Abstract
New luminescent organic-inorganic hybrid particles based on Tb-doped aluminates and asparagine (Asn) surface modifiers were investigated. The Tb3+ doped inorganic core was obtained by spray pyrolysis, at 200 ºC γ-AlOOH (BOE:Tbx%) or at 700 ºC γ-Al2O3 (γTA:Tbx%). The reaction of Asn with boehmite in water disaggregated the sub-micronic boehmite particles to give stable dispersion of surface modified nanoparticles Asn:BOE:Tbx% (x = 1 or 5). Concerning the Asn:γTA:Tbx% system, an Asn film wrapping alumina particles was observed. Photoluminescence spectra exhibited the bands assigned to Tb3+5D4 → 7FJ = 6-3 transitions. A broad absorption band (240 nm) was assigned to the host (aluminate) to ion (Tb3+) energy transfer. Efficient energy transfer was observed when active ions are incorporated in the defect-spinel structure of γTA, whereas it was relatively weak for BOE:Tb where Tb3+ are bonded to the hydroxyls groups at nanocrystals surface. It is noticeable that Asn strengthens the linkage of Tb3+ with the aluminate matrix, enhancing the host to dopant energy transfer.
Keywords:
hybrid; spray pyrolysis; alumina; rare earth; terbium luminescence
Introduction
“Alumina” is the general name encompassing a large number of products with different chemical formulas and/or structures. The thermodynamically stable phase at standard temperature and pressure conditions is α-Al2O3, having the hexagonal crystal structure of the mineral corundum. Lanthanide ions doped alumina (α) provide extremely versatile photoluminescent ceramics. The green emitter Tb3+ was for instance successfully incorporated into a dense alumina matrix, achieving a transparent light-emitting ceramic.11 Penilla, E. H.; Kodera, Y.; Garay, J. E.; Adv. Funct. Mater. 2013 , 23, 6036. Transitions alumina’s (partially dehydrated aluminum hydroxides) possess high surface areas which renders them appropriate for use as adsorbents, catalysts and catalyst carriers. The “γ-Al2O3” transition alumina is particularly important in catalysis and details of its structure and exact composition have been the subject of a number of controversial works.22 Zhou, R. S.; Snyder, R. L.; Acta Crystallogr.1991 , B47, 617.
3 Sohlberg, K.; Pennycook, S. J.; Pantelides, S. T.; J. Am. Chem. Soc.1999 , 121, 7493.
4 Pecharromán, C.; Sobrados, I.; Iglesias, J. E.; González Carreño, T.; Sanz, J.; J. Phys. Chem. B1999 , 103, 6160.
5 Krokidis, X.; Raybaud, P.; Gobichon, A.-E.; Rebours, B.; Euzen, P.; Toulhoat, H.; J. Phys. Chem. B2001 , 105, 5121.
6 Paglia, G.; Rohl, A. L.; Buckley, C. E.; Gale, J. D.; Phys. Rev. B2005 , 71, 224115.-77 Alvarez, L. J.; Leon, L. E.; Sanz, J. F.; Capitan, M. J.; Odriozola, J. A.; J. Phys. Chem.1995 , 99, 17872. Boehmite, γ-AlOOH, is actually the most important precursor for the synthesis of transition aluminas following the transformation sequence boehmite → γ → ∆ → θ → α. Crystallization degree, morphology and surface properties are observed to be highly dependent on the structure.
Nanocrystalline boehmite can be obtained through a sol-gel route as firstly described by Yoldas.88 Yoldas, B. E.; J. Mater. Sci.1975 , 10, 1856.,99 Yoldas, B. E.; Ceramic Bulletin1975 , 54, 289. Nanocrystalline boehmite may be employed as sorbent, for instance by removing potentially toxic metal from polluted waste water.1010 Granados-Correa, F.; Jiménez-Becerril, J.; J. Hazard. Mater.2009 , 162, 1178. The surface of boehmite particles is highly reactive towards carboxylic acids in aqueous media. The adsorption of low molecular weight carboxylic acids, ubiquitous in natural environments, on positively charged mineral surfaces, e.g., aluminium oxihydroxides, has also been the subject of many investigations.1111 Yoon, T. H.; Johnson, S. B.; Musgrave, C. B.; Brown Jr., G. E.; Geochim. Cosmochim. Acta2004 , 68, 4505.
Material chemists have taken benefit of this high affinity to synthesize alumoxanes from the reaction of boehmite with carboxylic acids.1212 Landry, C. C.; Pappé, N.; Mason, M. R.; Apblett, A. W.; Tyler, A. N.; MacInnes, A. N.; Barron, A. R.; J. Mater. Chem.1995 , 5, 331. Once formed, the carboxylate-substituted alumoxanes may be employed as precursors for a wide range of ceramics, including lanthanide-aluminium mixed-metal oxides.1313 Kareiva, A.; Harlan, C. J.; MacQueen, D. B.; Cook, R. L.; Barron, A. R.; Chem. Mater.1996 , 8, 2331.
Alumina-based nanoparticles or nano-structured objects with possible applications in the field of nano-bio-technology or nano-medicine have been described. Alumina membranes with highly ordered nanopores are proposed for instance in smart implantable drug delivery systems.1414 Kang, H.-J.; Kim, D. J.; Park, S.-J.; Yoo, J.-B.; Ryu, Y. S.; Thin Solid Films2007 , 515, 5184. Although far less described in literature than mesoporous silica, powdered mesoporous (hydrated) alumina is a potentially available substrate for controlled drug release, after suitable chemical functionalization of its surface.1515 Kapoor, S.; Hegde, R.; Bhattacharyya, A. J.; J. Control. Release2009 , 140, 34.
On the other hand, luminescent micro- or nano- particles are becoming of great importance in the field of bio-labeling. Several examples of crystalline lanthanide oxides as luminescent biolabels are also found in literature. Eu3+ and Tb3+ containing nanomaterials have also been reported as in nanocrystalline hydroxyapatite or in lanthanum phosphate for example.1616 Al-Kattan, A.; Santran, V.; Dufour, P.; Dexpert-Ghys, J.; Drouet, C.; J. Biomater. Appl.2014 , 28, 697.
