Abstract
Introduction:
Aging is understood as the sum of all biological, psychological and social changes that occur over the years. Associated with aging we list up the changes of morphological and functional order of the immune system: Immunosenescence.
Objective
This study's objective was to characterize the effect of a brief exercise program on the profile of cytokines and peripheral blood mononuclear cells of elderly individuals in Manaus, AM, Brazil.
Materials and methods:
Twelve subjects aged 66.8 (± 3.7) years old on average engaged in three weekly sessions of exercises for 16 weeks and, seven subjects aged 66.1 (± 6.7) years on average, who practiced only recreational activities, composed the control group. Serum levels of IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α and INF-γ were measured using the CBA technique (cytometric Bead Array) and the count of subpopulations of lymphocytes - B, B1, T/CD4, T/CD8, Treg, NK and NKT - was performed using flow cytometry.
Results:
The relative number of B lymphocytes, T/CD4+ and NKT (CD3+/CD16 +/CD56+) increased significantly (p <0.05) after physical activity, compared to the pre-exercise phase and the control group. In another analysis, each individual in the test group was classified either as major or minor producer of each cytokine; i.e., their values were above or below the cut-off point defined by the median of all measurements of that cytokine. Patterns of cytokine production were observed in the post-exercise group, which allowed defining sets ("signatures") of cytokines that were associated with the practice of short-term physical exercises.
Conclusion:
Our work showed that exercise induces changes in the count of immune cells, which allows us to infer that it can be used as an alternative to reverse or mitigate the implications of immunosenescence.
Keywords:
Aging; Fitness; Cytokine; Bead-array; Immunosenescence.
Resumo
Introdução:
O envelhecimento é compreendido como a soma de todas as alterações biológicas, psicológicas e sociais que ocorrem com o passar dos anos. Associadas ao envelhecimento elencam-se as alterações de ordem morfológica e funcional do sistema imunológico: Imunossenescência.
Objetivo:
Caracterizar o efeito do condicionamento físico breve sobre o perfil de citocinas e células mononucleares do sangue periférico de idosos na cidade de Manaus, AM.
Materiais e métodos:
Doze indivíduos com idade média de 66,8±3,7 anos realizaram 3 sessões semanais de exercícios físicos por 16 semanas e sete indivíduos com idade média de 66,1±6,7 anos, praticantes de atividades lúdicas, formaram um grupo controle. Os níveis séricos de IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α e INF-γ foram medidos pela técnica CBA (Cytometric Bead Array) e as contagens de subpopulações de linfócitos B, B1, T/CD4, T/CD8, TReg, NK e NKT foram realizadas por citometria de fluxo.
Resultados:
Observou-se que, após a atividade física, houve aumento significativo (p < 0,05) no número de linfócitos B, T/CD4+ e NKT (CD3+ /CD16+ /CD56+ ), quando comparados à fase pré-treinamento e ao grupo controle. Em outro modelo de análise, qualificando-se cada indivíduo do grupo teste como alto produtor ou baixo produtor das citocinas, observaram-se padrões na fase pós-treinamento que permitiram definir conjuntos ("assinaturas") de citocinas que se expressam associadas ao exercício. Conclusão: Nosso trabalho evidenciou que o exercício induz alterações na contagem de células imunes, o que nos permite inferir que pode ser usado como alternativa para reverter ou atenuar as implicações da imunossenescência.
Palavras-chave:
Envelhecimento; Exercício físico; Citocinas; Bead-array; Imunossenescência.
Introduction
Aging is seen as the sum of all biological, psychological, and social changes that take place over the years with various potential deleterious effects that hinder the quality of life of elderly individuals. Because aging is associated with physiological and functional limitations, preventive and therapeutic measures are needed to ensure well-being, healthiness and vitality among this population 1Zago AS, Gobbi S. Valores normativos da aptidão funcional de mulheres de 60 a 70 anos. Rev Bras Ciên Mov. 2003;11(2):77-86.. The increased population of elderly individuals in comparison to young individuals, is a common situation faced by both developed and developing societies 2Guimarães A, Rocha CAQC, Gomes ALM, Cader SA, Dantas EHM. Efeitos de um programa de atividade física sobre o nível de autonomia de idosos participantes do programa de saúde da família. Fitn Perform J. 2008;7(1):5-9. . Brazil is experiencing a phase of considerable transformations in the profile of its population. The nations of the so-called Third World have presented, in recent decades, a progressive decline in their mortality rates, and more recently, in their fertility rates.
The immunological system undergoes a series of changes over the course of an individual's life. These changes are of a morphological and functional nature and reach their peak at puberty, gradually declining with age. The immunological system can also be affected by factors such as inadequate nutritional conditions, exaggerated stress, infections and other diseases, eventually leading to dysfunction and impairment (3, 4). The term immunosenescence usually refers to age-related changes in the immunological system, that contribute to a greater incidence of infectious or even chronic-degenerative diseases, such as hypertension, cancer, rheumatic affections, atherosclerosis and heart diseases, all of which are prevalent among elderly individuals 5Tonet AC, Nóbrega O. Imunossenescência: a relação entre leucócitos, citocinas e doenças crônicas. Rev Bras Geriatr Gerontol. 2008;11(2):1-20 ..
