Abstract
The present study evaluated the main factors that influence the photocatalytic activity of titanium dioxide (TiO2) films grown by metalorganic chemical vapor deposition (MOCVD) at 400 and 500 °C, in different growth times. The photocatalytic behavior was analyzed by measuring the methyl orange dye degradation at different pH values. Structural and morphological characteristics, and the recyclability of the catalysts for several cycles were also investigated. Anatase phase was identified in all films. The higher photodegradation performances were obtained at acidic pH. The results demonstrated that the photocatalyst thickness is an important parameter in heterogenous photocatalysis. The best photocatalytic result occurred for the 395 nm-thick TiO2 film grown at 400 °C, which presented 65.3% of the dye degradation under UV light. The recyclability experiments demonstrated that the TiO2 films grown by MOCVD present a great stability after several photocatalytic cycles, which allows their practical application for water treatment with high efficiency.
Keywords:
titanium dioxide; MOCVD; dye degradation; heterogenous photocatalysis; recyclability
Introduction
In recent decades, the scientific interest using semiconductor materials in oxidative processes for water treatment has increased exponentially.11 Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D.; J. Cleaner Prod. 2018, 175, 38. Among them, titanium dioxide (TiO2) has been employed for being chemically and biologically inert.22 Duminica, F. D.; Maury, F.; Senocq, F.; Surf. Coat. Technol. 2004, 188-189, 255. It shows high photostability, being able to efficiently catalyze reactions, and does not present risks to the environment or humans.33 Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A.; Appl. Surf. Sci. 2016, 387, 183.,44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11. The crystalline structure of the TiO2 greatly influences on its photocatalytic characteristics. The photocatalytic degradation properties are different depending on the crystalline structure of the catalyst, anatase, rutile and brookite,55 Yang, J.; Gao, M. Z.; Jiang, S. B.; Huo, X. J.; Xia, R.; Mater. Lett. 2018, 232, 171. since their properties vary according to the obtained polymorph. Amorphous TiO2 does not present photocatalytic activity. Some studies66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33.,77 Jia, C.; Zhang, X.; Yang, P.; Appl. Surf. Sci. 2018, 430, 457. report that the anatase phase is more efficient in the photocatalytic process, while others88 Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M.; J. Catal. 2001, 203, 82.,99 Fleaca, C. T.; Scarisoreanu, M.; Morjan, I.; Luculescu, C.; Niculescu, A. M.; Badoi, A.; Vasile, E.; Kovacs, G.; Appl. Surf. Sci. 2015, 336, 226. describe that small amounts of rutile phase mixed with anatase increase the photocatalytic efficiency of the catalyst.
Several problems arise from the practical use of TiO2 during the photocatalytic process, especially when a suspension is utilized. The separation of the catalyst from the suspensions is difficult. Suspended particles tend to aggregate, mainly in high concentrations. Thus, great efforts have been made to prepare a TiO2-supported catalyst, in order to improve the photocatalyst recovery, and to obtain a high catalytic performance.1010 Zhang, X.; Zhou, M.; Lei, L.; Mater. Chem. Phys. 2005, 91, 73. TiO2-supported films exhibit different structural and morphological properties depending on the technique for obtention and the substrate used. These films can be obtained by various methods, such as sol-gel,1111 Krysiak, O. A.; Barczuk, P. J.; Bienkowski, K.; Wojciechowski, T.; Augustynski, J.; Catal. Today 2019, 321-322, 52. physical vapor deposition (PVD),66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33. and chemical vapor deposition (CVD).44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.
Among the advantages of CVD, it can be emphasized the formation of continuous and adherent films with well-defined stoichiometry, and small structural defects. Metalorganic chemical vapor deposition technique (MOCVD), a specific area of CVD, is an attractive process for the growth of TiO2 films, since it provides improvements in uniformity, presents high growth rates, allows the coating of substrates with complex geometries, and increases the range of substrates used, because in this process lower growth temperatures are used comparing to conventional CVD.1212 Thompson, A. G.; Mater. Lett.
1997, 30, 255.
13 Bento, R. T.; Ferrus Filho, A.; Pillis, M. F.; Rev. Bras. Inov. Tecnol. Saúde
2017, 7, 4.-1414 Krumdieck, S.; Gorthy, R.; Gardecka, A. J.; Lee, D.; Miya, S. S.; Talwar, S. D.; Polson, M. I. J.; Bishop, C.; Surf. Coat. Technol.
2017, 326, 402.
MOCVD process also allows a good control of the films thickness during their growth, promoting the synthesis of homogeneous films, important attribute for the study of its photocatalytic properties. Gardecka et al.1515 Gardecka, A. J.; Bishop, C.; Lee, D.; Corby, S.; Parkin, I. P.; Kafizas, A.; Krumdieck, S.; Appl. Catal., B 2018, 224, 904. grew TiO2 films by MOCVD at 525 °C. The obtained films were nanostructured compounds of anatase-rutile TiO2. X-ray photoelectron spectroscopy (XPS) results demonstrated that the thicker films contained high levels of carbon, inhibiting the photoactivity of the catalysts. Duminica et al.1616 Duminica, F. D.; Maury, F.; Hausbrand, R.; Surf. Coat. Technol. 2007, 201, 9304. produced TiO2 films by MOCVD in the temperature range of 400-600 °C. The authors observed that the films grown at low temperature (< 420 °C) are only constituted of anatase phase and exhibited high photocatalytic activity under UV light. However, at 430-600 °C the rutile starts growing leading to a progressive decrease of the photocatalytic efficiency. Krumdieck et al.1717 Krumdieck, S.; Gorthy, R.; Land, J. G.; Gardecka, A. J.; Polson, M. I. J.; Boichot, R.; Bishop, C. M.; Kennedy, J. V.; Phys. Status Solidi A 2018, 215, 1700578. synthesized TiO2 films by MOCVD at 375, 450 and 525 °C. The authors analyzed the effects of increasing the growth temperature on the microstructure of films, and its photocatalytic efficiency in the degradation of the methylene blue dye. All films presented good adhesion and uniform appearance. The results showed that with the increase of the growth temperature there was a proportional increase of the TiO2 photocatalytic activity. Bento and Pillis44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11. evaluated the influence of the thickness on the photocatalytic efficiency of TiO2 films grown by MOCVD on the degradation of methyl orange dye under UV light. The results suggested the existence of an ideal thickness in which the catalyst exhibits better photocatalytic performance.
Besides the structural, physical-chemical and morphological characteristics of the catalyst, the photocatalytic behavior of the TiO2 films is also influenced by several experimental parameters, which include the pH of the reaction medium, the initial concentration and type of pollutant, the amount of photocatalyst, the specific surface area of the catalyst, the reactor design, the reaction temperature, the luminous intensity of the radiation source, and the irradiation time.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33.,1818 Reza, K. M.; Kurny, A. S. W.; Gulshan, F.; Appl. Water Sci. 2017, 7, 1569.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. It is essential to know these parameters to define the best operating conditions for the use of TiO2 films in environmental applications. Recent researches have reported the significance of these operational parameters. Shao et al.2020 Shao, C.; Zhou, G.; Li, Z.; Wu, Y.; Xu, D.; Sun, B.; Chem. Eng. J. 2013, 230, 227. suggest that acid pH is more favorable for photocatalytic applications than neutral or alkaline ones. Ahmadpour et al.2121 Ahmadpour, A.; Zare, M.; Behjoomanesh, M.; Avazpour, M.; Adv. Environ. Technol. 2015, 3, 121. investigated the degradation of methyl orange dye under UV radiation using TiO2/Fe3O4 nanocomposites obtained by ultrasonic-assisted deposition-precipitation method. The pH of the solution ranged from 2 to 10. The results indicated that the best dye degradation occurred in acid pH (2 and 4) after 60 min of irradiation, with near-complete mineralization of the dye. Shang et al.2222 Shang, J.; Li, W.; Zhu, Y.; J. Mol. Catal. A: Chem. 2003, 202, 187. studied the photodegradation of the formaldehyde compound and observed that the photocatalytic activity of the TiO2 films is related to the crystallinity and thickness of the films.