17 Li, L.; Liu, Y.; Tao, J.; Zhang, M.; Pan, H.; Xu, X.; Tang, R.; J. Phys. Chem. C2008 , 112, 12219.-1818 Meiser, F.; Cortez, C.; Caruso, F.; Angew. Chem. Int. Ed.2004 , 43, 5954. Lanthanide emitters (Eu3+, Tb3+) are particularly interesting for the purpose of luminescent bio-labeling since the decay times for the main intra 4fn transitions are much longer than the decay times of the background fluorescence of biological samples. By employing well-suited experimental set-ups the photoluminescence signal from the optical probe may be discriminated from the background emission from all non-probed species.1919 Jin, D.; Connally, R.; Piper, J.; Cytom. Part A2007 , 71A, 783.
Concerning preparation methods spray pyrolysis (SP) is an aerosol process commonly used to form a variety of materials in powder form, including transition aluminas or α alumina.2020 Okada, K.; Tanaka, A.; Hayashi, S.; Otsuka, N.; J. Mater. Sci. Lett.1993 , 12, 854.
21 Vallet-Regi, M.; Rodriguez-Lorenzo, L. M.; Ragel, C. V.; Salinas, A. J.; Gonzalez-Calbet, J. M.; Solid State Ionics1997 , 101-103, 197.-2222 Kato, T.; J. Ceram. Soc. Jpn.2002 , 110, 146. A protocol based on the rapid drying of an aluminium alcoholate suspension was proposed in order to synthesize nanocrystalline γ-AlOOH or γ-Al2O3 in a single step, by the SP process.2323 Caiut, J. M. A.; Dexpert-Ghys, J.; Kihn, Y.; Vérelst, M.; Dexpert, H.; Ribeiro, S. J. L.; Messaddeq, Y.; Powder Technol.2009 , 190, 95. Moreover, by the addition of an optically active lanthanide ion Eu3+ or Tb3+, luminescent aluminates can be obtained in just one step.2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605.
25 Marques, R. F. C.; Caiut, J. M. A.; Paiva-Santos, C. O.; Ribeiro, S. J. L.; Messaddeq, Y.; Garcia, C.; Neumeyer, D.; Dexpert, H.; Verelst, M.; Dexpert-Ghys, J.; Braz. J. Phys.2009 , 39, 176.-2626 Caiut, J. M. A.; Bazin, L.; Mauricot, R.; Dexpert, H.; Ribeiro, S. J. L.; Dexpert-Ghys, J.; J. Non-Cryst. Solids2008 , 354, 4860.
Due to its high affinity for the boehmite surface, the amino-acid asparagines (Asn) strengthens the attachment of the luminescent europium ions at the boehmite nanoparticles after their suspension in water.2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605.
On the basis of these preceding observations, we present here a systematic investigation of Asn:aluminates:Tb nanostructured particles. Terbium-doped aluminates, having the boehmite (BOE) or the gamma alumina structure were obtained by spray pyrolysis, and will be noted BOE:Tbx% and γTA:Tbx%, respectively. The luminescence properties of these powders will be discussed regarding the possible sites of terbium ions in the aluminates and the effects of matrix-dopant electronic interactions. The possibility of modifying the surface of these aluminates by the amino acid Asn was then explored. Asparagine (Asn = H2N-CO-CH(NH3+)-COO− at pH = 7.0) was chosen to render the oxide particles bio-compatibles, because the carboxylic end will presumably attach the aluminate surface, with the formation of alumoxanes, whereas the amide group will be let free for further reaction. Hereafter, composites with Asn will be compared with the corresponding un-modified aluminate, to highlight the organic-inorganic interactions. Finally, the possibility to achieve luminescent nano-labels in the investigated systems will be discussed.
Experimental
Hydrated alumina samples were prepared by spray pyrolysis. The precursor (boehmite sol) was obtained by the methodology established by Yoldas modified to obtain the rare earth doped alumina.88 Yoldas, B. E.; J. Mater. Sci.1975 , 10, 1856.,99 Yoldas, B. E.; Ceramic Bulletin1975 , 54, 289. TbCl3 (0.37 g, 0.001 mol) was dissolved in water (300 mL) at 80 ºC. Aluminum tri-sec-butoxide (25.93 g, 0.1 mol) was added to terbium aqueous solution under stirring at 80 ºC. After 2 h stirring, nitric acid was added as a peptizing agent, up to 0.07 HNO3/Al3+ (molar ratio). The sol (with concentration adjusted to 0.2 mol L-1 Al3+ by water dilution) was spray dried in an experimental setup already described.2323 Caiut, J. M. A.; Dexpert-Ghys, J.; Kihn, Y.; Vérelst, M.; Dexpert, H.; Ribeiro, S. J. L.; Messaddeq, Y.; Powder Technol.2009 , 190, 95. In this setup, the spray was generated by a piezoelectric pellet oscillating at 2.4 MHz. The aerosol, made of fine droplets (Φ ca. 5 µm) from the precursor solution in air, was then driven into two heating zones, the first one around 100 ºC and the second one was the decomposition-densification zone where the temperature may be adjusted at a chosen temperature (TSYN). The powder was separated from the gas phase by an electrostatic collector. The drying and decomposition were accomplished within about 10 s. TSYN was fixed at 200 ºC for the synthesis of boehmite (BOE), and at 700 ºC for that of the “γ” transition alumina (γTA). Three dopant percentages were investigated: 1, 5 and 10% expressed in mol of Tb3+/mol of Al3+.
Amino acids
Aluminoxanes nanocomposites were obtained by dispersing the hydrated aluminas particles in Asn water solutions. Samples activated with Tb1% (BOE:Tb1% or γTA:Tb1%) were dispersed in water (300 mg in 25 mL). Asn was added (1/0.1, 1/0.3 or 1/0.5 mol Al/Asn) and kept under stirring at room temperature for 12 hours. The reaction mixture was centrifuged and the solid was rinsed three times with distilled water and ethanol. The particles were dried at 80 ºC and characterized by X-ray diffraction, electron microscopy, Fourier transform infrared spectroscopy and luminescence. Water suspensions were used for dynamic laser light scattering measurements and also to complete the analysis of Tb3+ luminescence. For the high Asn composition (0.5Asn:1BOE:Tb1%), the sample was observed to be composed off a mixture of nanoparticles and crystallized Asn; concerning the lower content (0.1 Asn), changes on alumina particles were not measureable. Therefore only results obtained for compositions 0.3Asn:1Al will be presented. Composite samples will be named Asn:BOE:Tb (%, mol Tb/Al) and Asn:γTA:Tb (%, mol Tb/Al).