A decline in the immune function is typically evidenced in thymus involution and decreased generation of naïve T cells, in the dysfunctional performance of the cascade of signaling of T cells, minor response to antigenic stimuli, including vaccines, decreased production of Interleukin-2 (IL-2), deficient activation and consequent proliferation of B and T cells, decreased expression of co-stimulatory molecules such as CD28, increased production of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α, and loss of balance between Th1 and Th2, among others 3Ongrádi J, Kövesdi V. Review Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010;7(7).,5Tonet AC, Nóbrega O. Imunossenescência: a relação entre leucócitos, citocinas e doenças crônicas. Rev Bras Geriatr Gerontol. 2008;11(2):1-20 .,6Ewers I, Rizzo LV, Kalil Filho J. Imunologia e envelhecimento. Einstein. 2008;6(Suppl 1): 13-20.,7Esquenazi DA. Imunossenescência: as alterações do sistema imunológico provocadas pelo envelhecimento. Rev HUPE. 2008;7(1):38-45. ,8Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. The J Pathol. 2007;211(2):144-56.,9Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6-41..
Due to some characteristics, such as lower cost and non-invasiveness, researchers have made an effort to clarify the effects of exercise on the organic systems of human beings and animals. Due to these studies, it is now known that regular exercise is an important factor in one's health status, including reduced body mass, lower blood pressure, improved glucose and lipid levels, and prevention of cancer, coronary artery disease, and self-immune diseases 10Hernandes ESC, Barros JF. Efeitos de um programa de atividades físicas e educacionais para idosos sobre o desempenho em testes de atividades da vida diária. Rev Bras Ciên Mov. 2004;12(2):43-50.. Individuals involved in exercise programs, even only aiming to improve quality of life, also benefit from a positive immunological system 11Dias RMR, Gurjão ALD, Marucci MFN. Benefícios do treinamento com pesos para aptidão física de idosos. Acta Fisiatr. 2006;13(2):90-95.,12Matsudo, SMM. Envelhecimento, atividade física e saúde. Rev Min Educ Fís. 2002;10(1):195-209 .. The effective participation in a exercise program - composed of aerobic exercises, using oxygen for the muscle to produce energy, or anaerobic exercises to strength the muscles - greatly contributes to a healthy aging process 13Da Silva MC, Rombaldi AJ, Campos ALP. Ordem dos exercícios físicos aeróbios e com pesos na aptidão física de mulheres acima dos 50 anos. Rev Bras Cineantropom Desempenho Hum. 2010;12(2):134-9..
Given the preceding discussion and, due to the few studies addressing the effect of exercise on the profile of immune cells among the elderly, this study's objective was to characterize the profile of cytokines and peripheral blood mononuclear cells in elderly individuals as a response to regular physical exercise in the city of Manaus, AM, Brazil.
Material and methods
Population
This prospective, longitudinal, controlled study included a group of elderly individuals who took part in a short physical conditioning program to verify the effect of this intervention in cell and humoral parameters of their immune systems. A total of 19 sedentary elderly volunteers enrolled in the elderly activities division of a park district in the city of Manaus, AM, Brazil. Participated on the study 14 women and 5 men, aged 66.57(± 4.90) years old, on average. Inclusion criteria were being between 60 and 75 years old, sedentary, lucid and having an independent active life. Exclusion criteria were having chronic diseases or cardiovascular, musculoskeletal, or cognitive impairment.
Twelve individuals composed the exercise-training group, 7 women and 5men aged 66.8 (± 3.7) years old, on average. These individuals were also enrolled in the elderly activities division of the park previously mentioned, and lived in the city of Manaus, had not practiced systematic or programmed exercise for at least six months, were physically independent in terms of self-care and mobility, had the time and were willing to become involved with the exercise program. All participants were instructed to maintain their daily routine activities.
Elderly individuals who had already attended the elderly recreational program at the park district for six months were selected for the control group. The activities they took part in this program included psychosocial or recreational activities, such as handcraft workshops, singing and sewing classes, and games (cards, dominoes, snooker).
Of the 25 elderly individuals invited for the exercise group, 4 refused to participate; 5 were excluded for having attended less than 75% of the intervention program; and 4 abandoned/withdrew the program.
The participants of the test group had their cardiovascular and aerobic capacity (VO2max) assessed at the Physical Education and Physical Therapy College at UFAM, before and after the intervention. Parameters related to quality of life (Medical Outcomes Study SF-36), concern over the risk of falls (Falls Efficacy FES-I-Brazil), and functional capacity (Timed Up & Go) were also addressed.
Physical and immunological assessments were performed among those included in the experimental group before and after the 16 weeks of exercise training, while the control group had only one immunological assessment performed at the beginning of the study. The participants had 10mL of venous blood collected with EDTA anticoagulant for the immunological tests. In the pre-exercise phase, blood was collected when the participants were under rest and 24hs after their last exercise session.
All the participants signed free and informed consent terms. Those from the experimental group were informed of the potential implications of the exercise protocol. This study was conducted in accordance to resolution 196/96, from the National Council of Health and was submitted to and approved by the Institutional Review Board of the Federal University of Amazonas. At the end of the exercise program, the elderly individuals from the experimental group were included in the sport and recreational modalities available through the park district.