The present work evaluated the main factors that influence the kinetics and mechanisms of the photocatalytic efficiency of TiO2 films. The films were grown by MOCVD technique at 400 and 500 °C, with growth times ranging from 11 to 60 min. The photocatalytic behavior of the catalysts was analyzed on the methyl orange dye degradation at different pHs. Structural and morphological characteristics of the TiO2 films, and the recyclability of the photocatalysts for several cycles were also investigated.
Experimental
Substrates
Borosilicate glass slides (25 × 76 × 1 mm) were used as substrate for the photocatalytic experimental tests. Silicon wafers substrates were also used for convenience in order to determine the films thickness. The surface preparation for both substrates consisted in the immersion of the samples in a 5% H2SO4 aqueous solution for 3 min, followed by rinsing in deionized water and drying in nitrogen prior of being immediately inserted into the MOCVD reactor.
Growth of TiO2 films
TiO2 films were grown by MOCVD process in a conventional homemade reactor previously described by Bento and Pillis,44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11. at the temperatures of 400 and 500 °C, under a pressure of 50 mbar. At 400 °C the growth time varied between 15 and 60 min, and for 500 °C it ranged from 11 to 60 min. The optimized process parameters based on previous work1313 Bento, R. T.; Ferrus Filho, A.; Pillis, M. F.; Rev. Bras. Inov. Tecnol. Saúde 2017, 7, 4.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. carried out by the research group are given in Table 1. For the synthesis of the films, only titanium(IV) isopropoxide (TTiP) (purity 99.999%, Sigma-Aldrich Co., St. Louis, USA) was used as the titanium and oxygen sources. Nitrogen was used as the carrier gas to transport the TTiP into the system, and as the purge gas. The flow rates were both fixed at 0.5 slm. The growth of the TiO2 films starts after the working temperature is reached, with release of the precursor flow in the line.
Characterization techniques
X-ray diffraction (XRD) analyses were performed for phases determination. The analyses were carried out in a Phillips MPD 1880 X-ray diffractometer with a monochromatized Cu Ka radiation (λ = 1.54148 Å). The measurements were performed in the θ-2θ configuration, from 5 to 90°, and step rate of 0.02°. The phases formed were identified with the JCPDS (Joint Committee on Powder Diffraction Standards) database. Surface morphology and mean grain size of the films grown on borosilicate glass substrates were evaluated by field emission scanning electron microscopy (FE-SEM) in a FEI Quanta 600 equipment. Films thicknesses were measured on the cross-section of the films grown on silicon substrates.
Evaluation of TiO2 heterogeneous photocatalytic performance
The photocatalytic activities of the TiO2 films in the methyl orange dye degradation under UV light were investigated in a homemade reactor setup previously described by Bento and Pillis.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11. The photoreactor consists of a glass chamber containing 50 mL of the dye solution, the photocatalyst, and the UV light source. These components were arranged in a box to prevent loss of photons and to protect users against the emitted radiation. The distance between the photocatalyst and the UV light source was set at 100 mm. TiO2 films grown by MOCVD were used as the photocatalysts. Two tubular black light lamps (General Electric; 15 W each one; λ = 365 nm) were used as UV radiation source. Methyl orange dye was employed as the model pollutant, since it is a simple compound of the azo dyes, extensively used in the industry. It is estimated that around 15 to 20% of the azo dyes used in the textile industries are disposed of in the water systems after the dyeing processing,2323 Jin, X. C.; Liu, G. Q.; Xu, Z. H.; Tao, W. Y.; Appl. Microbiol. Biotechnol. 2007, 74, 239. which represents a growing environmental danger. The methyl orange dye degradation was studied at different solution pH values (2, 7 and 10), with a concentration of 0.005 g L−1. The pH was adjusted by the addition of an adequate amount of H2SO4 or NaOH. The experiments were performed for a total test time of 300 min, under constant stirring and room temperature. Prior to the tests the dye solution was kept under bubbling with the catalyst for one hour into the dark to allow the adsorption-desorption equilibrium of the solution on the catalyst surface. The photolysis evaluation was carried out under UV radiation, without the catalyst, in order to verify the occurrence of dye photodegradation only under exposure to the light. The photocatalytic efficiency of the TiO2 films was analyzed for all growth conditions presented in the Table 1. The dye concentration changes were constantly monitored every 30 min using a Varian Carry 1E UV-Vis spectrophotometer. After the measurement, the aliquots were returned to the dye solution. The degree of dye photodegradation (X) is given by Beer-Lambert law (equation 1), where C0 is the initial dye concentration, and C is the dye concentration at different irradiation times.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498.
In terms of the kinetic study, the apparent pseudo-first order rate constant (k) was obtained to estimate in which condition the highest degradation of the dye occurred, according to a Langmuir-Hinshelwood model.2424 McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J. D.; Morris, M. A.; J. Mol. Catal. A: Chem. 2015, 406, 51. The k values were calculated from the linear relationship between ln (C0 / C) and irradiation time.
Recyclability experiments
Recycle tests on photocatalytic degradation of methyl orange dye by TiO2 films were performed to examine the photocatalytic behavior under UV light in each cycle. After finishing a photocatalytic cycle, the films were rinsed individually for 10 min at room temperature using different reactivation methods: deionized water, ethyl alcohol or acetone, and then dried in nitrogen. The recyclability experiment was carried out for twelve cycles.
Results and Discussion
Microstructural characterization
Morphology and cross-section analyses
The photocatalytic behavior of a semiconductor can vary with the different morphological characteristics and impurities present on the catalyst surface.1818 Reza, K. M.; Kurny, A. S. W.; Gulshan, F.; Appl. Water Sci. 2017, 7, 1569.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. A high specific surface area may favor a greater formation of hydroxyl radicals (HO•), which results in the increase of the photocatalytic efficiency and regeneration time of the catalyst.66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33. The surface area of TiO2 films is related to the grain size. The growth temperature is an important parameter that influences the morphological characteristics of the TiO2 films obtained by MOCVD.2525 Antunes, R. A.; Oliveira, M. C. L.; Pillis, M. F.; Int. J. Electrochem. Sci. 2013, 8, 1487.
Figure 1 shows the surface and cross-section FE-SEM micrograph of the TiO2 films grown at 400 °C on Si(100). It is observed that there is a flat interface between film and substrate. The films grow perpendicular to the substrate surface, and present dense structure and uniform thickness. Film thickness increases proportionally with the growth time. The films grown for 15, 30 and 60 min presented mean thickness of 184, 395 and 762 nm, respectively. The growth rate was estimated by dividing the film thickness by the time of growth. The value found was of 13 nm min−1 for TiO2 films grown at 400 °C. The surface of all the catalysts is formed by grains of heterogeneous size. The mean grain size also increases with the growth time. Other studies44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,1616 Duminica, F. D.; Maury, F.; Hausbrand, R.; Surf. Coat. Technol. 2007, 201, 9304. showed a similar trend.
