Abstracts
OBJECTIVES: The present study was designed to identify the effect of positive end expiratory pressure (PEEP) and the ideal pulmonary tidal volume to ventilate animals with a surgically produced bronchopleural fistula, aiming to reduce fistula output without affecting gas exchange. METHODS: Hemodynamic and respiratory assessment of gas exchange was obtained in five, healthy, young, mechanically ventilated Large White pigs under volume controlled ventilation with FiO2 of 0.4 and an inspiration:expiration ratio of 1:2, keeping respiratory rate at 22 cpm. A bronchopleural fistula was produced by resection of the lingula. Underwater seal drainage was installed and the thorax was hermetically closed. Gas exchange and fistula output were measured with the animals ventilated sequentially with tidal volumes of 4 ml/kg, 7 ml/kg and 10 ml/Kg alternating zero of positive end expiratory pressure (ZEEP) and PEEP of 10 cmH2O, always in the same order. RESULTS: These findings are attributed to reduced alveolar ventilation and ventilation/perfusion abnormalities and were attenuated with larger tidal volumes. PEEP increases air leak, even with low volume (of 2.0 ± 2.8mL to 31 ± 20.7mL; p= 0.006) and decreases alveolar ventilation in all tidal volumes. Alveolar ventilation improved with larger tidal volumes, but increased fistula output (10 mL/kg - 25.8 ± 18.3mL to 80.2 ± 43.9mL; p=0.0010). Low tidal volumes result in hypercapnia (ZEEP - Toneloto MGC, Terzi RGG, Silva WA, Moraes AC, Moreira MM 83.7± 6.9 mmHg and with PEEP 10 - 93 ± 10.1mmHg) and severely decreased arterial oxygen saturation, about of 84%. CONCLUSIONS: The tidal volume of 7 ml/Kg with ZEEP was considered the best tidal volume because, despite moderate hypercapnia, arterial oxygen saturation is sustained around 90%, alveolar ventilation improves and the fistula output is reduced when compared with a tidal volume of 10ml/Kg. A low tidal volume results in hypercapnia and severe desaturation. Finally, at any tidal volume, PEEP increases the fistula leak and decreases alveolar ventilation.
Positive-pressure respiration; Bronchial fistula; Respiratory tract fistula; Respiration, artificial; Tidal volume; Hypercapnia; Swine
OBJETIVO: O presente estudo foi desenhado para identificar o efeito da pressão expiratória final positiva (PEEP) e o volume corrente pulmonar ideal para ventilar animais com fístula broncopleural produzida cirurgicamente, com o intuito de reduzir a vazão da fístula sem afetar a troca gasosa. MÉTODOS: Avaliação hemodinâmica e respiratória da troca gasosa foi obtida em cinco porcos jovens, saudáveis, da linhagem Large White, ventilados mecanicamente no modo ventilatório volume controlado com FiO2 de 0.4 e relação inspiração:expiração em torno de 1:2, com freqüência respiratória mantida em 22 cpm. A fístula broncopleural foi produzida pela ressecção da língula. Um sistema de drenagem a selo d'água foi instalado e o tórax foi hermeticamente fechado. A troca gasosa e o débito da fístula broncopleural foram medidos com animais ventilados sequencialmente com volumes correntes de 4 ml/kg, 7 ml/kg e 10 ml/Kg alternando zero de pressão expiratória final positiva (ZEEP) e PEEP de 10 cmH2O, sempre na mesma ordem. RESULTADOS: Esses dados são atribuídos à ventilação alveolar reduzida e às anormalidades da ventilação/perfusão que foram atenuadas com volumes correntes mais altos. PEEP aumentou o vazamento de ar pela fístula, mesmo com baixos volumes, de 2.0 ± 2,8mL para 31 ± 20,7mL (p= 0,006) e diminuiu a ventilação alveolar em todos os volumes correntes. A ventilação alveolar melhorou com altos volumes correntes, mas aumentou o débito da fístula (4 ml/kg - 2,0 ± 2,8mL e 10 mL/kg - 80,2 ± 43,9mL; p=0,001). Baixos volumes correntes resultaram em hipercapnia (ZEEP - 83,7± 6,9 mmHg e com PEEP 10 -93 ± 10,1mmHg) e diminuição significativa da saturação de oxigênio arterial, em torno de 84%. CONCLUSÃO: O volume corrente de 7 ml/kg com ZEEP foi considerado o melhor volume corrente, visto que, apesar da hipercapnia moderada, a saturação de oxigênio arterial é sustentada em torno de 90%. A ventilação alveolar melhora e o débito da fístula é reduzido quando comparado ao volume corrente de 10ml/Kg. Um baixo volume resulta em hipercapnia e grave dessaturação. Finalmente, em qualquer volume corrente, PEEP aumenta o débito da fístula e diminui a ventilação alveolar.