Immunological assessment
The samples were processed in the Research Laboratory at the Amazon Hematology and Hemotherapy Foundation (FHEMOAM), where complete blood count and flow cytometry to count mononuclear cells were performed, and serum was separated in aliquots for later quantification of cytokines.
Flow cytometry - Flow cytometry was performed using the following panel of monoclonal antibodies, marked with specific fluorochromes against cell surface antigens: (Anti-CD3 PERCP, Anti-CD4 PE, Anti-CD8 FITC and Anti-CD69 APC, BD(r) Biosciences), to identify subpopulations of T/CD4+ (CD3+ and CD4+) and T/CD8+ (CD3+ and CD8+) lymphocytes; (Anti-CD19 PE and Anti-CD5 FITC, BD(r) Biosciences), to identify total B lymphocytes (CD19+) and B1 lymphocytes (CD19+/CD5+);(Anti-CD3 PERCP, Anti-CD56 PE, Anti-CD69 APC and Anti-CD16 FITC,BD(r) Biosciences), to identify Natural Killer NK cells (CD3-/CD16+/CD56+) and NKT (CD3+/CD16/CD56-); (Anti-CD4 FITC, Anti-CD25 APC and Anti- FoxP3 PE, BD(r) Biosciences), to identify Treg cells (CD4+/CD25+/FoxP3+) (Becton-Dickinson, California). The different cells populations were identified based on the acquisition of 10,000 events and analyzed in gates, according to the markers previously described, using FACSCalibur flow cytometry (Becton-Dickinson Immunocytometry Systems, Palo Alto, California), CellQuest software (v. 3.1) and Flow Jo software (v.9.0).
Serum cytokines - The dosage of IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α and INF-γ cytokines was performed using the CBA (Cytometric Bead Array) technique or flow cytometry with arrangement of microparticles, using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine Kit BD(tm) (Becton-Dickinson Immunocytometry Systems, Palo Alto, California). Manufacturer's technical instructions were followed concerning the preparation of the microparticles, detection antibodies and cytokines standards.
In this trial, seven populations of microparticles with different fluorescence intensities were conjugated with a specific capture antibody for each cytokine, to form the CBA. The populations of microparticles were visualized according to their respective fluorescence intensities. The samples of microparticles of cytokine capture were mixed with the detection antibody, conjugated with the PE fluorochrome and, then, incubated with the test samples in sandwich assays. The acquisition tubes were prepared with 50 µL of sample, 50 µL of the microparticles mixture and 50 µL of the detection reagent Th1/Th2 PE (Human Th1/Th2 PE Detection Reagent/1 vial, 4mL). The same procedure was used to obtain the standard curve. The tubes were homogenized and incubated for three hours in the dark and at an ambient temperature. After the incubation, the samples and standards were washed and read using FACSCalibur flow cytometry (Becton Dickinson Immunocytometry Systems, Palo Alto, California), and data analysis was performed using F Cap-Array software (v.3.0). The results were compared to positive controls in ten serial dilutions of a standard curve with known and detectable minimal and maximum concentrations of each cytokine. The standard calibration curve for each cytokine was input into the cytometer before the samples were analyzed. At the end of the analyses, graphs and tables were generated using GraphPad Prism software (v.5.0).
Cytokine signature - the cut off points for major and minor producers of cytokines were established based on a global median computation of all participants for each cytokine. Based on this approach, the individuals of each group were classified as major or minor producers 14Ferreira GF, Moraes C, Silveira AMS, Correa-Oliveira R, Teixeira-Carvalho A, Martins-Filho AO, et al. Distinct cytokine profiles of circulating mononuclear cells stimulated with Staphylococcus aureus enterotoxin A in vitro during early and late episodes of chronic osteomyelitis. Mem Inst Oswaldo Cruz. 2012;107(3):48 - 55. ,15Almeida C, Lima TA, Castro DB, Torres KL, Silva WB, Peruhype-Magalhães V, et al. Immunological/Virological Peripheral Blood Biomarkers and Distinct Patterns of Sleeping Quality in Chronic Hepatitis C Patients. Scand J Immunol. 2011; 73(5):486 - 95.
Activities protocol
The exercise program was performed in the morning (between 7am and 11am) for 60 minutes, three times a week with three hours per week at most during four consecutive months, totalizing 50 sessions distributed in 16 weeks. The program comprised 10 minutes of stretching, 40 minutes of exercises, and 10 minutes of cooling down. The participants' blood pressure was measured before the exercises and during the cooling period.
The elderly individuals moved the lower and upper limbs in the upright and supine positions, stretched the brachial biceps, triceps, carpal flexors and fingers, paraspinal, triceps sural, quadriceps, biceps femoris, iliopsoas, hamstrings, tensor fasciae latae and iliotibial band, which were administered in 10-second series during each movement. After warming up, the individuals performed active, resistance and isotonic exercises in circuits using weights ranging from 0.5Kg up to 4Kg, medium and strong elastic bands, sticks, and balls in a climate-controlled room at an average of 22ᵒC. After the exercises, the individuals performed a guided walk on a 700m track. In the first week, they walked two laps, then three laps in the second week, and ended the program walking eight laps, always respecting each individual's conditioning threshold.