FE-SEM images of TiO2 films grown at 400 °C substrate by MOCVD for: 15 min (a) surface and (b) cross-section; 30 min (c) surface and (d) cross-section; and 60 min (e) surface and (f) cross-section. The surface images are related to the films grown on borosilicate and the cross-section images are from films grown on Si(100) substrates.
Figure 2 shows the surface and cross-section FE-SEM micrograph of the TiO2 films grown at 500 °C. The films show uniform thickness, well-defined columnar structure, and grow perpendicular to the substrate surface. Antunes et al.2525 Antunes, R. A.; Oliveira, M. C. L.; Pillis, M. F.; Int. J. Electrochem. Sci. 2013, 8, 1487. and Jung et al.2626 Jung, C. K.; Kang, B. Y.; Chae, H. Y.; Kim, Y. S.; Seo, M. K.; Kim, S. K.; Lee, S. B.; Boo, J. H.; Moon, Y. J.; Lee, J. Y.; J. Cryst. Growth 2002, 235, 450. also observed the formation of well-defined columnar structures at 500-750 °C. The growth rate of the films by the MOCVD technique in the studied temperature is linear. The mean growth rate for TiO2 films grown at 500 °C is 37 nm min−1. From the surface analyses, it can be observed the formation of grains of well-defined size for all of the growth times at 500 °C. The grain size increases linearly with the growth time and the film thickness. The results revealed that in the growth of TiO2 films at 400 and 500 °C the films thickness increases linearly with the deposition time, demonstrating a constant growth rate, which allows a good reproducibility of the deposition process.22 Duminica, F. D.; Maury, F.; Senocq, F.; Surf. Coat. Technol. 2004, 188-189, 255.,44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. Table 2 exhibits the morphological characteristics of the TiO2 films grown at 400 and 500 °C.
FE-SEM images of TiO2 films grown at 500 °C by MOCVD for: 15 min (a) surface and (b) cross-section; 30 min (c) surface and (d) crosssection; and 60 min (e) surface and (f) cross-section. The surface images are related to the films grown on borosilicate and the cross-section images are from films grown on Si(100) substrates.
Summary of the morphological characteristics of the TiO2 films grown by MOCVD at 400 and 500 °C for different growth times
Phase structures
The crystallinity and phases formed in the TiO2 films grown on borosilicate substrates at 400 and 500 °C by the MOCVD process were investigated by XRD, as shown in Figure 3. The results suggested that the films present good crystallinity. All the peaks are in great agreement with the standard spectra (JCPDS No. 21-1272). From XRD pattern it can be seen that the films are formed only by anatase crystalline phase.1515 Gardecka, A. J.; Bishop, C.; Lee, D.; Corby, S.; Parkin, I. P.; Kafizas, A.; Krumdieck, S.; Appl. Catal., B 2018, 224, 904.,2727 Hanaor, D. A. H.; Sorrell, C. C.; J. Mater. Sci. 2011, 46, 855. No evidences of rutile or brookite phases were found. The films grown at 400 °C exhibit the most intense peak at 2θ = 47.8°, which corresponds to [200] of anatase-TiO2.1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. The films grown at 500 °C present a crystallographic preferential orientation in [112] at 2θ = 38.1°.2525 Antunes, R. A.; Oliveira, M. C. L.; Pillis, M. F.; Int. J. Electrochem. Sci. 2013, 8, 1487. The intensity of the peaks increases with the films thickness. Khalifa et al.2828 Khalifa, Z. S.; Lin, H.; Shah, S. I.; Thin Solid Films 2010, 518, 5457. obtained similar results. Lee et al.2929 Lee, H. Y.; Park, Y. H.; Ko, K. H.; Langmuir 2000, 16, 7289. observed that at 400 °C different crystalline plans appear in relation to these present at 500 °C, behavior also showed in the present study.
XRD patterns of the TiO2 films grown at 400 °C on borosilicate substrates by MOCVD: (a) 184; (b) 395; (c) 762 nm; and at 500 °C: (d) 600; (e) 1100; (f) 2100 nm.
Photocatalytic performance of the methyl orange dye
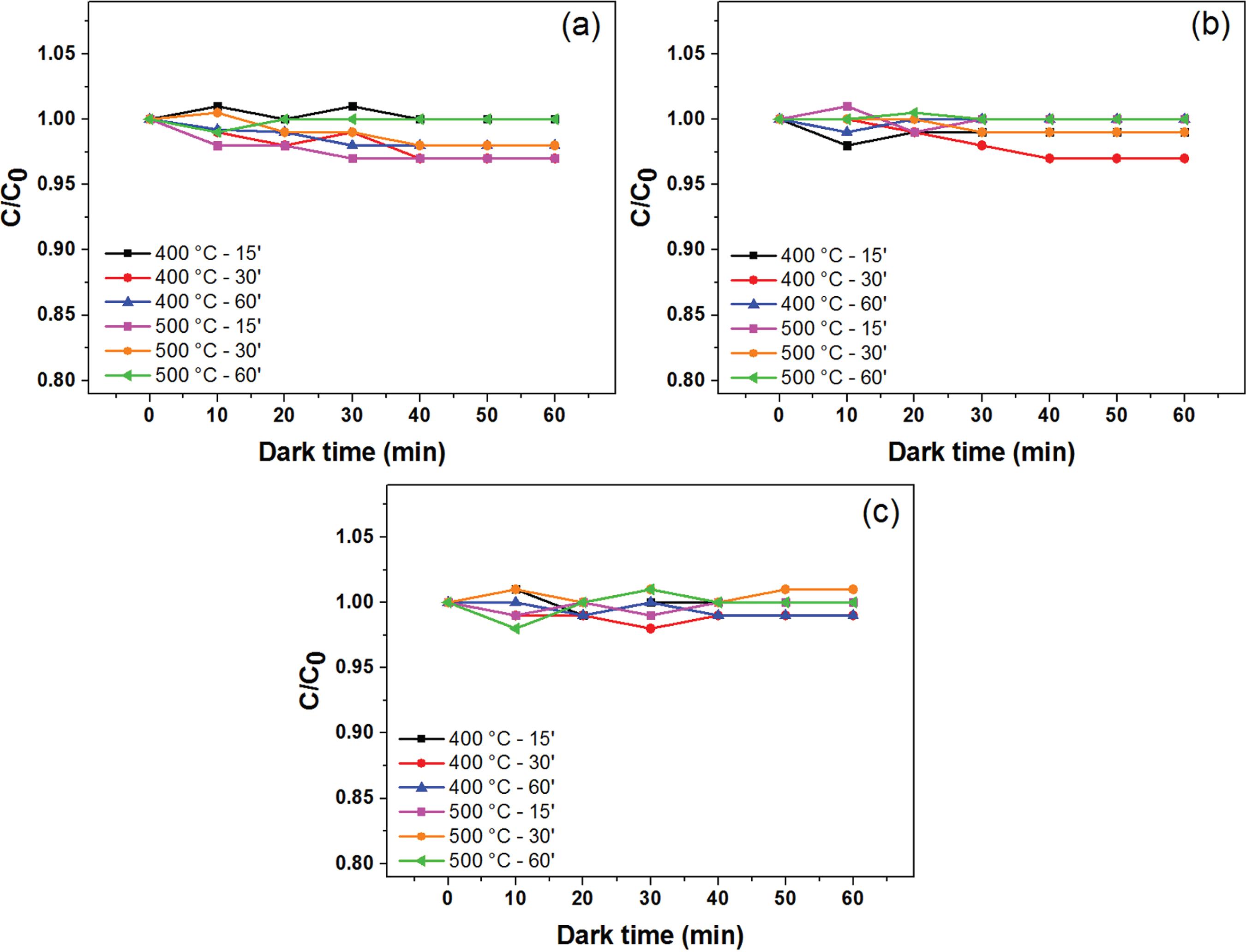
Before inducing the photocatalytic reactions with light, it is essential for the organic pollutants-model to get adsorbed on the catalyst surface in the dark. More adsorption on the catalyst improves the dye degradation. Therefore, the solution was allowed into the dark for 60 min to achieve the equilibrium between desorption of dye molecules and ion adsorption on the TiO2 film, at different pH values. As shown in Figure 4, the adsorption-desorption saturation was reached after approximately 40 min for all conditions.