Respiração com pressão positiva; Fístula bronquial; Fístula do trato respiratório; Respiração artificial; Volume de ventilação pulmonar; Hipercapnia; Suínos
ORIGINAL ARTICLE
Effects of different tidal volumes and positive end expiratory pressure on gas exchange in experimental bronchopleural fistula
Maria Gabriela Cavicchia TonelotoI; Renato Giuseppe Giovanni TerziII; William Adalberto SilvaIII; Ana Cristina de MoraesIII; Marcos Mello MoreiraI
IPost-Graduate Student from the Faculdade de Ciências Médicas, Universidade Estadual de Campinas - UNICAMP, Campinas (SP), Brazil
IIFull Professor from the Department of Surgery from the Faculdade de Ciências Médicas, Universidade Estadual de Campinas - UNICAMP, Campinas (SP), Brazil
IIIBiologist from the Faculdade de Ciências Médicas, Universidade Estadual de Campinas - UNICAMP, Campinas (SP), Brazil
Address for correspondence
ABSTRACT
OBJECTIVES: The present study was designed to identify the effect of positive end-expiratory pressure (PEEP) and the ideal pulmonary tidal volume to ventilate animals with a surgically produced bronchopleural fistula, aiming to reduce fistula output without affecting gas exchange.
METHODS: Hemodynamic and respiratory assessment of gas exchange was obtained in five, healthy, young, mechanically ventilated Large White pigs under volume controlled ventilation with FiO2 of 0.4 and an inspiration:expiration ratio of 1:2, keeping respiratory rate at 22 cpm. A bronchopleural fistula was produced by resection of the lingula. Underwater seal drainage was installed and the thorax was hermetically closed. Gas exchange and fistula output were measured with the animals ventilated sequentially with tidal volumes of 4 ml/kg, 7 ml/kg and 10 ml/Kg alternating zero of positive end expiratory pressure (PEEP)-(ZEEP) and PEEP of 10 cmH2O, always in the same order.
RESULTS: These findings are attributed to reduced alveolar ventilation and ventilation/perfusion abnormalities and were attenuated with larger tidal volumes. PEEP increases air leak, even with low volume (of 2.0 ± 2.8mL to 31 ± 20.7mL; p= 0.006) and decreases alveolar ventilation in all tidal volumes. Alveolar ventilation improved with larger tidal volumes, but increased fistula output (10 mL/kg -25.8 ± 18.3mL to 80.2 ± 43.9mL; p=0.0010). Low tidal volumes result in hypercapnia (ZEEP -83.7± 6.9 mmHg and with PEEP 10 -93 ± 10.1mmHg) and severely decreased arterial oxygen saturation, about of 84%.
CONCLUSIONS: The tidal volume of 7 ml/Kg with ZEEP was considered the best tidal volume because, despite moderate hypercapnia, arterial oxygen saturation is sustained around 90%, alveolar ventilation improves and the fistula output is reduced when compared with a tidal volume of 10ml/Kg. A low tidal volume results in hypercapnia and severe desaturation. Finally, at any tidal volume, PEEP increases the fistula leak and decreases alveolar ventilation.
Keywords: Positive-pressure respiration; Bronchial fistula; Respiratory tract fistula; Respiration, artificial; Tidal volume; Hypercapnia; Swine
INTRODUCTION
Bronchopleural fistula (BPF) is a serious complication with a high rate of hospital morbidity and mortality, especially when associated with mechanical ventilation (MV). Persistent air leakage occurring after thoracic drainage for pneumothorax is the early clinical sign.1 Diagnosis is confirmed when an active air leak is observed for more than 24 hours.2 The etiology of BPF is multifactorial: pneumonia, radiotherapy, tumors, tuberculosis, pulmonary resection and thoracic trauma.