Characterization
The powders were characterized by X-ray diffraction (XRD), with a Seifert XRD3000 diffractometer. The coherent length was estimated with the Scherrer equation (D = 0.9l (FWHM) cosθ) with the full width at half maximum measured in (2θ) and expressed in radians. The size distributions of powders dispersed in water were determined by dynamic laser light scattering (DLS) using a Malvern Instruments ZETASIZER 3000, model DTS5300.
Samples were observed by transmission electron microscopy (TEM) with a Philips-CM120 and by scanning electron microscopy (SEM) with a SEM-JEOL 6700F. Fourier transform infrared (FTIR) spectra were recorded in KBr pellets using a model 2000 Perkin-Elmer spectrometer. Room temperature luminescence excitation and emission spectra were measured with a Hitachi-Fl100 spectrofluorimeter (at 2.5 nm spectral resolution) for solids samples. The 5D4 lifetime measurements were obtained by coupling a phosphorimeter to a spectrofluorimeter SPEX Fluorolog, model F212I, monitoring the emission intensity at 543 nm. Finally, a Varian ECLIPSE (model El05123699) operating in time-resolved mode with excitation and emission band-passes of 10 nm was employed to record the spectra of particles in suspension.
Results and Discussion
XRD and FTIR: structural investigations
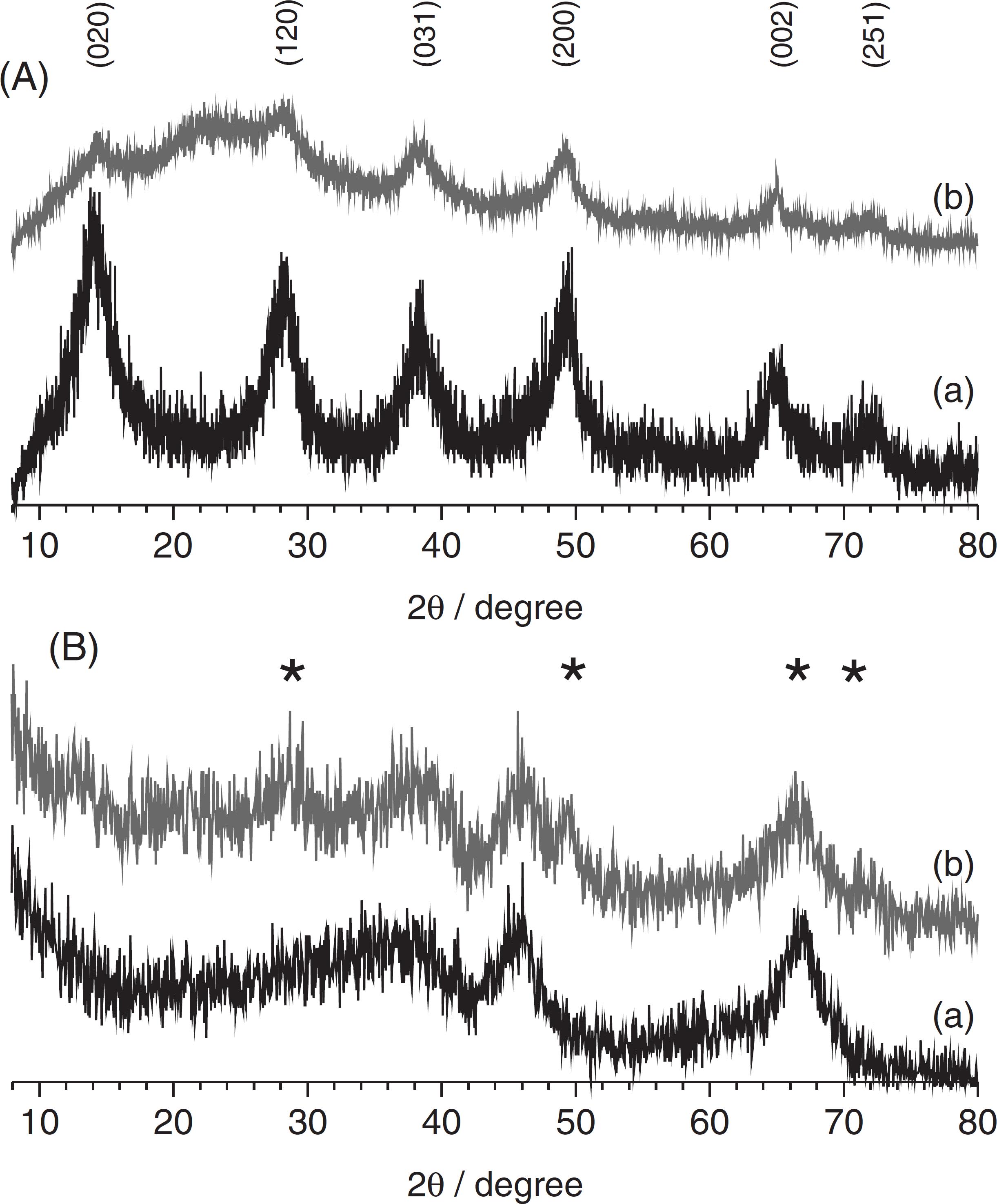
Investigations by X-ray diffraction (Figure 1) and FTIR spectroscopy (Figure 2) confirmed that powders synthesized at 200 and 700 ºC were boehmite γ-AlOOH and γ-Al2O3, respectively, both being weakly crystallized and with a high degree of hydration. Surface modification by Asn induced tenuous changes as discussed hereafter.
Powder X-ray diffraction (A) (a) BOE:Tb1% and (b) Asn:BOE:Tb1%. (B) (a) γTA:Tb1% and (b) Asn:γTA:Tb1%. (*) denote γ-AlOOH phase in γ-Al2O3.
(A) FTIR spectra of (a) BOE:Tb1% and (b) Asn:BOE:Tb1%. (B) FTIR spectra of (a) γTA:Tb1% and (b) Asn:γTA:Tb1%.