Adsorption-desorption equilibrium curves of the dye solution on the TiO2 films surface. The films were grown at 400 and 500 °C for 15, 30 and 60 min: (a) pH = 2; (b) pH = 7; and (c) pH = 10.
Effect of pH
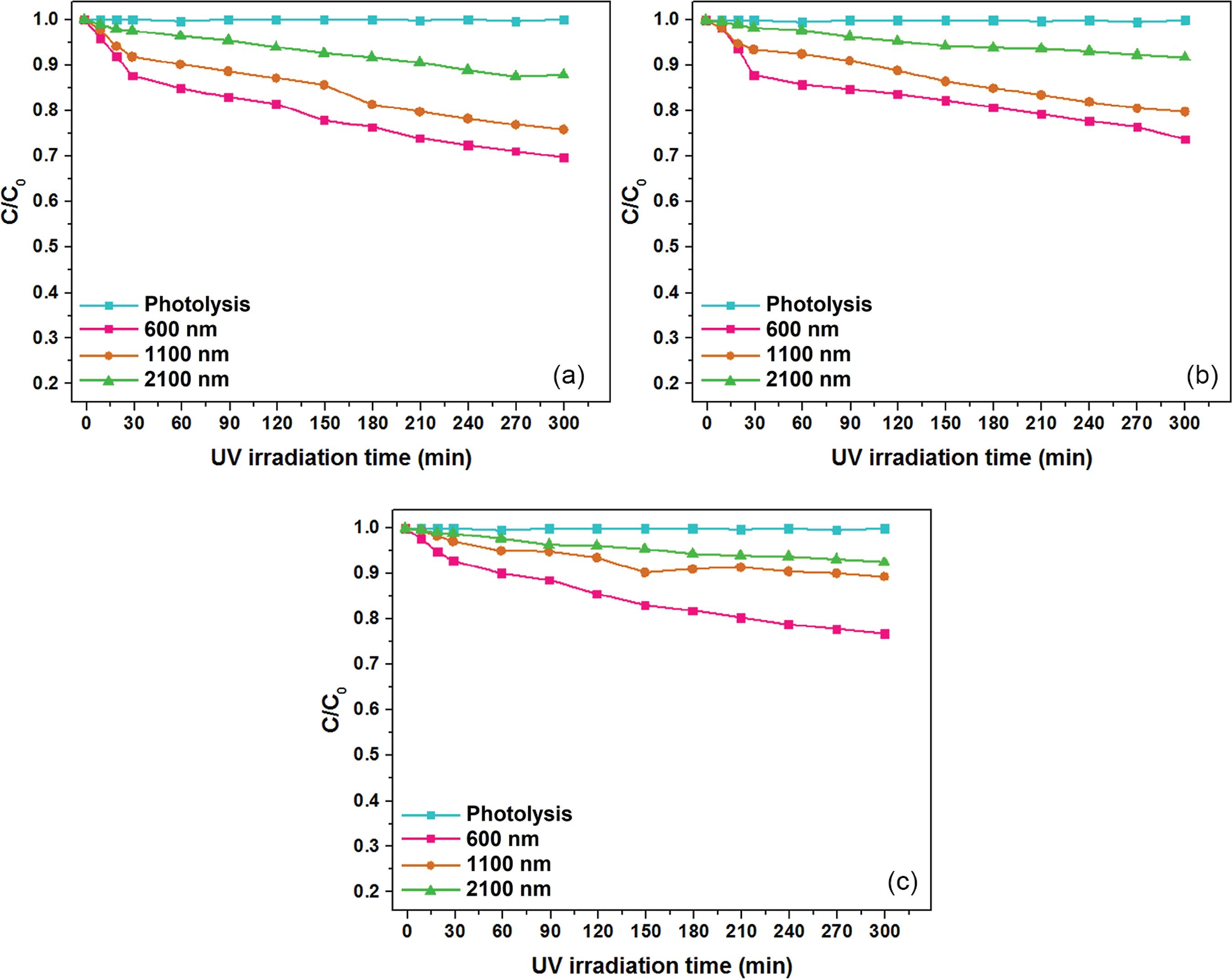
Figure 5 shows the photocatalytic activity of the TiO2 films grown on borosilicate substrates by MOCVD at 400 °C with 184, 395 and 762 nm-thick. The photodegradation efficiency of the catalysts was evaluated by the methyl orange dye degradation under UV light for 300 min in different pH solutions. The C/C0 graph exhibits the dye degradation as a function of the time of exposition to the light source, with and without the presence of TiO2 films. The photolysis curves indicated that without the presence of the photocatalysts there was no methyl orange dye degradation under UV radiation. Previous studies44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,3030 Guettai, N.; Amar, H. A.; Desalination 2005, 185, 427. confirm that the degradation of the methyl orange dye occurs only in the presence of TiO2. It is observed that, among the pH values used, the best result occurred for the TiO2 film with 395 nm-thick at pH = 2, with 65.3% of dye degradation. The films with 184 and 762 nm-thick showed less efficiency on the dye degradation in all the pHs range. At pH = 2, for all the growth times, the photocatalytic efficiency of TiO2 films on the methyl orange dye degradation was slightly better than that observed for pH = 7. For pH = 10 the films presented minor efficiency, which suggests that lower pH dye solution results in higher efficiencies.
Methyl orange dye degradation as a function of the time of exposure to UV light with and without the presence of the 184, 395 and 762 nm-thick TiO2 films grown at 400 °C: (a) pH = 2; (b) pH = 7; and (c) pH = 10.
Figure 6 exhibits the photocatalytic behavior of the TiO2 films grown at 500 °C with 600, 1100 and 2100 nm-thick, on the methyl orange dye degradation under UV light for 300 min at pH dye solutions of 2, 7 and 10. The TiO2 film with 600 nm-thick presented the best photocatalytic performance, showing 30.2% of the dye degradation at pH = 2. The results show a similar trend to that obtained by the TiO2 films grown at 400 °C for all of the thicknesses (Table 3). However, for the films grown at 500 °C the photocatalytic activity decreases as the film thickness increases. Guettai and Amar3030 Guettai, N.; Amar, H. A.; Desalination 2005, 185, 427. observed that the photodegradation is more intense at acidic pH than at alkaline pH, since the adsorption of the methyl orange dye to the photocatalyst surface is better at pH < 4. Dai et al.3131 Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H.; Chemosphere 2007, 69, 1361. and Niu3232 Niu, P.; Asian J. Chem. 2013, 25, 1103. obtained the same effect of pH on the methyl orange dye degradation using TiO2 as catalyst.