In intensive care units (ICU), it is usually associated with barotrauma and prolonged mechanical ventilation.3 BPF also occurs in patients with acute respiratory distress syndrome (ARDS), associated with poor prognosis due to incomplete lung expansion, the need of positive end expiratory pressure (PEEP) and inability to remove CO2. However, the true frequency of this type of complication seems to be low and its magnitude as well as clinical impact remains uncertain.4
Regardless of the cause, when BPF is associated with MV with significant air leakage, morbidity and mortality rates are high.5
The distribution of pulmonary ventilation, the ventilation/perfusion ratio and the arterial blood gases may alter significantly in critical patients with BPF. When these patients cannot be treated surgically, mechanical ventilation has to be adjusted in order to achieve optimal gas exchange. This intervention is imposed when profound hypoxemia and hypercapnia are observed in high flow BPF - a condition in which the reduction of the volume of gas through the fistula is considered an important part of therapy.6 It has been shown that respiratory acidosis and mortality rate are directly related to the magnitude of the air leak, particularly when over 50% of the minute volume.1
Despite several proposals of innovative measures for reducing the air leak, the literature is limited to case reports, revealing the need for more comprehensive and controlled studies.4 With regard to strategic ventilation in BPF, mechanical ventilation does not follow the medical technological progress of recent years due to the fact that primitive methods such as drainage system pressurization are still used along with the more sophisticated methods as high frequency ventilation (HFV).
The literature is very consistent in not recommending PEEP when ventilating patients with BPF. High PEEP levels to optimize blood oxygenation may perpetuate air leak through BPF.1 However, should be imperative the use of PEEP, it has been suggested to associated it with low tidal volumes in order to reduce air leak.1,7 To reduce the air leak and to permit the closure of the BPF, low tidal volumes, low peak pressures, low respiratory rates and low PEEP levels have been advocated.7-10
Independent lung ventilation (ILV) and HFV have currently been more frequently cited in the literature. However, in clinical practice, very few hospitals are able to provide these resources. Therefore, mechanical ventilation in BPF has been performed with low mean pressure in the airways independently of the ventilator mode, accepting hypercapnia in an indiscriminate and random manner.
Since hypercapnia is, partly, a consequence of the fistula itself, a choice of the ideal tidal volume and ventilator mode becomes even more difficult. Moreover, besides the adverse effects attributed to hypercapnia11,12 the administration of sodium bicarbonate and curarization may prolong the use of mechanical ventilation and delay possible fistula closure.
Hence, a strategy that would reduce BPF leakage without, or with tolerable, hypercapnia aimed to improve alveolar ventilation and reduce air leakage is desirable. It should be expected enhancement of BPF closure without the inherent risks of hypoventilation. To date, no systematic assessment of the effect of zero of PEEP (ZEEP) and PEEP at different tidal volumes on gas exchange in BPF has been reported.
The purpose of this study was to detect the ideal tidal volume and evaluate the effect of PEEP in order reduce BPF leakage to acceptable levels with the least interference on pulmonary gas exchange. This work is part of a more comprehensive study that includes the assessment of an inspiratory occlusion valve and its association with PEEP, which should be the object of future publications.
METHODS
Five young Large White male pigs with a mean weight of 25 kg underwent anesthesia (fentanyl, ketamine and thiopental) endotracheal intubation (6F) and were mechanically ventilated with a BIRD 8400 respirator with a tidal volume sufficient to maintain end tidal carbon dioxide partial pressure (PETCO2) around 45 mmHg. A Swan-Ganz catheter was inserted for hemodynamic assessment and a multiparametric respiratory monitoring system (CO2SMOPlus® - Novametrix-Respironics-Dixtal) was installed. An arterial line was placed to record blood pressure and collect blood for blood gases (IL-1640®, Instrumentation Laboratory) and hemo-oximetry (OSM-3® Radiometer) calibrated for swine blood. Whenever needed, curare (pancuronium) was administered in order to avoid respiratory efforts.
After left thoracotomy, a BPF was induced by the surgical resection of the lingula at the level required expose a bronchial stump with a mean diameter of 4mm. Subsequently, the thorax was drained (28F) and hermetically closed.
In the original completed work, the comparison between ventilatory modes, volume and controled pressure was made and since there was no statistically significant difference between them, we chose to describe the present results using the volume controlled ventilation (VCV). This mode was used with 40% FiO2, a variable squared flow and inspiration:expiration ratio approximately 1:2 maintaining the respiratory rate at 22 cpm. After thoracotomy, the animals were ventilated with tidal volumes of 4 mL/kg, 7 mL/kg and 10 mL/kg, always in the same order, initially with ZEEP and then with PEEP of 10 cmH2O; effects on pulmonary gas exchange and fistula output were observed.
Each sequential step was called treatment. The transition from one treatment to the other occurred every five minutes. The PEEP of 10 cmH2O was chosen in order to highlight its effect, because PEEP of 5 cmH2O is considered physiological.