At TSYN = 200 ºC the diffractogram matched with the reference JCPD No. 21-1307. In a former paper, details of X-ray data of boehmite samples elaborated by SP have been analyzed.2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605. The average nanocrystal size following the orthorhombic axis b does not exceed one cell, so that half the hydroxyl groups are at the external surface of nanocrystals. At TSYN = 700 ºC, the diffractogram corresponded to reference data JCPD No. 10-0425: with peaks labeled (400) and (440) at 2θ = 46 and 66.5º, respectively, and a very broad and asymmetrical band between 30 and 40º, which contains the badly resolved contributions from (220) and (311) planes. The peak positions matched with the gamma-alumina standard apart from the absence of (111) and (222), which should be observed in well crystallized transition aluminas.22 Zhou, R. S.; Snyder, R. L.; Acta Crystallogr.1991 , B47, 617.,55 Krokidis, X.; Raybaud, P.; Gobichon, A.-E.; Rebours, B.; Euzen, P.; Toulhoat, H.; J. Phys. Chem. B2001 , 105, 5121. The coherent length evaluated was about 3 nm for the SP transition aluminas, but the important background observed under the peaks supports that the size of most nanocrystals in the samples were below this value. On reacting with asparagine, an additional broad band appeared at 2θ ca. 20º for boehmite samples, assigned to the organic counterpart of the composite. The reaction of γ alumina with Asn has lead to the partial transformation of γ-Al2O3 into γ-AlOOH, the diffraction peaks of γ-AlOOH being marked by stars in Figure 1B.
FTIR spectrum of boehmite γ-AlOOH has been described in literature.2727 Fripiat, J. J.; Bosmans, H.; Rouxhet, P. G.; J. Phys. Chem.1967 , 71, 1097.,2828 Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J. B.; Berthet, P.; Huntz, A. M.; Roy, P.; Tétot, R.; J. Solid State Chem.2009 , 182, 1171.In agreement with these previous papers, the spectrum recorded for the powder TSYN = 200 ºC (Figure 2A) displayed vibrational modes of [AlO6] at 481, 632, 734 cm-1 and ∆OH at 1072 and 1157 cm-1. Samples were highly hydrated, as were amorphous or nanocrystallized boehmite described in references.2828 Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J. B.; Berthet, P.; Huntz, A. M.; Roy, P.; Tétot, R.; J. Solid State Chem.2009 , 182, 1171.,2929 Wang, S.-L.; Johnston, C. T.; Bish, D. L.; White, J. L.; Hem, S. L.; J. Colloid Interface Sci.2003 , 260, 26. The two ∆OH modes at 3083 and 3310 cm-1 were partly obscured by the broad band around 3400 cm-1, this last one assigned to adsorbed hydroxyls. The presence of adsorbed water molecules was evidenced by the ∆HOH at 1630 cm-1. FTIR spectrum of sample TSYN = 700 ºC (Figure 2B) was quite in agreement, in the wave number range 400-1400 cm-1, with the one of γ-Al2O3,2828 Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J. B.; Berthet, P.; Huntz, A. M.; Roy, P.; Tétot, R.; J. Solid State Chem.2009 , 182, 1171. with relatively broad bands peaking at 635 cm-1 (stretching AlO6) and 785 cm-1 (stretching AlO4). Adsorbed water and hydroxyls were attested at 1630 cm-1 and around 3400 cm-1.
Boehmite and aluminates from spray pyrolysis displayed features around 1385 cm-1 from residual nitrates but no trace of organics from the preparation mixture were detected. The band at 1673 cm-1 (C=O) and CH vibrations at 1360, 1316 and 1236 cm-1 were well isolated for sample 0.3Asn:BOE:Tb1%. Additional features were observed at 1500 cm-1 and superimposed with the hydroxyls modes in the region 3000-3400 cm-1, but were not assigned. In the spectrum of Asn:γTA:Tb1%, shown in Figure 2B, only very weak effect from the organic part was detectable, as a low intensity band at 1416, 1428 and 1511 cm-1 ∆ CN, υS CO2– and ∆ NH3+ vibration, respectively.3030 Cooper, S. J.; Langmuir2002 , 18, 3749. Important changes were observed in the aluminate response since bands characteristic of boehmite were observed proving the partial transformation of γ-Al2O3 into γ-AlOOH, also evidenced by XRD.
Morphology and water dispersion
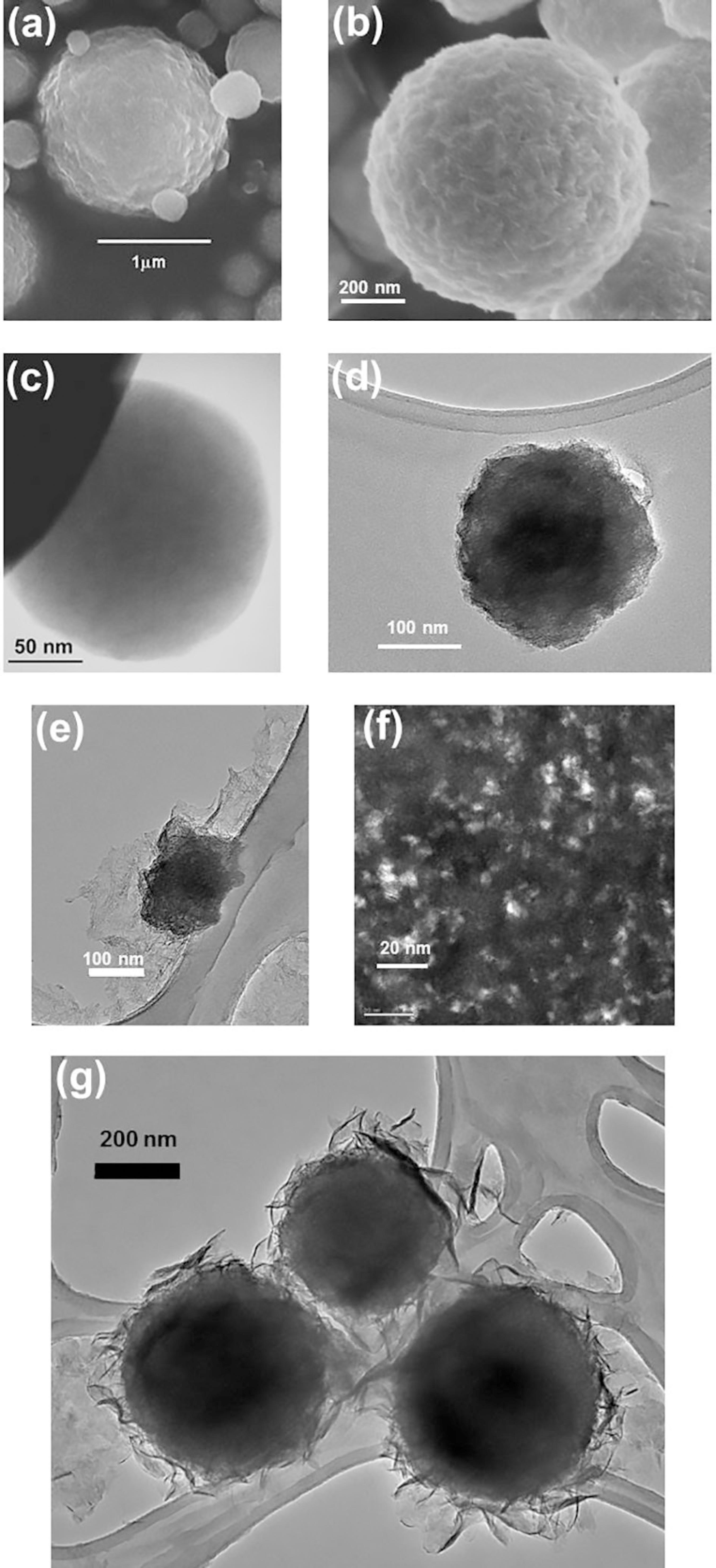
The particles prepared by spray pyrolysis were always spherical and sub-micrometric with a moderately dispersion in size, whatever their composition. The major fraction of hydrated alumina particles presents diameters between 100 and 500 nm. Some characteristic electron microscope images are gathered in Figure 3. SEM images recorded on BOE:Tb1%, Figure 3a, and on γTA:Tb1%, Figure 3b, evidence irregular surfaces, and spherical particles consisting of smaller (nanometric) sub-particles, aggregated in the spray pyrolysis processing. TEM observations, Figures 3c and 3d, give additional information since the spheres were homogeneously packed with sub-particles, and not hollow, although hollow particles can be obtained by SP.2222 Kato, T.; J. Ceram. Soc. Jpn.2002 , 110, 146.