The control group had attended recreational and psychosocial activities for six months and did not perform any physical activity. They took part in handcraft workshops, singing or sewing classes, or games (e.g., cards, dominoes, snooker) three times a week.
Statistical treatment
Kolmogorov-Smirnov was employed to assess data normal distribution. The Mann-Whitney U test was used to compare non-Gaussian data between groups, even after logarithmic transformation. The Chi-square test, with Pearson correction when necessary, was used for the pairwise comparison of categorical data. Student's t test was used to compare continuous data. Data were analyzed using GraphPad Prism(r) 5.0. Level of significance was p < 0.05 and confidence interval (CI) was 95%.
Results
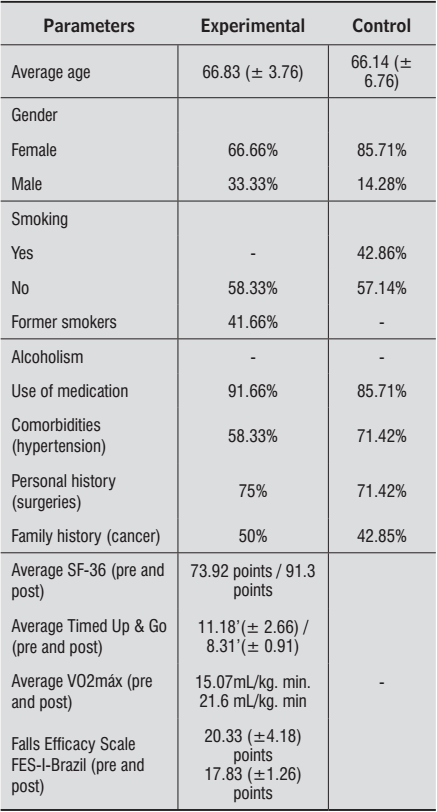
Demographic and physiological data of the studied population, both from the exercise training and control groups, are presented in Table 1. Women (66.66%) were the majority in the experimental and control (85.71%) groups. The exercise group was 66.83 (± 3.76) years old on average and the control group was 66.14 years old on average (± 6.76), with a maximum and minimum of 74 and 64 years old, respectively. Other epidemiological data are presented in Table 1.
Demographic and physiological data of the population in the experimental and control groups. Manaus, AM, Brazil - 2012
The lymphocyte total count presented in the blood cell count increased from 27.5% (± 5.5) at baseline to 36.6 (± 4.1) in the post-exercise phase (p = 0.0012). The hemoglobin concentration also increased from 12.59g/dL (± 2.9) to 13.8 (± 1.3), though without statistical significance.
Quantification of cytokines
The average fluorescence intensity (AFI) of IL-2, IL-4, IL-10 and INF-y cytokines presented in Figure 1A, 1B, 1C and 1D, revealed no statistical significance between the test group in the pre-exercise and post-exercise phases. The AFI of IL-2 in the pre-exercise phase was 7.74 (± 0.39) and 7.67 (± 0.55) in the post-exercise phase, while it was 7.44 (± 0.44) in the control group. The AFI of IL-4 in the pre-exercise phase was 4.76 (± 0.65), 4.66 (± 0.64) in the post-exercise phase and 4.88 (± 0.66) in the control group. The AFI of IL-10 in the pre-exercise phase was 2.36 (± 0.94), 2.39 (± 0.61) in the post-exercise phase, and 2.41 (± 0.52) in the control group. The AFI of IFN-γ in the pre-exercise phase was 1.73 (± 0.61), 1.27 (± 0.78) in the post-exercise phase, and 1.57 (± 0.34) in the control group.
Values expressed by the MFI of the cytokines IL-2, IL-4, IL-10, IFN-γ, IL-6, IL-17 and TNF-α present in the serum of the subjects of physical activities program in the pre and post training and the control group
The AFI of IL-6, IL-17 and TNF-α cytokines (Figure 1E, 1F and 1G) showed no statistical significance between the test group in the pre and post-exercise phases. The AFI of IL-6 in the pre-exercise phase was 5.86 (± 1.54), 6.07 (± 9.85) in the post-exercise phase, and 7.80 (±5.6) in the control group. The AFI of IL-17 in the pre-exercise phase was 3.52 (± 0.63), 3.41 (± 0.81) in the post-exercise phase, and 3.41 (± 0.56) in the control group. The AFI of TNF-α in the pre-exercise phase was 3.52 (± 0.63), 3.64 (± 0.81) in the post-exercise, and 2.78 (± 0.48) in the control group.
The absolute values in pg/mL of the seven cytokines under study were extremely low, close to the minimum detection threshold: 3.92 (IL-17); 2.55 (IFN-γ); 3.96 (TNF-α); 2.29 (IL-10); 5.38 (IL-6); 5.73 (IL-4) and 8.06 (IL-2), with values expressed according to AFI. To give an idea of the possible range, the maximum level of detection the method provided was 3,133.96 (IL-17); 2,525.48 (IFN-γ); 2,813.32 (TNF-α); 2,819.72 (IL-10); 3,586.64 (IL-6); 3,651.74 (IL-4) and 4,332.30 (IL-2).