Fistula output was calculated as the difference between the inspired and the expired volumes as recorded by the respiratory monitoring system (COSMO Plus ® Novametrix-Respironics-Dixtal). Alveolar oxygen partial pressure (PAO2) was calculated for the three studied tidal volumes employing the alveolar air equation:13 PAO2 = (Pb-47) * FiO2 - PaCO2/0.8 where: FiO2 = 0.4 and Pb = 700 mmHg (in Campinas, SP, 693 m altitude), (FiO2 - oxygen inspired fraction, PaCO2 arterial carbon dioxide partial pressure, Pb, barometric pressure).
A descriptive analysis evaluated the position and dispersion of the variables. The variance analysis (ANOVA) with three repeated factors (PEEP and ZEEP/ ventilation mode - pressure and controlled volume/ tidal volume - 4, 7 e 10 mL/kg) was applied to explain the response variability between treatments with repeated measures. Due to the size of the sample and variability of the answers or measures, a ranking transformation was performed. The significance level adopted was 5% (p < 0.05).14
RESULTS
Table 1 presents the mean and standard deviation of each variable recorded before thoracotomy and after the induction of the BPF at each tidal volume with ZEEP and with PEEP.
Bronchopleural fistula leak increases with larger tidal volume at ZEEP. When PEEP is applied the air leak increases significantly even with small tidal volumes (Figure 1), reaching an air loss that affects alveolar ventilation (Figure 2). However, PaCO2 does not change at higher tidal volumes when PEEP is applied (Figure 3).
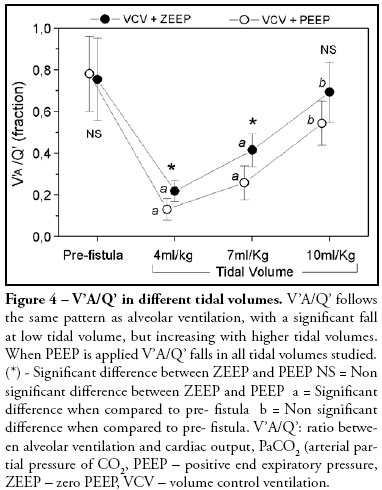
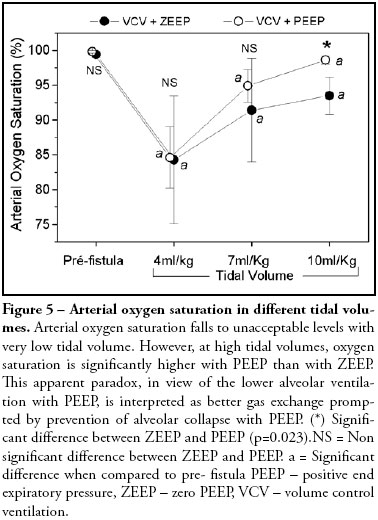
V'A/Q' (ratio between alveolar ventilation and cardiac output) follows the same pattern as alveolar ventilation, with a significant fall at low tidal volume, but increasing with higher tidal volumes. When PEEP is applied V'A/Q' falls in all tidal volumes studied (Figure 4). Arterial oxygen saturation falls to unacceptable levels with very low tidal volume. However, at higher tidal volumes, oxygen saturation is significantly higher with PEEP than with ZEEP (Figure 5). Cardiac output and oxygen delivery were not affected by changing tidal volumes up to 10 mL/kg associated or not with the application of PEEP of 10 cmH2O.
DISCUSSION
Regardless of the cause, when a BPF of significant air leakage is associated with mechanical ventilation it evolves with significant morbidity and mortality 5. The ideal strategy in BPF should be to conform and adjust mechanical ventilation to support adequate gas exchange without interference with hemodynamics in order to reduce air leakage, permitting fistula closure. We can reach this with the tidal volume of 7mL/kg with ZEEP despite moderate hypercapnia. Adequate arterial oxygen saturation is obtained, alveolar ventilation improves and the fistula output is reduced. When mechanical ventilation with conventional parameters - tidal volume, respiratory rate, inspiratory time and PEEP - is applied the air flow through the fistula is maintained, affecting gas exchange and impeding fistula closure.
In order to significantly control air leakage in the low frequency controlled mode, the intermittent mandatory ventilation (IMV) mode has been proposed to allow spontaneous respiration 6. The superiority of the IMV over totally controlled ventilation was reported by Powner and Grenvik 6, suggesting that the use of low tidal volumes, low respiratory rate, low PEEP and low inspiratory time lead to decreased BPF flow 6,15,16. However, this strategy may worsen pulmonary ventilator distribution, retain CO2 and result in hypoxemia. In this study, controlled ventilation mode was used to avoid the occurrence of spontaneous respiration, a factor that may interfere in data analysis.