Methyl orange dye degradation as a function of the exposure time to UV light with and without the presence of the 600, 1100, 2100 nm-thick TiO2 films grown at 500 °C: (a) pH = 2; (b) pH = 7; and (c) pH = 10.
Photocatalytic activity of the TiO2 films grown on borosilicate substrates by MOCVD at 400 and 500 °C with different thicknesses and under different pH values. The photocatalytic experiments concern to the methyl orange dye degradation under UV light for 300 min
The pH is an important parameter to be considered in the processes of azo dyes photodegradation. It influences the dye adsorption on the TiO2 surface, and it depends on the electrostatic force between the dye and the catalyst, among other factors. For the methyl orange dye, an anionic dye, a high negative charge density in the molecule occurs when it is dissolved in water.3030 Guettai, N.; Amar, H. A.; Desalination 2005, 185, 427. The ionization state of the TiO2 surface can be protonated or deprotonated, respectively, under acidic or alkaline pH,3333 Qamar, M.; Muneer, M.; Desalination 2009, 249, 535. according to the following reactions:
At pH > 6, strong adsorption of the dye on the TiO2 surface is observed, result of electrostatic attraction. Under alkaline dye solution, the methyl orange molecules are negatively charged, which did not favor this process since the competitive adsorption by hydroxyl groups and the dye molecule suffer Coulombic repulsion.3434 Mozia, S.; Morawski, A. W.; Toyoda, M.; Inagaki, M.; Desalination 2009, 241, 97. Under higher pH solution, the HO• radicals are rapidly absorbed, having no opportunity to react with the dye. In this way, anionic dyes, such as methyl orange dye, suffer better photodegradation in acidic pH.3030 Guettai, N.; Amar, H. A.; Desalination 2005, 185, 427.
Effect of the film thickness
According to several studies,44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,2222 Shang, J.; Li, W.; Zhu, Y.; J. Mol. Catal. A: Chem. 2003, 202, 187.,3535 Feltrin, J.; Sartor, M. N.; de Noni Jr., A.; Bernardin, A. M.; Hotza, D.; Labrincha, J. A.; Ceramica 2013, 49, 620. the photocatalytic activity of the TiO2 films improves with increasing film thickness until a limit, from which the degradation reaction remains almost constant or decreases. This limit corresponds to the increase in film opacity, limiting the diffraction of the light through the catalyst and the consequent diffusion of charge carriers.3636 Jung, S. C.; Kim, S. J.; Imaishi, N.; Cho, Y. I.; Appl. Catal., B 2005, 55, 253. However, thinner films present a higher recombination rate of the photogenerated charges, due to the difficulty of transferring the electron (e−)/hole (h+) pair, which reduces its photocatalytic performance.3737 Malagutti, A. R.; Mourão, H. A. J. L.; Garbin, J. R.; Ribeiro, C.; Appl. Catal., B 2009, 90, 205. In this way, the study of the influence of the TiO2 film thickness on the methyl orange dye degradation was developed, in order to ensure an efficient photocatalytic activity. The best behavior was reached for the 395 nm-thick film (30 min). Thus, in order to evaluate the performance of films with thicknesses close to that, 260 and 520 nm-thick TiO2 films were grown, which allowed to analyze the behavior of films with thicknesses near to the 395 nm-thick synthesized film. Figure 7 shows the photocatalytic behavior of the TiO2 films grown on borosilicate substrates by MOCVD at 400 °C. The photocatalytic experimental tests for these films were carried out at pH = 2, which is the best observed condition. The results suggest an ideal thickness value of TiO2 films for photocatalytic applications. The best result was maintained for the film grown for 30 min, with a thickness of 395 nm, which presented 65.3% of methyl orange dye degradation under UV light for 300 min. The other films presented lower photocatalytic efficiency in the same test period, under the same conditions. From the values obtained, the photocatalytic rate constant (k) of the TiO2 films were calculated, as shown in Table 4. Increasing the k values an increase in the degradation rate of the dye was observed.
Influence of TiO2 film thickness on the methyl orange dye degradation under UV light for 300 min at pH = 2. The films were grown on borosilicate substrates by MOCVD process at 400 °C.
Evaluation of the photocatalytic performance of the TiO2 films grown on borosilicate substrates by MOCVD at 400 °C for different growth times. The photocatalytic experiments were observed on the methyl orange dye degradation at pH = 2, under UV light for 300 min
The results demonstrated that there is a TiO2 film thickness range where the best photocatalytic behavior occurs and, consequently, the highest percentage of dye degradation. The studies developed by Duminica et al.1616 Duminica, F. D.; Maury, F.; Hausbrand, R.; Surf. Coat. Technol. 2007, 201, 9304. and Wu et al.3838 Wu, C. Y.; Lee, Y. L.; Lo, Y. S.; Lin, C. J.; Wu, C. H.; Appl. Surf. Sci. 2013, 280, 737. exhibited similar results with 300 nm thick films approximately. Carp et al.66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33. suggest that there is an optimum thickness for the photocatalytic activity of TiO2 films. When the film is very thin, only a small portion of incident light is absorbed by the catalyst.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11. The increase in thickness is favorable for the photocatalytic performance, since thinner films have a higher electron recombination rate than thicker films. Nevertheless, increased film thickness interferes in the electron mobility, due to the increased depth of light penetration required for the photons to activate the electrons of the semiconductor, and consequently to form the HO• radicals.3939 Horáková, M.; Klementová, S.; Kříž, P.; Balakrishna, S. K.; Špatenka, P.; Golovko, O.; Hájková, P.; Exnar, P.; Surf. Coat. Technol. 2014, 241, 154. Moreover, in very thick and dense films the majority of electron (e−)/hole (h+) pairs are generated inside the catalyst, not reaching the surface,66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33.,1616 Duminica, F. D.; Maury, F.; Hausbrand, R.; Surf. Coat. Technol. 2007, 201, 9304.,3737 Malagutti, A. R.; Mourão, H. A. J. L.; Garbin, J. R.; Ribeiro, C.; Appl. Catal., B 2009, 90, 205. as schematized in Figure 8. In both cases the films present low photocatalytic activity, which suggests a minimum and a maximum limiting thickness where the photocatalytic activity is greater.
Schematic representation of cross-sections of the TiO2 films with different thickness illustrating the photocatalytic behavior on the methyl orange dye degradation under UV light when the film thickness increases. The diagram also demonstrates the electron-hole recombination in this condition, and the mechanism of semiconductor heterogeneous photocatalysis.