Quantification of cell subpopulations
The quantification of mononuclear cells is expressed in percentage form related to the total lymphocytes count. The quantification of B lymphocytes (CD19+) showed an increase: 10.5% (± 3,8) in the pre-exercise phase and 15.4% (± 4.1) in the post-exercise phase (p = 0.02) (Figure 2A). No statistical significance (p > 0.05) was found concerning the percentage of B1 cells (CD19+/CD5+), though a slight increase was observed (Figure 2B). The percentage of T-helper/inductors lymphocytes (CD4+) showed an increase of 36.8% (± 12.3), to 49.2% (± 7.3) in the post-exercise phase (Figure 2C). The percentage of T-cytotoxic lymphocytes (CD8+) did not vary, in statistical terms, between the pre (16.6% ±9.0) and post-exercise (19.9 ± 6.4) phases (Figure 2D). The regulatory T cells (Treg), CD4+/CD25+/FoxP3+ presented a slight increase from 9.03% (±2.340) in the pre-exercise phase to 12.4% (±4.0) in the post-exercise phase (p>0.05) (Figure 2E). There was, in the population of NK cells (CD3-/CD16+/CD56+), an increase in the average percentage, from 8.99% (±7.59) in the pre-exercise phase to 11.55% (±4.43) in the post-exercise phase (p>0.05) (Figure 2F).
Immunophenotyping of CD19 + cell subsets, CD19 + / CD5 +, CD4 +, CD8 +, FoxP3, CD3, CD16 + / CD56 + with expressed in percentage values for the total peripheral blood lymphocytes in the experimental group (pre training/post training) and control group
The subpopulation of NKT cells (CD3+/CD16+/CD56+) expressed the major changes in the brief exercise. The lymphocytes total percentage increased from 1.05% (±0.67) in the pre-exercise phase to 1.87% (±0.53) in the post-exercise phase (p=0.01) (Figure 2G).
Major and minor producers of cytokines
The characterization of serum cytokines was based on the frequency of individuals classified as major and minor producers. That is, the values obtained by the experimental (pre-exercise and post-exercise) and control groups were compiled into a single group and the median was calculated and adopted as the cut off point for each cytokine under study (Figure 3A), based upon which the participants were classified as minor or major producers 14Ferreira GF, Moraes C, Silveira AMS, Correa-Oliveira R, Teixeira-Carvalho A, Martins-Filho AO, et al. Distinct cytokine profiles of circulating mononuclear cells stimulated with Staphylococcus aureus enterotoxin A in vitro during early and late episodes of chronic osteomyelitis. Mem Inst Oswaldo Cruz. 2012;107(3):48 - 55. ,15Almeida C, Lima TA, Castro DB, Torres KL, Silva WB, Peruhype-Magalhães V, et al. Immunological/Virological Peripheral Blood Biomarkers and Distinct Patterns of Sleeping Quality in Chronic Hepatitis C Patients. Scand J Immunol. 2011; 73(5):486 - 95.
(A) Median Mfi Il-17, IFN-y, TNF-α, IL-10, IL-6, IL-4 and IL-2. (B) Cytokine according to the percentage of individuals "high" and "low" producer of serum cytokines. The overall median value of each serum cytokine was calculated using the entire study population. The cut-off point set each individual as low producer and senior producer in the pre training phase, post training and control
We opted to categorize the participants as minor or major producers, given the difficulty in analyzing extremely low levels of cytokines, and also to make the results more homogeneous. In Figure 3B, the major producers - those with values greater than the median - are represented by the black area, while the minor producers are represented by the white area. A total of 67% of the individuals in the pre-exercise group were major producers of IL-17; 58% were major producers of IFN-γ and IL-4; and 50% were major producers of IL-10 and IL-2. In the post-exercise group, 67% were major producers of IL-10; 50% were major producers of IL-17 and IL-6; and were minor producers of the remaining cytokines. The participants in the control group were major producers of IL-6 (86%), TNF-α (71%), IFN-γ (57%) and IL-4 (57%).
Frequency of major producers of serum cytokines
The intra-group comparison showed that the frequency of major producers of IL-2 in the experimental group was the same (50%) in the pre-exercise and post-exercise phases, though the frequency of the remaining cytokines changed. The frequency of major producers of IL-4 decreased from 58%, in the pre-exercise phase, to 42% in the post-exercise phase. A similar decrease was observed with IFN-γ, from 58% to 33%. In contrast, the frequency of major producers of IL-10 in the post-exercise phase increased from 50% to 67%. In regard to the percentage of major producers of IL-6, there were 42% in the pre-exercise phase, while in the post-exercise phase there were 50%. In regard to the IL-17 and TNF-α cytokines, a decrease was observed in the frequency of major producers. The percentage of major producers of IL-17 in the pre-exercise phase was 67%, while in the post-exercise phase it was 50%. Similarly, 50% of the individuals were major producers of TNF-α in the pre-exercise phase and 42% in the post-exercise. A dominant pattern of major production of cytokines was observed according to the exercise phase or group. Figure 4 shows that major levels of IFN-γ, IL-4 and IL-17 cytokines were produced by more than 50% of the participants in the pre-exercise phase. In the post-exercise phase, only IL-10 was produced at major levels by more than 50% of these participants. In the control group, major levels of IFN-γ, IL-10, Il-4, TNF-α and IL-6 cytokines were produced by more than 50% of the participants.
