
An ETV-ICP OES method for direct determination of metals and metalloids in solid pharmaceuticals was developed. High sample throughput, good sensitivity, low or no sample preparation that reduces the risk of contamination and analyte losses are the main advantages of the proposed method.
Freon was used as reaction gas, which promoted the analytes vaporization at lower temperature. By adequate optimization of ETV-ICP OES parameters, 13 elements were accurately determined in 10 pharmaceutical samples. From the list of 16 elements that must be controlled in pharmaceuticals, only Ir, Hg and Os could not be included in the method, due to low sensitivity and/or losses in the pyrolysis step. The investigated elements were not detected in the analyzed samples, with the exception of Cr. Cyclobenzaprine hydrochloride was the only sample where Cr was detected, but the concentration found was lower than the concentration limit recommended by the pharmacopoeias. The LODs of Cd, Cr, Cu, Mn, Ni, Pd, Pt, Rh, Ru and V are lower than the maximum limits recommended by the FBRAS.55 Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010. However, the method does not attend the limits established by USP44 The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011. for As, Mo, Pb, Pd, Pt and V.
Acknowledgements
The authors gratefully acknowledge the support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
-
1World Health Organization (WHO), http://www.who.int/topics/pharmaceutical_products/en/ accessed in December 2014.
» http://www.who.int/topics/pharmaceutical_products/en/ -
2Meermann, B.; Sperling, M.; Anal. Bioanal. Chem. 2012, 403, 1501.
-
3International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH Harmonized Tripartite Harmonized, Guideline for Elemental Impurities; ICH Q3D Guideline, Geneva, 2014.
-
4The United States Pharmacopoeia 35; Elemental Impurities-Limits 232, Pharm. Forum, 37, 2011.
-
5Agência Nacional de Vigilância Sanitária - Farmacopeia Brasileira, 5ª ed.; Brasília, 2010.
-
6European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, 2005.
-
7Scripcariu, M.; Tanase, I. G.; Fleschin, S.; Magearu, V.; Rev. Roum. Chim. 2008, 53, 149.
-
8Tanase, A.; Miu, A.; Rev. Roum. Chim. 2012, 57, 971.
-
9Raghuram, P.; Soma Raju, I. V.; Sriramulu, J.; Pharmazie 2010, 65, 15.
-
10Antosz, F. J.; Xiang, Y.; Diaz, A. R.; Jensen, A. J.; J. Pharm. Biomed. Anal. 2012, 62, 17.
-
11Carvalho, G. G. A.; Nunes, L. C.; Souza, P. F.; Krug, F. J.; Alegre, T. C.; Santos Jr., D.; J. Anal. At. Spectrom. 2010, 25, 803.
-
12Lewen, N.; Mathew, S.; Schenkenberger, M.; Raglione, T.; J. Pharm. Biomed. Anal. 2004, 35, 739.
-
13Van Hoecke, K.; Catry, C.; Vanhaecke, F.; J. Anal. At. Spectrom. 2012, 27, 1909.
-
14Mester, Z.; Sturgeon, R. In Comprehensive Analytical Chemistry - Sample Preparation for Trace Element Analysis; Barceló, D.; ed.; Elsevier: Amsterdam, 2003, p. 1338.
-
15Mitra, S. In Sample Preparation Techniques in Analytical Chemistry; Kebbekus, B. B.; ed.; John Wiley & Sons: New Jersey, 2003, ch. 5.
-
16Barin, J. S.; Tischer, B.; Picoloto, R. S.; Antes, F. G.; Silva, F. E. B.; Paula, F. R.; Flores, E. M. M.; J. Anal. At. Spectrom. 2014, 29, 352.
-
17Pereira, J. S. F.; Pereira, L. S. F.; Schmidt, L.; Moreira, C. M.; Barin, J. S.; Flores, E. M. M.; Microchem. J. 2013, 109, 29.
-
18Antes, F. G.; Duarte, F. A.; Mesko, M. F.; Nunes, M. A. G.; Pereira, V. A.; Müller, E. I.; Dressler, V. L.; Flores, E. M. M.; Talanta 2010, 83, 364.
-
19Kurfürst, U.; Solid Sample Analysis Direct and Slurry Sampling Using GF-AAS and ETV-ICP, Springer: Heidelberg, 1998.
-
20Grindlay, G.; Mora, J.; Gras, L.; Loos-Vollebregt, M. T. C.; Anal. Chim. Acta 2009, 652, 154.
-
21Masson, P.; Dauthieu, M.; Trolard, F.; Denaix, L.; Spectrochim. Acta, Part B 2007, 62, 224.
-
22Wu, Y.; Hu, B.; Jiang, Z.; Chen, S.; J. Anal. At. Spectrom. 2002, 17, 121.
-
23Resano, M.; Aramendíaz, M.; Devos, W.; Vanhaecke, F.; J. Anal. At. Spectrom. 2006, 21, 891.
-
24Barth, P.; Hassler, J.; Kudrik, I.; Krivan, V.; Spectrochim. Acta, Part B 2007, 62, 924.
-
25Asfaw, A.; Wibetoe, G.; Beauchemin, D.; J. Anal. At. Spectrom. 2012, 27, 1928.
-
26Lin, M. -L.; Jiang, S. -J.; J. Anal. At. Spectrom. 2011, 9, 1813.
-
27Majidi, V.; Smith, R. G.; Xu, N.; McMahon, M. W.; Bossio, R.; Spectrochim. Acta, Part B 2000, 55, 1787.
-
28Flores, E. M. M.; Barin, J. S.; Mesko, M. F.; Knapp, G.; Spectrochim. Acta, Part B 2007, 62, 1051.
-
29Detcheva, A.; Barth, P.; Hassler, J.; Anal. Bioanal. Chem. 2009, 394, 1485.
-
30Matousek, J. P.; Satumba, R. T.; Spectrochim. Acta, Part B 1989, 44, 1005.
-
31Schäffer, U.; Krivan, V.; Anal. Chem. 1999, 71, 849.
-
32Ren, J. M.; Salin, E. D.; Spectrochim. Acta, Part B 1994, 49, 555.
-
33Kántor, T.; Maestre, S.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B 2005, 60, 1323.
-
34Kántor, T.; Güçer, S.; Spectrochim. Acta, Part B 1999, 54, 763.
-
35Grégoire, D. C.; Sturgeon, R. E.; Spectrochim. Acta, Part B 1999, 54, 773.
-
36Kántor, T.; Loos-Vollebregt, M. T. C.; Spectrochim. Acta, Part B 2003, 58, 1901.
-
37Lu, H. H.; Jiang, S. J.; Anal. Chim. Acta 2001, 429, 247.
-
38Ho, C. Y.; Jiang, S. J.; J. Anal. At. Spectrom. 2002, 17, 688.
-
39Li, Y. C.; Jiang, S. J.; Chen, S. F.; Anal. Chim. Acta 1998, 372, 365.
-
40Houk, R. S.; Jarvis, K. E.; Gray, A. L.; Handbook of Inductively Coupled Plasma Mass Spectrometry, Blackie & Son Ltd.: Glasgow, 1992.
-
FAPERGS has sponsored the publication of this article.
Publication Dates
-
Publication in this collection
Mar 2015
History
-
Received
11 Sept 2014 -
Accepted
13 Jan 2015