The mean grain size is also an important parameter in photocatalytic applications, which influences the behavior of TiO2 films on water treatment. The smaller grain size promotes the increase of the superficial area and, consequently, the photocatalytic efficiency of the films.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,1919 Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498. Tian et al.4040 Tian, G.; Fu, H.; Jing, L.; Tian, C.; J. Hazard. Mater. 2009, 161, 1122. studied the photocatalytic activity of TiO2 on the methyl blue dye and phenol degradation under UV light. The results showed that higher crystallinity, larger superficial area and smaller grain size result in higher photocatalytic activity of TiO2. In our work, the films presented mean grain size values between 160 and 300 nm (Table 2). The grain size increases with the film thickness. The results suggest that, for TiO2 films grown by MOCVD process, there is the existence of an ideal grain size, and an optimum thickness value; the latter being the principal variable for films employed on advanced oxidation processes.
Effect of growth temperature
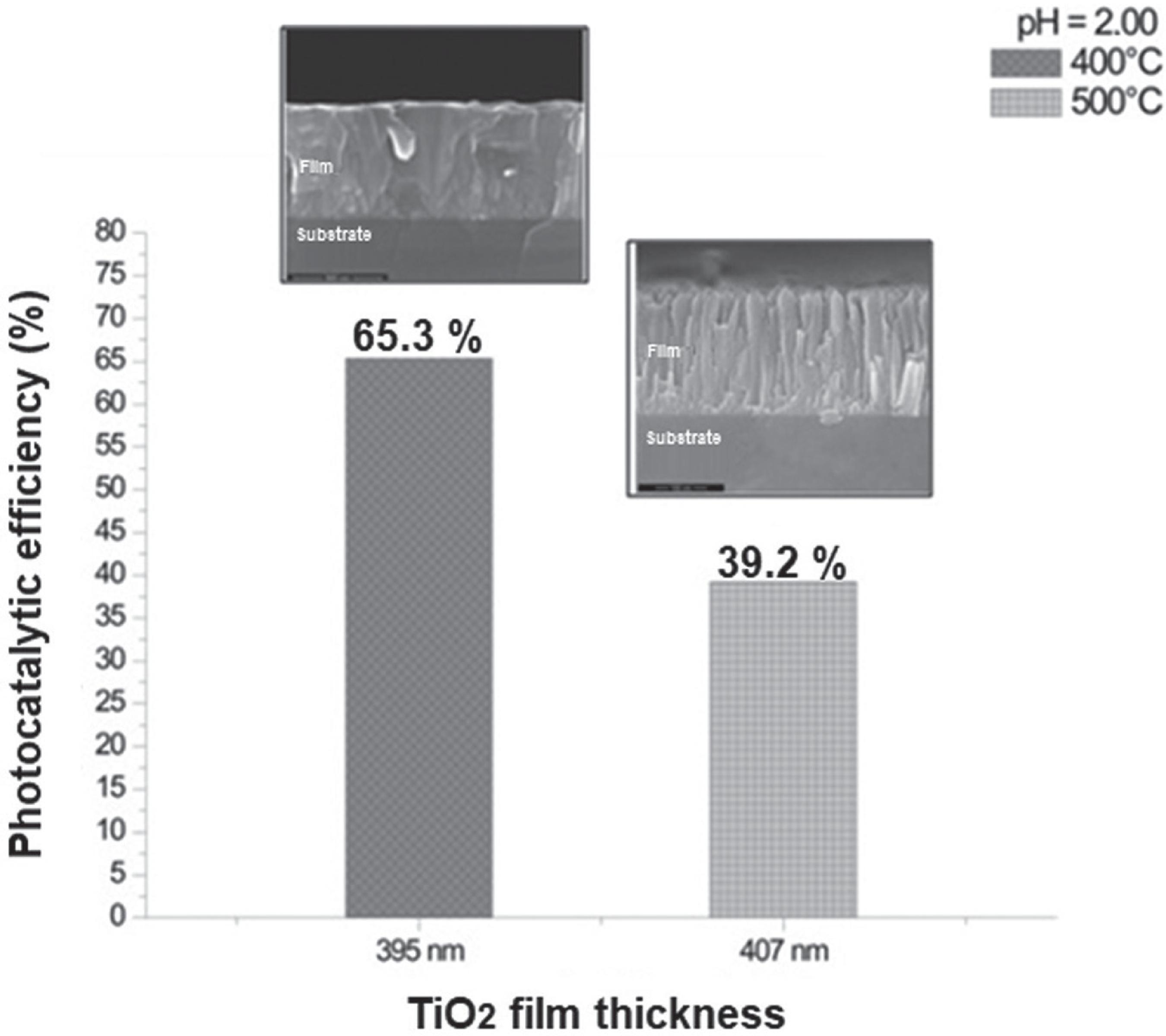
To evaluate the influence of growth temperature on photocatalytic activity of the catalysts on the methyl orange dye degradation, the TiO2 films with thickness of around 400 nm were grown by MOCVD at 400 and 500 °C. Previously, the 395 nm-thick film obtained at 400 °C exhibited the best photocatalytic performance, and its thickness was used as a parameter for the growth at 500 °C. Figure 9 shows the photocatalytic behavior of the films under UV light for 300 min at pH dye solution of 2. The catalyst grown at 400 °C presented a photocatalytic activity 66.5% more efficient than the TiO2 film grown at 500 °C, as shown in Table 5. The results demonstrate that the structure is an important parameter to obtain the better methyl orange dye degradation. TiO2 films grown at 400 °C tend to present better photocatalytic activity than those grown at 500 °C.
Influence of growth temperature of the TiO2 films on the methyl orange dye degradation under UV light for 300 min at pH = 2. The films were grown on borosilicate substrates by MOCVD process at 400 °C for 30 min, and 500 °C for 11 min, which resulted in catalysts with thickness of 395 and 407 nm, respectively.
Summary of the photocatalytic activity results for the TiO2 films grown on borosilicate substrates by MOCVD at 400 and 500 °C with 395 and 407 nm-thick, respectively
Based on structural calculations, the facets {101}, {111}, {100} and {001} present the lower formation energy.4141 Cho, C. H.; Han, M. H.; Kim, D. H.; Kim, D. K.; Mater. Chem. Phys. 2005, 92, 104. According to the literature,4242 Min-Kyo, L.; Young-Chun, P.; Thin Solid Films 2017, 638, 9.,4343 Lun, P.; Xiangwen, Z.; Li, W.; Ji-Jun, Z.; Mater. Lett. 2015, 160, 576. some exposed facets are more reactive than others. The photocatalytic properties of anatase-TiO2 depend on the crystallinity, shape, size, and exposed facets.66 Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33. Usually, the TiO2 nanocrystals facets have specific physical and chemical properties, such as adsorption and photocatalytic reactivity.4444 Jiang, J.; Zhao, K.; Xiao, X. Y.; Zhang, L. Z.; J. Am. Chem. Soc. 2012, 134, 4473. Besides that, the facets could facilitate the electron (e-)/hole (h+) separation. X-ray diffraction pattern shows the enhanced (200) peak (see Figure 3) which indicates that the TiO2 structures are dominant in {100} facets at 400 °C, and a peak at (112) for the growth temperature of 500 °C. Previous studies4545 Zhang, D. Q.; Li, G. S.; Yang, X. F.; Yu, J. C.; Chem. Commun. 2009, 4381.,4646 Min, S.; Shijie, X.; Xinglong, W.; Chengyu, H.; Tinghui, L.; Paul, K. C.; Adv. Mater. 2013, 25, 2035. showed that anatase-TiO2 catalysts with {001}, {101}, or {100} exposed facets exhibit enhanced photocatalytic activity, similar behavior obtained in the present work.