Comparison of the frequency of high producers of serum cytokines in experimental groups (pre training/post training) and control. The shaded areas indicate the cytokines that had a higher frequency of high producers
Discussion
Exercise is, in general, associated with a modulation of immunological responses, as it increases the dynamics and kinetic of cells and humoral mediators. This study revealed that the elderly individuals who took part in the moderate, brief, gradual and systematic protocol of exercises, during 16 weeks, presented changes in the profile of cytokines and cell-immunes in the post-exercise phase. This indicates a positive balance in the immunological activity of these individuals, which resulted from the acquisition of functional aptitude. Because this is a study with internal control, performed at two points in time (pre and post-exercise phases), with a control group who did not take part in the physical activities, it enabled observing the responses of immune and humoral cells to active and systematic physical activities in comparison to recreational activities without important muscle energy expenditure. Additionally, we also had the opportunity to observe such a response in a region that, due to its geographical location and intrinsic and remarkable climatic characteristics, is peculiar.
The immune system undergoes substantial changes in the course of human development. These changes become even more evident with aging, which characterizes immunosenescence. The benefit of exercise for an individual's health is a concept rooted in popular imagery. The main benefits related to the practice of regular exercise, regardless of age, include weight control or weight loss, reduced risk of occurrence or recurrence of diseases, especially metabolic and cardiovascular conditions, diseases of the musculoskeletal system, decreased stress or depression, improved self-esteem and socialization 16Caromano FA, Candeloro JM. Fundamentos da hidroterapia para idosos. Arq Ciências Saúde UNIPAR. 2001;l(5) : 187 - 95.. This study's results reinforce the data reported by the scientific literature that shows the sensitivity of some cells of the immunological system in response to exercise.
The humoral immunity of elderly individuals is mainly due to a decreased production of B-lymphocytes that produce immunoglobulin in the long term. This decrease, which accrues from events related to dysfunctions inherent to the individual or to environmental factors, results in the interruption of the germinative formation of these cells in the secondary lymphoid tissues 8Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. The J Pathol. 2007;211(2):144-56.,17Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ , et al. Constitutive pro-and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100(4):1124-33.,18Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23(2):64-74..
Even though the responses of specific antibodies tend to decrease with age, our results show that the regular practice of exercise caused an expressive increase in the number of B CD19+ lymphocytes in the post-exercise phase, confirming findings reported by other studies 4Senchina DS, Kohut ML. Immunological outcomes of exercise in older adults. Clin Interv Aging. 2007;2(1):3-16.,9Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6-41.,19Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435-46.,20Bruunsgaard H, Pedersen BK. Effects of exercise on the immune system in the elderly population. Immunol Cell Biol. 2000;78(5):523-31.. In this sense, we believe that moderate, brief or even prolonged exercise programs are able to stimulate positively B-lymphocytes in elderly individuals. With the increase of antibody precursor cells in peripheral blood, we can infer that exercise reduces or reverts senescence of these cells, enabling the natural acquisition of immunity during this phase of life. Further studies are needed to address the functional capacity of these cells in response to vaccines in physically active elderly individuals compared to sedentary elderly individuals.
Prior reports have shown that human aging is also associated with a decrease in the number and function of total T cells and a deficiency in the functionality of subpopulations of T CD4+ and T CD8+ cells 4Senchina DS, Kohut ML. Immunological outcomes of exercise in older adults. Clin Interv Aging. 2007;2(1):3-16.,6Ewers I, Rizzo LV, Kalil Filho J. Imunologia e envelhecimento. Einstein. 2008;6(Suppl 1): 13-20.,16Caromano FA, Candeloro JM. Fundamentos da hidroterapia para idosos. Arq Ciências Saúde UNIPAR. 2001;l(5) : 187 - 95.. This study's results reveal a favorable effect of exercise through physiological bio-adaptations in the area of immunity, a fact generated by the stimulation and proliferation of subpopulation of TCD4+ lymphocytes. An elderly individual with preserved immune functions is capable to orchestrate more efficient responses against various pathogens or antigenic stimuli. Considering the differentiation of T naïve lymphocytes in T CD4+, we have an effector cell largely participative in immunological responses, expressing cytokines (IL-2, IL-4) and surface molecules (CD40-binder) that activate B-lymphocytes in responses optimized to protein antigens 9Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6-41..
NK and NKT cells play an important role in the immune responses against tumors through immune-regulatory cytokines and perforin, showing the greatest changes in the immune profile in response to exercise 21Yan J, Greer JM, Hull R, O'Sullivan JD, Henderson RD, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7(4):1-10.,22Rosa LFPBC, Vaisberg MW. Influências do exercício na resposta imune. Rev Bras Med Esporte. 2002;8(4):167-72.. This study enabled observing that these cells seem to be more sensitive to exercise, since there was a mobilization of the CD16+/CD56+ sub-population to the area of peripheral circulation in the post-exercise phase, confirming the findings of Drela et al. 23Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr. 2004;4(1):8.. Other studies also show the role of these cells in the immunological bio-adaptation of young and athletic individuals. Horn et al. 24Horn P, Kalz A, Lim CL, Pyne D, Saunders P, Mackinnon L, et al. Exercise-recruited NK cells display exercise-associated e HSP-70. Exerc Immunol Rev. 2007; 13:100-11. investigated the immune profile of trained athletes, during and after exercise, and verified an increase of 83% in the count of NK cells immediately after the activity, showing that there is an important connection between exercise and the innate immune system, in addition to the reinforcement of immune surveillance.