Electron microscopy images, SEM (a) BOE:Tb1%; (b) γTA:Tb1%; TEM (c) BOE:Tb1%; (d) γTA:Tb1%; (e) Asn:BOE:Tb1%; (f) (dark field) Asn:BOE:Tb1%; (g) Asn:γTA:Tb1%.
In composite materials, the organic and inorganic counterparts could be well observed, the effect of the dispersant being more noticeable on boehmite than on transition alumina particles. Most of the boehmite spheres had disaggregated during reaction with Asn. Sample Asn:BOE:Tb1%, shows an organic film in which the nanoparticles appear dispersed. TEM image Figure 3e shows the composite film and one bigger particle not completely disaggregated. Aluminum was identified by local analysis (EDXS in TEM) throughout the film. Figure 3f was recorded on the film in dark field conditions: all bright parts are due to crystallized boehmite nanoparticles (a few nm in size), embedded in the amorphous polymer. For sample Asn:γTA:Tb1% (TEM image in Figure 3g), the organic film was observed to wrap the sub micrometric alumina particles.
Asn molecules were observed to interact differently with boehmite and Al2O3. Boehmite2323 Caiut, J. M. A.; Dexpert-Ghys, J.; Kihn, Y.; Vérelst, M.; Dexpert, H.; Ribeiro, S. J. L.; Messaddeq, Y.; Powder Technol.2009 , 190, 95.,2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605. micrometric spheres obtained by SP had been spontaneously dispersed in water. The same remark was made here for BOE:Tb1% by DLS analysis. 60% of the particles fall in the range 25-40 nm, while higher values (up to 85 nm) were measured for the remaining 40%. Spherical sub-micrometric particles were disaggregated in water and nanometric sub-particles remained very stable. At 5 or 10% Tb3+ particles were noticeably bigger: higher contents in Ln3+ lead to more tightly linked sub-particles. Once formed, these spherical microparticles had not been fragmented in water. In addition, all particles synthesized at TSYN = 700 ºC with the γ-Al2O3 structure, remained stable in water. The addition of Asn did not change the particles form and the final composite presented low concentration of Asn as corroborated by the low organic content detected by FTIR.
Photoluminescence
The two hydrated aluminates under consideration have quite different crystal structures, in particular when one considers the way that bigger cations as Ln3+ could be accommodated on the Al3+ sites. γ-Al2O3 has a defect-spinel structure with most of Al atoms in octahedral coordination and the minority ones in tetrahedral coordination.33 Sohlberg, K.; Pennycook, S. J.; Pantelides, S. T.; J. Am. Chem. Soc.1999 , 121, 7493. At crystal surface, more distorted tetrahedral Al sites may occur with penta- and hepta-coordinated Al.3131 Alvarez, L. J.; Leon, L. E.; Sanz, J. F.; Capitan, M. J.; Odriozola, J. A.; J. Phys. Chem.1995 , 99, 17872. γ-Alumina has a lot of structural defects or empty cationic sites, especially near the surface of nano-crystals in poorly crystallized sample, and it has been admitted that Eu3+ ions may be accommodated in γ-Al2O3, most probably at these defect sites.2525 Marques, R. F. C.; Caiut, J. M. A.; Paiva-Santos, C. O.; Ribeiro, S. J. L.; Messaddeq, Y.; Garcia, C.; Neumeyer, D.; Dexpert, H.; Verelst, M.; Dexpert-Ghys, J.; Braz. J. Phys.2009 , 39, 176.,2626 Caiut, J. M. A.; Bazin, L.; Mauricot, R.; Dexpert, H.; Ribeiro, S. J. L.; Dexpert-Ghys, J.; J. Non-Cryst. Solids2008 , 354, 4860.,3232 Monteiro, M. A. F.; Brito, H. F.; Felinto, M.; Brito, G. E. S.; Teotonio, E. E. S.; Vichi, F. M.; Stefani, R.; Micropor. Mesopor. Mat.2008 , 108, 237. The crystal structure of boehmite,3333 Milligan, W. O.; McAtee, J. L.; J. Phys. Chem.1956 , 60, 273. is orthorhombic. The unit cell consists of two double layers of aluminium-centered distorted octahedral AlO4(OH)2. OH groups locate at the outer surface of the double layers and interact to hold the layers together. There is no substitution of Eu3+ on the Al3+ octahedral sites in boehmite for Eu3+,2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605. but that partly hydrated Eu3+ ions are directly bonded to OH groups at the boehmite surface. It is highly probable that Tb3+ behave the same way as Eu3+, hereafter we discuss characteristic features from the terbium luminescence data that support these hypotheses.