The photocatalytic mechanisms of the TiO2 films on the methyl orange dye photodegradation under UV light can be explained as follows: electrons (e−) in the valence band (VB) of TiO2 are photo-excited (3.2 eV for anatase-TiO2) to its conduction band (CB), which leads to the formation of electronic holes (h+), as shown in equation 4.1818 Reza, K. M.; Kurny, A. S. W.; Gulshan, F.; Appl. Water Sci. 2017, 7, 1569.,4747 Chiou, C. H.; Wu, C. Y.; Juang, R. S.; Chem. Eng. J. 2008, 139, 322. The electronically excited semiconductor reacts with the adsorbed water, the hydroxyl ions, and the oxygen. The electrons (e−) can reduce ions from Ti4+ to Ti3+. The water and hydroxyl ions are oxidized to HO• radicals by the holes (h+) in the VB and the Ti4+ sites present on the catalyst surface (equation 5). The oxygen behaves like an electronic trap for the electrons.4848 Zhao, X.; Zhao, Q.; Yu, J.; Liu, B.; J. Non-Cryst. Solids 2008, 354, 1424. Since the irradiation time is prolonged, the dissolved oxygen molecules can capture the electron (e−) on the TiO2 surface to generate reactive superoxide radical anions, as shown in equation 6. The formation of hydrogen peroxide (H2O2) can occur by the adsorption of the molecular oxygen in the Ti3+ sites, according to equation 7, and subsequent reduction of H2O2 (equation 8). The HO• and O2• radicals can oxidize the adsorbed dye compounds on the TiO2 surface (equation 9), which occurs only in the presence of light and oxygen, which can lead to complete mineralization of the contaminant.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,2727 Hanaor, D. A. H.; Sorrell, C. C.; J. Mater. Sci. 2011, 46, 855.
There is a competition between the recombination of the electron (e−)/hole (h+) pairs and the transfer of charges in the catalyst/dye solution interface. The first inhibits the photocatalytic activity of the TiO2, and the second will continue the photodegradation process. At this point, the oxygen performs an important function as electron acceptor, decreasing the electronic recombination, and as responsible for continuing the reactions initiated by the holes (h+) and the HO• radicals, reacting with the products of the primary reactions and leading to the formation of CO2 and H2O.44 Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.,1818 Reza, K. M.; Kurny, A. S. W.; Gulshan, F.; Appl. Water Sci. 2017, 7, 1569.,2727 Hanaor, D. A. H.; Sorrell, C. C.; J. Mater. Sci. 2011, 46, 855.
Durability and reactivation of TiO2 films
The durability and recyclability of the photocatalytic materials are important requirements for practical applications of heterogeneous photocatalysis in water treatment. Therefore, TiO2 films grown by MOCVD at 400 °C were subjected to methyl orange dye degradation for 120 min and retrieved by individually applying different reactivation methods: deionized water, ethyl alcohol or acetone for up to five cycles (Figure 10). The reactivation method using ethyl alcohol presented the most stable photocatalytic behavior, without showing any significant loss of efficiency under UV light. Nevertheless, the methods using deionized water and acetone were not as attractive. After five photocatalytic cycles, the efficiency of the film rinsed with deionized water decreased from 40.1 to 24.3%, while the cleaning method with acetone reduced the photodegradation efficiency to 8% at the same test condition. Lin et al.4949 Lin, L.; Wang, H.; Luo, H.; Xu, P.; J. Photochem. Photobiol., A 2015, 307-308, 88. used ethanol in the reactivation of Fe-TiO2 films to perform the durability experiments. The authors obtained satisfactory results after 28 catalytic cycles, and they proposed that ethanol, besides removing the dye adsorbed on the catalyst surface, could eliminate residues derived from the synthesis process, which results in increased surface area and adsorption sites. Xu et al.5050 Xu, J.; Ao, Y.; Fu, D.; Yuan, C.; Appl. Surf. Sci. 2008, 255, 2365. performed recyclability tests on TiO2 films to evaluate their photocatalytic behavior for six cycles. The catalysts were rinsed with deionized water at end of each cycle. The results indicated a reduction of 15% in the photocatalytic activity of the films, a similar trend to that obtained in the present work.
Recyclability of photocatalytic degradation of the methyl orange in the presence of the TiO2 films under UV light for 120 min at pH = 2. The films were grown on borosilicate substrates by MOCVD process at 400 °C for 30 min. The photocatalytic reactivation of the TiO2 films was performed under three different methods: deionized water, ethyl alcohol or acetone for up to five cycles.
Figure 11 shows the durability experiments of photocatalytic reaction of original and ten photodegradation cycles of TiO2 film grown by MOCVD at 400 °C by using the ethanol reactivation method. The experiments were performed on the methyl orange dye degradation under UV light for 300 min at pH = 2. The efficiency of the film was 65.3% until the seventh cycle. The photocatalytic activity slightly dropped in the eighth and ninth cycle, in which the photodegradation efficiencies were of 64.8 and 63.6%. In the tenth cycle, a photocatalytic activity of 62.9% was obtained. The results for the recycled TiO2 films indicated a high possibility of reuse of the photocatalyst. The TiO2 films grown by MOCVD exhibited a great stability after several photocatalytic cycles, which suggests that these catalysts have a high potential to be used in the practical applications of heterogenous photocatalysis, and for the removal of dye contaminants in real wastewater treatment with high efficiency.
Ten cycles of photocatalytic reaction of the TiO2 films grown by MOCVD at 400 °C under UV light for 300 min.
Conclusions
In this work, TiO2 catalysts were grown at 400 and 500 °C by MOCVD process. This method was efficient for the growth of TiO2 films, using only TTiP as precursor as both titanium and oxygen. The thickness of the films as well as the mean grain size increase linearly with the growth time. The films obtained in both temperatures exhibited high crystallinity, and anatase-TiO2 phase was the only crystalline phase identified. The films obtained at 400 °C showed a dense columnar morphology, and a crystalline preferential orientation at [200]. The films grown at 500 °C presented a well-defined columnar structure, and crystalline preferential orientation at [112]. The methyl orange dye was used as pollutant-model to evaluate the performance of the catalysts under UV light. Increasing the thickness of the films grown at 500 °C, there is a decrease in the photocatalytic activity. The results suggested the existence of a thickness range in which the catalyst presents the highest dye photodegradation performance. The best photocatalytic result occurred for the 395 nm-thick TiO2-film grown at 400 °C which presented 65.3% of methyl orange dye degradation after 300 min under UV light exposition. The photocatalytic efficiency of the catalysts increases for acidic pH. This behavior is independent of the structural and morphological characteristics of the films. The recyclability of the catalysts was evaluated. After five photocatalytic cycles, the TiO2 film rinsed with ethyl alcohol presented the most stable photocatalytic behavior. The TiO2 films grown by MOCVD are promising for the application as photocatalysts for water treatment by a green method, and can be used in the organic pollutants degradation, such as azo compounds, widely used in the textile industry.
Acknowledgments
This work was supported by the Brazilian agencies CAPES, CNPq (process 168935/2018-0), and FAPESP (process 05/55861-4).
References
-
1Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D.; J. Cleaner Prod. 2018, 175, 38.
-
2Duminica, F. D.; Maury, F.; Senocq, F.; Surf. Coat. Technol. 2004, 188-189, 255.