According to Rosa and Vaisberg 22Rosa LFPBC, Vaisberg MW. Influências do exercício na resposta imune. Rev Bras Med Esporte. 2002;8(4):167-72., these cells increase from 150% to 300% in number in the peripheral blood supply, in the period immediately after physical exertion. This increase is transitory and, after 30 minutes, their numbers return to the standards prior to exercising. Longer duration of activity (above 90 minutes) is associated with a lower increase in the number of NK cells. There is an increase from 40% to 100% in the cytotoxic activity of NK cell after heavy-intensity exercise 18Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23(2):64-74.. Wang and Weng 25Wang JS, Weng TP. Hypoxic exercise training promotes antitumour cytotoxicity of natural killer cells in young men. Clin Sci. 2011; 121(8): 343 - 53. show that it is possible to promote an improvement in the parameters that predict physical conditioning, such as aerobic capacity, and simultaneously improve the percentage and natural cytotoxicity of NK cells, which was possible through standardized exercise using ergonometric bicycles for four months with young and sedentary male individuals.
With the exception of IL-6 and TNF-α, which has more systemic effects in the body, with specific telocrine actions, this study's results show that there was no statistically significant difference in regard to the quantification of serum cytokines (IL-2, IL-4, IL-10 and IL-17, IFN-γ) in the post-exercise phase, compared to the pre-exercise phase and control group. We believe that this lack of significance was due to the small sample size, short period of physical activity (four months), and short life of most cytokines that were elected for investigation and their predominantly autocrine and paracrine action. Another fact that may have directly influenced the results was the time of collection: the second sample was obtained 24 hours after the last session of exercise. Perhaps, if we had collected data immediately after the last exercise session, we would be able to observe detectable levels of serum in the trained individuals. Or, if we had stimulated ex-vivo cytokines, challenging them with an antigen or mitogen, we could more effectively identify higher levels and observe the responses of these mediators.
Given the difficulties to perform individual analyses of the participant's cytokines, we opted to use a new approach for the joint characterization of cytokines - the signature - with the inclusion of all the participants. The definition of signatures was possible due to the stratification of the individuals into major and minor producers, which facilitates our understanding regarding the repercussions of these mediators during aging, and their association with exercise.
The signature of cytokines showed that the frequency of major producers of IFN-γ, IL-4 and IL-17 was greater in the pre-exercise phase. There was a higher frequency of major producers of IL-10 in the post-exercise phase. This result may be associated with the increase in helper T cells, since IL-10 is involved in the activation of B-lymphocytes subpopulation and in the control of homeostasis of innate and cell immune reactions. Comparison with the control group showed that recreational or psychosocial activities are involved with serum increases of IFN-γ, IL-10, IL-4, TNF-α and IL-6. Due to the scarcity of data related to the practice of recreational activities by elderly individuals and their relationship to immune responses, and our small sample, it was not possible to generalize the results. However, we infer that recreational or psychosocial tools can regulate some immune parameters, through the production of IFN-γ (the main activator of macrophages in cell and adaptive immune responses), IL-10 (control of immune homeostasis) and IL-4 (inducer of B cell proliferation). Additionally, the inhibitor effect of IL-4 and IL-10 on Th1 response favors an important regulatory action against chronic inflammatory diseases that are prevalent among elderly individuals.
To assess the effects of exercise on pro and anti-inflammatory cytokines and on the growth factors of leukocytes, Zaldivar et al. 17Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ , et al. Constitutive pro-and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100(4):1124-33. collected blood samples of 11 men aged between 11 and 30 years old, who engaged in strenuous biking exercise. The results showed that the number of leukocytes, including T lymphocytes and B-lymphocytes, NK cells, monocytes and neutrophils, immediately after 30 minutes of exercise, had increased, as expected. A predominantly pro-inflammatory pattern, however, was not observed, as there was an increase in the number of anti-inflammatory cytokines, which reflects a mixed response. The same study also showed a major expression of IL-6 in the circulation immediately after exercise, showing that this cytokine is undoubtedly the most expressive mediator during heavy physical exercise, even though it did not show statistically significant differences in this study.
Natale 26Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. 2003;121(1):9-14. et al. verified the effects of three types of aerobic exercises with intensities that ranged from 60%-70% of VO2máx to 90%-97% of VO2máx in the leucocytes count [total leucocytes, total T cells (CD3+), helper T lymphocytes (CD3+CD4+), T lymphocytes cytotoxic (CD3+CD8+), B-lymphocytes (CD19+) and natural killer NK cells (CD3-/CD16+/56+) and NKT (CD3+/CD16+/56+)], during and after exercise in the case of individuals aged 24.9 (±2.3), on average, and verified that three types of exercises caused leukocytosis, which persisted for three hours after exercise. The leukocytosis was especially due to the increase in the number of circulating neutrophils and monocytes, though there was also a small increase in the lymphocyte count. Additionally, the authors verified that the three types of exercise induce to a significant increase in the count of total T lymphocytes (CD3+) immediately after exercise.