In clinical practice, VCV has been used more frequently, but if intra-thoracic pressures increase due to reduced compliance of the respiratory system, fistula closure may be jeopardized. In our study, significant statistical difference were not found in any variables between the two ventilatory modes used, volume and controlled pressure, therefore, we use the results from VCV in a aleatory form. Authors have observed that in ARDS associated BPF, the use of pressure controlled ventilation requires the pressure adjustment to assure the preset volume15,16. On the other hand, Denis et al. observed that the use of high peak pressure to maintain good lung expansion and open new pulmonary units do not significantly increase air leakage through the fistula 17. Our observation is that high tidal volumes increase air leakage and PEEP critically amplifies this effect (Figure 1) concomitantly reducing alveolar ventilation (Figure 2). Controlled comparative data between pressure and volume controlled modes related to BPF output are not available in the literature. This study revealed that the greater the volume applied and, consequently, higher the intrathoracic pressure, greater is the fistula output. Nevertheless, it should be emphasized that these animals had normal lungs without any previous or acute pulmonary impairment.
Although there is a consensus in the literature that during conventional ventilation, permissive hypercapnia should be established to reduce BPF, this intervention is not free from adverse pulmonary, cardiovascular and neurological effects, besides being highly related to morbidity and mortality 18. In the present study, pre-fistula PaCO2 of 48.3 ± 3.5 mmHg increased significantly at low tidal volume (4 mL/kg) and remained elevated up to 10ml/Kg. No major difference was noted in PaCO2 between ZEEP and PEEP (Figure 3) as was noted for fistula volume (Figure 1) and alveolar ventilation (Figure 2). The expected hypercapnia due to reduced alveolar ventilation prompted by PEEP (Figure 2) did not occur. The probable explanation is that, despite reduction of the alveolar ventilation, CO2 escapes through the bronchopleural fistula, as has been demonstrated by Bishop et al. 2.
Arterial oxygen saturation fell from 99.4% before the fistula to 84.3% after induction of the BPF with the tidal volume of 4 mL/kg and to 91.4% and 93.5% with tidal volumes of 7 mL/kg and 10 mL/kg respectively, all with statistical significant difference when compared with the pre-fistula value. With the tidal volume of 10 mL/kg, alveolar ventilation per breath improved significantly with the addition of PEEP (Figure 4). The low oxygen saturation could be attributed to the increase in PCO2 in the alveolar air.
Calculated PAO2 was always higher than 150 mmHg at all tidal volumes, suggesting that the expected saturation should approach 100%. The increased alveolar to arterial PO2 gradient indicates that arterial hypoxemia cannot be explained only by hypoventilation. A factor that could explain the arterial hypoxemia is pulmonary vasoconstriction due to hypercabia as previously observed 12. A second factor is the occurrence of collapsed alveoli due to low tidal volumes. As a matter of fact, with higher tidal volumes, arterial oxygen saturation improves with PEEP when compared with ZEEP, nevertheless without statistical significant difference (Figure 4) despite reduction of alveolar ventilation (Figure 2). This apparent paradox, is interpreted as a better gas exchange prompted by prevention of alveolar collapse with PEEP.
It was also observed that venous oxygen saturation (SvO2) fell from 75% to 65% in the three tidal volumes studied. The low venous oxygen saturation could be attributed to a higher oxygen uptake due to decreased tissue perfusion. However, cardiac output did not fall after BPF and oxygen delivery did not exhibit statistical significant difference among the three volumes used, which remained relatively constant after induction of the BPF.
Alveolar ventilation in every breath fell effectively from 167ml before the fistula to 50 ml with a tidal volume of 4 mL/kg and to 107 and 159 with tidal volumes of 7 mL/kg and 10 mL/kg respectively. Alveolar ventilation per breath worsened with the addition of PEEP in all tidal volumes (Figure 2) and difference in alveolar ventilation was statistically significant among the three tidal volumes. The relation of the minute alveolar ventilation to cardiac output results in the V'A/Q' ratio. This ratio fell from approximately 0.70 to 0.22 with the tidal volume of 4ml/Kg and to 0.41 and 0.69 with tidal volumes of 7 mL/kg and 10 mL/kg respectively. VÁ/Q' ratio worsened with the addition of PEEP in all tidal volumes (Figure 5).