-
3Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A.; Appl. Surf. Sci. 2016, 387, 183.
-
4Bento, R. T.; Pillis, M. F. In Titanium Dioxide - Material for a Sustainable Environment; Yang, D., ed.; InTech: London, England, 2018, ch. 11.
-
5Yang, J.; Gao, M. Z.; Jiang, S. B.; Huo, X. J.; Xia, R.; Mater. Lett. 2018, 232, 171.
-
6Carp, O.; Huisman, C. L.; Reller, A.; Solid State Chem. 2004, 32, 33.
-
7Jia, C.; Zhang, X.; Yang, P.; Appl. Surf. Sci. 2018, 430, 457.
-
8Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M.; J. Catal. 2001, 203, 82.
-
9Fleaca, C. T.; Scarisoreanu, M.; Morjan, I.; Luculescu, C.; Niculescu, A. M.; Badoi, A.; Vasile, E.; Kovacs, G.; Appl. Surf. Sci. 2015, 336, 226.
-
10Zhang, X.; Zhou, M.; Lei, L.; Mater. Chem. Phys. 2005, 91, 73.
-
11Krysiak, O. A.; Barczuk, P. J.; Bienkowski, K.; Wojciechowski, T.; Augustynski, J.; Catal. Today 2019, 321-322, 52.
-
12Thompson, A. G.; Mater. Lett. 1997, 30, 255.
-
13Bento, R. T.; Ferrus Filho, A.; Pillis, M. F.; Rev. Bras. Inov. Tecnol. Saúde 2017, 7, 4.
-
14Krumdieck, S.; Gorthy, R.; Gardecka, A. J.; Lee, D.; Miya, S. S.; Talwar, S. D.; Polson, M. I. J.; Bishop, C.; Surf. Coat. Technol. 2017, 326, 402.
-
15Gardecka, A. J.; Bishop, C.; Lee, D.; Corby, S.; Parkin, I. P.; Kafizas, A.; Krumdieck, S.; Appl. Catal., B 2018, 224, 904.
-
16Duminica, F. D.; Maury, F.; Hausbrand, R.; Surf. Coat. Technol. 2007, 201, 9304.
-
17Krumdieck, S.; Gorthy, R.; Land, J. G.; Gardecka, A. J.; Polson, M. I. J.; Boichot, R.; Bishop, C. M.; Kennedy, J. V.; Phys. Status Solidi A 2018, 215, 1700578.
-
18Reza, K. M.; Kurny, A. S. W.; Gulshan, F.; Appl. Water Sci. 2017, 7, 1569.
-
19Bento, R. T.; Correa, O. V.; Pillis, M. F.; J. Eur. Ceram. Soc. 2019, 39, 3498.
-
20Shao, C.; Zhou, G.; Li, Z.; Wu, Y.; Xu, D.; Sun, B.; Chem. Eng. J. 2013, 230, 227.
-
21Ahmadpour, A.; Zare, M.; Behjoomanesh, M.; Avazpour, M.; Adv. Environ. Technol. 2015, 3, 121.
-
22Shang, J.; Li, W.; Zhu, Y.; J. Mol. Catal. A: Chem. 2003, 202, 187.
-
23Jin, X. C.; Liu, G. Q.; Xu, Z. H.; Tao, W. Y.; Appl. Microbiol. Biotechnol. 2007, 74, 239.
-
24McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J. D.; Morris, M. A.; J. Mol. Catal. A: Chem. 2015, 406, 51.
-
25Antunes, R. A.; Oliveira, M. C. L.; Pillis, M. F.; Int. J. Electrochem. Sci. 2013, 8, 1487.
-
26Jung, C. K.; Kang, B. Y.; Chae, H. Y.; Kim, Y. S.; Seo, M. K.; Kim, S. K.; Lee, S. B.; Boo, J. H.; Moon, Y. J.; Lee, J. Y.; J. Cryst. Growth 2002, 235, 450.
-
27Hanaor, D. A. H.; Sorrell, C. C.; J. Mater. Sci. 2011, 46, 855.
-
28Khalifa, Z. S.; Lin, H.; Shah, S. I.; Thin Solid Films 2010, 518, 5457.
-
29Lee, H. Y.; Park, Y. H.; Ko, K. H.; Langmuir 2000, 16, 7289.
-
30Guettai, N.; Amar, H. A.; Desalination 2005, 185, 427.
-
31Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H.; Chemosphere 2007, 69, 1361.
-
32Niu, P.; Asian J. Chem. 2013, 25, 1103.
-
33Qamar, M.; Muneer, M.; Desalination 2009, 249, 535.
-
34Mozia, S.; Morawski, A. W.; Toyoda, M.; Inagaki, M.; Desalination 2009, 241, 97.
-
35Feltrin, J.; Sartor, M. N.; de Noni Jr., A.; Bernardin, A. M.; Hotza, D.; Labrincha, J. A.; Ceramica 2013, 49, 620.
-
36Jung, S. C.; Kim, S. J.; Imaishi, N.; Cho, Y. I.; Appl. Catal., B 2005, 55, 253.
-
37Malagutti, A. R.; Mourão, H. A. J. L.; Garbin, J. R.; Ribeiro, C.; Appl. Catal., B 2009, 90, 205.
-
38Wu, C. Y.; Lee, Y. L.; Lo, Y. S.; Lin, C. J.; Wu, C. H.; Appl. Surf. Sci. 2013, 280, 737.
-
39Horáková, M.; Klementová, S.; Kříž, P.; Balakrishna, S. K.; Špatenka, P.; Golovko, O.; Hájková, P.; Exnar, P.; Surf. Coat. Technol. 2014, 241, 154.
-
40Tian, G.; Fu, H.; Jing, L.; Tian, C.; J. Hazard. Mater. 2009, 161, 1122.
-
41Cho, C. H.; Han, M. H.; Kim, D. H.; Kim, D. K.; Mater. Chem. Phys. 2005, 92, 104.
-
42Min-Kyo, L.; Young-Chun, P.; Thin Solid Films 2017, 638, 9.
-
43Lun, P.; Xiangwen, Z.; Li, W.; Ji-Jun, Z.; Mater. Lett. 2015, 160, 576.
-
44Jiang, J.; Zhao, K.; Xiao, X. Y.; Zhang, L. Z.; J. Am. Chem. Soc. 2012, 134, 4473.
-
45Zhang, D. Q.; Li, G. S.; Yang, X. F.; Yu, J. C.; Chem. Commun. 2009, 4381.
-
46Min, S.; Shijie, X.; Xinglong, W.; Chengyu, H.; Tinghui, L.; Paul, K. C.; Adv. Mater. 2013, 25, 2035.
-
47Chiou, C. H.; Wu, C. Y.; Juang, R. S.; Chem. Eng. J. 2008, 139, 322.
-
48Zhao, X.; Zhao, Q.; Yu, J.; Liu, B.; J. Non-Cryst. Solids 2008, 354, 1424.
-
49Lin, L.; Wang, H.; Luo, H.; Xu, P.; J. Photochem. Photobiol., A 2015, 307-308, 88.
-
50Xu, J.; Ao, Y.; Fu, D.; Yuan, C.; Appl. Surf. Sci. 2008, 255, 2365.
Publication Dates
-
Publication in this collection
08 June 2020 -
Date of issue
June 2020
History
-
Received
14 Sept 2019 -
Accepted
28 Jan 2020