The photoluminescence emission spectra of samples BOE:Tb1% and γTA:Tb1% are displayed in Figures 4a and 4b, respectively. Characteristic emission bands assigned to the 5D4 → 7FJ transitions (J = 6, 5, 4, 3) with the dominant green band at 540 nm are observed. The shape of Tb3+ emission is generally little sensitive to the local environment and no additional information could be extracted from their comparison in the two hydrated aluminates. Considering photoluminescence excitation a number of narrow lines assigned to Tb3+ (4f8 configuration) 7F6 → 2S + 1LJ transitions could be observed.3434 Carnall, W. T.; Crosswhite, H.; Crosswhite, H. M.; Energy Level Structure and Transition Probabilities in the Spectra of the Trivalent Lanthanides in LaF3, 1st ed.; Argonne National Laboratory: Argonne, USA, 1977. Of particular interest is the broad contribution observed at wavelengths 320-400 nm since it matches the excitation wavelengths most often encountered in laboratory fluorescence microscopes and supports the consideration of Tb3+ as fluorescent bio-label. In addition, an intense broad band at around 240 nm was observed for γTA:Tb1%, but not for BOE:Tb1%. The strength to the way terbium ions are connected with the host may be responsible for the differences. Two assignments are conceivable for the band around 240 nm. The first assignment is the of Tb3+ interconfigurational parity-allowed absorption. From the ground state 7F6 (4f8), transitions occur to the multiplets 9DJ and 7DJ (4f7 5d1). The former transition (ending at 9DJ) is spin forbidden and occurs at lower energy than the spin allowed one ending at 7DJ.
Photoluminescence of (a) BOE:Tb1%; (b) γTA:Tb1%; (c) BOE:Eu2%; (d) γTA:Eu2%; (e) Asn:BOE:Tb1%; (f) Asn:γTA:Tb1%. Excitation spectra monitored at λ emission = 543 nm (a, b, e, f) or at λ emission = 592 nm (c, d); emission spectra by excitation at 351 nm (a), 250 nm (host at b, e, f) and 394 nm (c, d).
Dorenbos3535 Dorenbos, P.; J. Phys. Condens. Matter2003 , 15, 6249. initiated a shift model in the analysis of 5d levels of Tb3+. The spin-allowed transition energies for Tb3+ in some mixed oxides (borates or silicates) are actually reported at around 5.20 eV (238 nm). Consistently, Zawadski et al.3636 Zawadski, M.; Hreniak, D.; Wrzyszcz, J.; Mista, W.; Grabowska, H.; Malta, O. M.; Strek, W.; Chem. Phys.2003 , 291, 275. assigned the absorption band at 240 nm observed in mixed oxides Al2O3-ZrO2:Tb3+ to (4f8) → 7DJ (4f7 5d1). All these values agree with the band at 240 nm (5.17 eV) observed in γTA:Tb1%. Considering BOE:Tb1%, (Figure 4a) no strong absorption band could be observed, but rather the low energy tail of a stronger excitation truncated by inner filter effect. This would mean that the spin allowed band is shifted towards higher energies, as it is in the aquo ion [Tb(OH)2]8 where absorption maximum is at 5.71 eV (217 nm)3737 Carnall, W. T.; J. Chem. Phys.1968 , 49, 4412. supporting the existence of hydrated terbium ions in boehmite.
The second possible assignment is the host sensitization, which may be described according to the Förster-Dexter energy transfer. A spectral overlap of the host emission with the acceptor absorption is necessary. The visible blue photoluminescence of γ-Al2O3 nanoparticles has been investigated in detail by Yu et al.3838 Yu, Z. Q.; Li, C.; Zhang, N.; J. Lumin.2002 , 99, 29. An emission band peaking at 405 nm, associated with excitations at 236, 245, 255 nm has been observed for γ-Al2O3 calcined at 500 ºC, and assigned to electron-hole recombination at oxygen defects in the transition alumina. Indeed, the emission background observed for our samples may be assigned to that of the aluminate matrices. The broad emission from alumina overlaps the wavelengths of Tb3+ intra 4f8 absorption lines (7F6 → 5G6, 5D3, 5L10, 5L8, 5D2, 5L8, 5H7), so that γ-Al2O3 to acceptor Tb3+ energy transfer would occur favorably. Inversely, although a similar photoluminescene occurs for boehmite,3939 Yu, Z. Q.; Wang, C. X.; Gu, X. T.; Li, C.; J. Lumin.2004 , 106, 153.,4040 Bai1, X.; Caputo, G.; Hao, Z.; Freitas, V. T.; Zhang, J.; Longo, R. L.; Malta, O. L.; Ferreira, R. A. S.; Pinna, N.; Nat. Commun. 2014 , 5, 5702. there would be no boehmite to terbium energy transfer in BOE:Tb. This can be understood if the bonding of Tb3+ with the boehmite is weaker than it is with the transition alumina. So finally both mechanisms would easily explain our observations. An argument in favor of the second assignment that of host sensitization, is given by the consideration of Eu3+ in same hosts. The photoluminescence spectra from our preceding work2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605. are also shown in Figures 4c and 4d. At wavelengths below 300 nm, a broad absorption band was detected for γTA:Eu1% but not for BOE:Eu1%. This band was assigned to absorption in the localized LMCT state (ligand to metal charge transfer),2424 Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605. but it could also be due to non radiative energy transfer between the host and Eu3+, since the visible emission of aluminates matches the group of absorption lines 7F0 → 5D3, 5L6, 5L7, 5D4.
Also in line with terbium ions having a much more hydrated environment in boehmite than in transition alumina, the 5D4 emission lifetime was shortened due to the enhanced probability of non-radiative de-excitation by the OH vibrators. The 5D4 luminescence decay curves were fitted with first-order exponential functions and the decay time was found to be markedly lower in sample BOE:Tb1% (1.00 ± 0.10 ms) than in sample γTA:Tb1% (2.56 ± 0.26 ms). Ishizaka et al.4141 Ishizaka, T.; Nozaki, R.; Kurokawa, Y.; J. Phys. Chem. Solids2002 , 63, 613. have measured the 5D4 decay times in Tb3+ doped alumina films prepared by sol-gel method, then heat-treated to remove residual water molecules: our observations agree rather well with those reported on films after heat treatment at 25 ºC and at 500 ºC, respectively.