One limitation of this study may be associated to our healthy animal model. As most BPF in the ICU setting occur in ARDS, respiratory compliance and resistance may differ, so that clinical application of our data should be cautious. A second limitation is the sequence of the different treatments applied, always in the same order of 4 mL/kg, 7 mL/kg e 10 mL/kg, with ZEEP and then with PEEP. It could be argued that the history of one could interfere in the following treatment. However, it would have been impossible to randomize specific treatment because many parameters were to be changed and recorded within a limited time.
CONCLUSIONS
The tidal volume of 7 mL/kg with ZEEP was considered the best tidal volume because, despite moderate hypercapnia, arterial oxygen saturation is sustained around 90%, alveolar ventilation improves and the fistula output is reduced when compared with a tidal volume of 10 mL/ kg. A low tidal volume results in hypercapnia and severe arterial oxygen desaturation. Finally, at any tidal volume, PEEP increases the fistula leak and decreases alveolar ventilation. Further studies in animals or patients with abnormal compliance and resistance are necessary.
REFERENCES
- 01. Pierson DJ, Horton CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation. A review of 39 cases. Chest. 1986;90(3):321-3.
- 02. Bishop MJ, Benson MS, Pierson DJ. Carbon dioxide excretion via bronchopleural fístulas in adult respiratory distress syndrome. Chest. 1987;91(3):400-2.
- 03. Willatts SM. Alternative modes of ventilation. Part I. Disadvantages of controlled mechanical ventilation: intermittent mandatory ventilation. Intensive Care Med. 1985;11(2):51-5.
- 04. Pierson DJ. Management of bronchopleural fístula in the adult respiratory distress syndrome. New Horiz. 1993;1(4):512-21.
- 05. Martin WR, Siefkin AD, Allen R. Closure of a bronchopleural fístula with bronchoscopic instillation of tetracycline. Chest. 1991;99(4):1040-2. Comment in: Chest. 1992;101(6):1737-8.
- 06. Powner DJ, Grenvik A. Ventilatory management of life-threatening bronchopleural fístulae. A summary. Crit Care Med. 1981;9(1):54-8.
- 07. Rafferty TD, Palma J, Motoyama E, et al. Management of a bronchopleural fistula with differential lung ventilation and positive end-expiratory pressure. Respir Care. 1980;25:654-7.
- 08. Tietjen CS, Simon BA, Helfaer MA. Permissive hypercapnia with high-frequency oscillatory ventilation and one-lung isolation for intraoperative management of lung resection in a patient with multiple bronchopleural fistulae. J Clin Anesth.1997;9(1):69-73. 09. Bevelaqua FA, Kay S. A modified technique for the management of bronchopleural fistula in ventilator-dependent patients: a report of two cases. Respir Care. 1986;31:904-8.
- 10. Barringer M, Meredith J, Prough D, Gibson R, Blinkhorn R. Effectiveness of high-frequency jet ventilation in management of an experimental bronchopleural fistula. Am Surg. 1982;48(12):610-3.
- 11. Mas A, Saura P, Joseph D, Blanch L, Baigorri F, Artigas A, Fernández R. Effect of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. 2000;28(2):360-5.
- 12. Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109(5):1215-21.
- 13. West JB. Respiratory physiology - the essentials. 5th ed. Baltimore: Williams & Wilkins; 1994. p.51-2.
- 14.Montgomery DC. Design and analysis of experiments. 3rd ed. New York: John Wiley; 1991.
- 15. Schinco MA, Formosa VA, Santora TA. Ventilatory management of a bronchopleural fistula following thoracic surgery. Respir Care. 1998;43(12):1064-9.
- 16. Litmanovitch M, Joynt GM, Cooper PJ, Kraus P. Persistent bronchopleural fistula in a patient with adult respiratory distress syndrome. Treatment with pressure-controlled ventilation. Chest. 1993;104(6):1901-2.
- 17. Dennis JW, Eigen H, Ballantine TV, Grosfeld JL.- The relationship between peak inspiratory pressure and positive end expiratory pressure on the volume of air lost through a bronchopleural fistula. J Pediatr Surg. 1980;15(6):971-6.
- 18. Dries DJ. Permissive hypercapnia. J Trauma. 1995;39(5):984-9.
Publication Dates
-
Publication in this collection
04 Nov 2008 -
Date of issue
Sept 2008
History
-
Accepted
29 Sept 2008 -
Received
22 Jan 2008