It is worth noting that the experiment performed only allows for claims based on the study's internal validity. In this sense, considering the limitations of the design adopted and the small sample size, we suggest the conduction of further studies, with control for other variables, the adoption of physical exercise programs for periods longer than 16 weeks and with individuals that practice other psychosocial modalities.
In summary, this study and most of the previous studies show that aerobic exercises, both of moderate and strong intensity, and prolonged submaximal exercise, induce important changes in the immune cell count, which allow us to infer that one of the alternatives that can be used to revert or reduce implications of immunosenescence is physical activity. In face of the new profile of the Brazilian population, the participation in regular and supervised physical activities constitutes an important measure for individuals to acquire an immunological system prepared for the challenges of aging.
Acknowledgements
The authors thank the Federal University of Amazonas, FAPEAM (Support Research Foundation of the State of Amazonas), CNPq, Parque Municipal do Idoso , Amazon Hematology and Hemotherapy Foundation, and are grateful for the technical support generously provided by Lucas Teixeira, Alex Nascimento, Mateus Rossato and Ariel Arnon. All the authors declare no conflict of interest.
References
- Zago AS, Gobbi S. Valores normativos da aptidão funcional de mulheres de 60 a 70 anos. Rev Bras Ciên Mov. 2003;11(2):77-86.
- Guimarães A, Rocha CAQC, Gomes ALM, Cader SA, Dantas EHM. Efeitos de um programa de atividade física sobre o nível de autonomia de idosos participantes do programa de saúde da família. Fitn Perform J. 2008;7(1):5-9.
- Ongrádi J, Kövesdi V. Review Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010;7(7).
- Senchina DS, Kohut ML. Immunological outcomes of exercise in older adults. Clin Interv Aging. 2007;2(1):3-16.
- Tonet AC, Nóbrega O. Imunossenescência: a relação entre leucócitos, citocinas e doenças crônicas. Rev Bras Geriatr Gerontol. 2008;11(2):1-20 .
- Ewers I, Rizzo LV, Kalil Filho J. Imunologia e envelhecimento. Einstein. 2008;6(Suppl 1): 13-20.
- Esquenazi DA. Imunossenescência: as alterações do sistema imunológico provocadas pelo envelhecimento. Rev HUPE. 2008;7(1):38-45.
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. The J Pathol. 2007;211(2):144-56.
- Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6-41.
- Hernandes ESC, Barros JF. Efeitos de um programa de atividades físicas e educacionais para idosos sobre o desempenho em testes de atividades da vida diária. Rev Bras Ciên Mov. 2004;12(2):43-50.
- Dias RMR, Gurjão ALD, Marucci MFN. Benefícios do treinamento com pesos para aptidão física de idosos. Acta Fisiatr. 2006;13(2):90-95.
- Matsudo, SMM. Envelhecimento, atividade física e saúde. Rev Min Educ Fís. 2002;10(1):195-209 .
- Da Silva MC, Rombaldi AJ, Campos ALP. Ordem dos exercícios físicos aeróbios e com pesos na aptidão física de mulheres acima dos 50 anos. Rev Bras Cineantropom Desempenho Hum. 2010;12(2):134-9.
- Ferreira GF, Moraes C, Silveira AMS, Correa-Oliveira R, Teixeira-Carvalho A, Martins-Filho AO, et al. Distinct cytokine profiles of circulating mononuclear cells stimulated with Staphylococcus aureus enterotoxin A in vitro during early and late episodes of chronic osteomyelitis. Mem Inst Oswaldo Cruz. 2012;107(3):48 - 55.
- Almeida C, Lima TA, Castro DB, Torres KL, Silva WB, Peruhype-Magalhães V, et al. Immunological/Virological Peripheral Blood Biomarkers and Distinct Patterns of Sleeping Quality in Chronic Hepatitis C Patients. Scand J Immunol. 2011; 73(5):486 - 95.
- Caromano FA, Candeloro JM. Fundamentos da hidroterapia para idosos. Arq Ciências Saúde UNIPAR. 2001;l(5) : 187 - 95.
- Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ , et al. Constitutive pro-and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100(4):1124-33.
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23(2):64-74.
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435-46.
- Bruunsgaard H, Pedersen BK. Effects of exercise on the immune system in the elderly population. Immunol Cell Biol. 2000;78(5):523-31.
- Yan J, Greer JM, Hull R, O'Sullivan JD, Henderson RD, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7(4):1-10.
- Rosa LFPBC, Vaisberg MW. Influências do exercício na resposta imune. Rev Bras Med Esporte. 2002;8(4):167-72.
- Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr. 2004;4(1):8.
- Horn P, Kalz A, Lim CL, Pyne D, Saunders P, Mackinnon L, et al. Exercise-recruited NK cells display exercise-associated e HSP-70. Exerc Immunol Rev. 2007; 13:100-11.
- Wang JS, Weng TP. Hypoxic exercise training promotes antitumour cytotoxicity of natural killer cells in young men. Clin Sci. 2011; 121(8): 343 - 53.
- Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. 2003;121(1):9-14.
Publication Dates
-
Publication in this collection
Apr-Jun 2016
History
-
Received
16 July 2013 -
Accepted
18 Aug 2015