Photoluminescence spectra of the nanocomposites Asn:BOE:Tb1% and Asn:γTA:Tb1% are displayed in Figures 4e and 4f, respectively. Concerning PLE spectra, the main difference between Asn modified or unmodified aluminates were the intensities ratios between the band at 240 nm and 7F6 → 2S + 1LJ lines. The broad band appeared clearly in Asn:BOE:Tb1%, whereas it was absent in un-modified boehmite. This observation supports the assumption that host to terbium energy transfer is the cause of this excitation band: indeed, it may be understood that the amine acid strengthens the linkage of hydrated Tb ions with the boehmite surface and favors energy transfer.
Photoluminescence in aqueous medium
After the screening of various amine acid/aluminate/terbium ratios it was established that compositions Tb3+/BOE equal 1 or 5 percent and Asn/BOE equal 0.3/1 may be considered as luminescent labels in aqueous media since they remain well dispersed at the nanometric scale in solution. We have thus evaluated the possibility to record the Tb3+ luminescence with the measurement conditions usually employed in rare earth luminescent bio-assays: using proper excitation wavelength and optimized time-resolution conditions. Figure 5 shows the emission of Asn:BOE:Tb1% at concentration 0.16 mol L-1 (in mol of Al3+) under excitation at 250 or 351nm and with a signal detection fixed from 0.1 milliseconds to 0.5 milliseconds after the exciting pulse.
Time resolved photoluminescence of 0.3Asn:BOE:Tb1% 0.16 mol L-1 in water (a) excitation spectra monitored at λ emission = 543 nm and emission spectra by excitation at (b) 250 nm; (c) 351 nm. Delay time at 0.1 ms, sample window as 0.4 ms.
It is important to establish the lower concentration compatible with the unambiguous observation of time-resolved spectra. In that work, it was checked the luminescence emission up to 0.1 mol L-1 for hybrid in water medium, however the results let us to affirm at the 0.05 mol L-1 as the low limit concentration to time-resolution analysis. The water stability and luminescence behavior could enable the hybrid system to monitor wastewater treatment, e.g., labeling Escherichia coli.4242 Duarte, A. P.; Mauline, L.; Gressier, M.; Dexpert-Ghys, J.; Roques, C.; Caiut, J. M. A.; Deffune, E.; Maia, D. C. G.; Carlos, I. Z.; Ferreira, A. A. P.; Ribeiro, S. J. L.; Menu, M.-J.; Langmuir2013 , 29, 5878. Since, Escherichia coli and enterococci are used as regulatory tools to monitor water and presence of potential enteric pathogens yet their source (human or animal) cannot be determined with routine methods.4343 Srinivasan, S.; Aslan, A.; Xagoraraki, I.; Alocilja, E.; Rose, J. B.; Water Res.2001 , 45, 2561.
Conclusions
The spray pyrolysis (SP) of aluminium tri-sec-butoxide and TbCl3 in aqueous medium allowed synthesizing nano-structured powders having the crystalline phase of boehmite (noted BOE) or transition alumina γ-Al2O3 (noted γTA). Particles were spherical and sub-micrometric, consisting of aggregated nanometric sub-particles. The photoluminescence emission spectra of samples BOE:Tb1% and γTA:Tb1% exhibited the well-known green emission 5D4 → 7FJ = 6-3 of Tb3+. The strong near-UV absorption (around 240 nm) is assigned to the host to ion energy transfer. We associate its presence in γTA:Tb1% to the incorporation of Tb3+ in the defect-spinel structure of the γ-Al2O3 matrix. In BOE:Tb1%, in contrast, the energy transfer was very weak, confirming our earlier hypothesis that Ln3+ ions do not enter the aluminate network in boehmite, but are bonded to the hydroxyls groups at boehmite surface.
The surface of luminescent terbium doped boehmite particles was modified by asparagine Asn. The particles, in (water + Asn), disaggregated into nanoparticles (hydrodynamic diameter from 5 to 100 nm). Asn is localized at the surface of the particles and at the same time the linkage of hydrated terbium ions with boehmite was strengthened, enhancing the aluminate → Tb energy transfer. The goal to get modified aluminate nanoparticles with enhanced luminescence starting from the γTA:Tb1% was not achieved since it was not possible to disaggregate the sub-micronic spheres in (water + Asn). Besides, γ-Al2O3 hydrolysed partially into γ-AlOOH during the reaction with Asn. When homogeneous suspensions in aqueous solution of nanoparticles were achieved, the limit of detection in time-resolution conditions analysis was estimated to 0.05 mol L-1 AlOOH:Tb1%. Spray pyrolysis is a cheap, fast and reliable production process. It is well adapted to the synthesis of hydrated aluminate particles. Moreover aluminates particles may be doped with any luminescent lanthanide Ln leading to strong photoluminescence in the visible, with a millisecond lifetime appropriated for efficient time gated detection. With a highly reactive surface, terbium-doped boehmite nanoparticles may be easily modified and therefore fulfill several of the key features for their actual use for in vitro or in vivo applications.
-
FAPESP has sponsored the publication of this article.
Acknowledgments
Authors thank Y. Kihn (CEMES-CNRS) and C. Galaup (SPCMIB-Université de Toulouse) for their support in electron microscopy and time-resolved luminescence investigations, respectively. The Brazilian agencies FAPESP (2007/55723-6, 2011/21551-0), CNPq, CAPES, and the CAPES-COFECUB Brazil-France cooperation program for the grant to J. M. A. C. are also acknowledged.
References
-
1Penilla, E. H.; Kodera, Y.; Garay, J. E.; Adv. Funct. Mater 2013 , 23, 6036.
-
2Zhou, R. S.; Snyder, R. L.; Acta Crystallogr.1991 , B47, 617.
-
3Sohlberg, K.; Pennycook, S. J.; Pantelides, S. T.; J. Am. Chem. Soc.1999 , 121, 7493.
-
4Pecharromán, C.; Sobrados, I.; Iglesias, J. E.; González Carreño, T.; Sanz, J.; J. Phys. Chem. B1999 , 103, 6160.
-
5Krokidis, X.; Raybaud, P.; Gobichon, A.-E.; Rebours, B.; Euzen, P.; Toulhoat, H.; J. Phys. Chem. B2001 , 105, 5121.
-
6Paglia, G.; Rohl, A. L.; Buckley, C. E.; Gale, J. D.; Phys. Rev. B2005 , 71, 224115.
-
7Alvarez, L. J.; Leon, L. E.; Sanz, J. F.; Capitan, M. J.; Odriozola, J. A.; J. Phys. Chem.1995 , 99, 17872.
-
8Yoldas, B. E.; J. Mater. Sci.1975 , 10, 1856.
-
9Yoldas, B. E.; Ceramic Bulletin1975 , 54, 289.
-
10Granados-Correa, F.; Jiménez-Becerril, J.; J. Hazard. Mater.2009 , 162, 1178.
-
11Yoon, T. H.; Johnson, S. B.; Musgrave, C. B.; Brown Jr., G. E.; Geochim. Cosmochim. Acta2004 , 68, 4505.
-
12Landry, C. C.; Pappé, N.; Mason, M. R.; Apblett, A. W.; Tyler, A. N.; MacInnes, A. N.; Barron, A. R.; J. Mater. Chem.1995 , 5, 331.
-
13Kareiva, A.; Harlan, C. J.; MacQueen, D. B.; Cook, R. L.; Barron, A. R.; Chem. Mater.1996 , 8, 2331.
-
14Kang, H.-J.; Kim, D. J.; Park, S.-J.; Yoo, J.-B.; Ryu, Y. S.; Thin Solid Films2007 , 515, 5184.
-
15Kapoor, S.; Hegde, R.; Bhattacharyya, A. J.; J. Control. Release2009 , 140, 34.
-
16Al-Kattan, A.; Santran, V.; Dufour, P.; Dexpert-Ghys, J.; Drouet, C.; J. Biomater. Appl.2014 , 28, 697.
-
17Li, L.; Liu, Y.; Tao, J.; Zhang, M.; Pan, H.; Xu, X.; Tang, R.; J. Phys. Chem. C2008 , 112, 12219.
-
18Meiser, F.; Cortez, C.; Caruso, F.; Angew. Chem. Int. Ed.2004 , 43, 5954.
-
19Jin, D.; Connally, R.; Piper, J.; Cytom. Part A2007 , 71A, 783.
-
20Okada, K.; Tanaka, A.; Hayashi, S.; Otsuka, N.; J. Mater. Sci. Lett.1993 , 12, 854.
-
21Vallet-Regi, M.; Rodriguez-Lorenzo, L. M.; Ragel, C. V.; Salinas, A. J.; Gonzalez-Calbet, J. M.; Solid State Ionics1997 , 101-103, 197.
-
22Kato, T.; J. Ceram. Soc. Jpn.2002 , 110, 146.
-
23Caiut, J. M. A.; Dexpert-Ghys, J.; Kihn, Y.; Vérelst, M.; Dexpert, H.; Ribeiro, S. J. L.; Messaddeq, Y.; Powder Technol.2009 , 190, 95.
-
24Caiut, J. M. A.; Ribeiro, S. J. L.; Messaddeq, Y.; Dexpert-Ghys, J.; Verelst, M.; Dexpert, H.; Nanotechnology2007 , 18, 455605.
-
25Marques, R. F. C.; Caiut, J. M. A.; Paiva-Santos, C. O.; Ribeiro, S. J. L.; Messaddeq, Y.; Garcia, C.; Neumeyer, D.; Dexpert, H.; Verelst, M.; Dexpert-Ghys, J.; Braz. J. Phys.2009 , 39, 176.
-
26Caiut, J. M. A.; Bazin, L.; Mauricot, R.; Dexpert, H.; Ribeiro, S. J. L.; Dexpert-Ghys, J.; J. Non-Cryst. Solids2008 , 354, 4860.
-
27Fripiat, J. J.; Bosmans, H.; Rouxhet, P. G.; J. Phys. Chem.1967 , 71, 1097.
-
28Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J. B.; Berthet, P.; Huntz, A. M.; Roy, P.; Tétot, R.; J. Solid State Chem.2009 , 182, 1171.
-
29Wang, S.-L.; Johnston, C. T.; Bish, D. L.; White, J. L.; Hem, S. L.; J. Colloid Interface Sci.2003 , 260, 26.
-
30Cooper, S. J.; Langmuir2002 , 18, 3749.
-
31Alvarez, L. J.; Leon, L. E.; Sanz, J. F.; Capitan, M. J.; Odriozola, J. A.; J. Phys. Chem.1995 , 99, 17872.
-
32Monteiro, M. A. F.; Brito, H. F.; Felinto, M.; Brito, G. E. S.; Teotonio, E. E. S.; Vichi, F. M.; Stefani, R.; Micropor. Mesopor. Mat.2008 , 108, 237.
-
33Milligan, W. O.; McAtee, J. L.; J. Phys. Chem.1956 , 60, 273.
-
34Carnall, W. T.; Crosswhite, H.; Crosswhite, H. M.; Energy Level Structure and Transition Probabilities in the Spectra of the Trivalent Lanthanides in LaF3, 1st ed.; Argonne National Laboratory: Argonne, USA, 1977.
-
35Dorenbos, P.; J. Phys. Condens. Matter2003 , 15, 6249.
-
36Zawadski, M.; Hreniak, D.; Wrzyszcz, J.; Mista, W.; Grabowska, H.; Malta, O. M.; Strek, W.; Chem. Phys.2003 , 291, 275.
-
37Carnall, W. T.; J. Chem. Phys.1968 , 49, 4412.
-
38Yu, Z. Q.; Li, C.; Zhang, N.; J. Lumin.2002 , 99, 29.
-
39Yu, Z. Q.; Wang, C. X.; Gu, X. T.; Li, C.; J. Lumin.2004 , 106, 153.
-
40Bai1, X.; Caputo, G.; Hao, Z.; Freitas, V. T.; Zhang, J.; Longo, R. L.; Malta, O. L.; Ferreira, R. A. S.; Pinna, N.; Nat. Commun 2014 , 5, 5702.
-
41Ishizaka, T.; Nozaki, R.; Kurokawa, Y.; J. Phys. Chem. Solids2002 , 63, 613.
-
42Duarte, A. P.; Mauline, L.; Gressier, M.; Dexpert-Ghys, J.; Roques, C.; Caiut, J. M. A.; Deffune, E.; Maia, D. C. G.; Carlos, I. Z.; Ferreira, A. A. P.; Ribeiro, S. J. L.; Menu, M.-J.; Langmuir2013 , 29, 5878.
-
43Srinivasan, S.; Aslan, A.; Xagoraraki, I.; Alocilja, E.; Rose, J. B.; Water Res.2001 , 45, 2561.
Publication Dates
-
Publication in this collection
Nov 2015
History
-
Received
17 June 2015 -
Accepted
25 Aug 2015