Abstracts
Candida albicans is present in the oral cavity and in the whole digestive tract of humans and other animals, being frequently related to endodontic treatment failure. The present study determined the incidence of C. albicans in the oral cavity and the susceptibility of isolates to different pH values and saturated calcium hydroxide aqueous solution at pH 12.5. Sixty-five patients attending the Endodontic Clinic at the Sagrado Coração University participated in the study. The collected samples were cultivated in selective media for C. albicans and the isolates were tested in terms of resistance to both alkaline pH and saturated aqueous solution of calcium hydroxide. In relation to time variables, yeast viability was assessed by the Sabouraud's agar culture and fluorescein diacetate and ethidium bromide fluorescent staining method. Results from the different pHs and experimental times, including those from different techniques measuring fungal viability, were compared using the chi-square and Fisher's exact tests (α=0.05). The yeasts became completely inviable after 48 h of contact with the calcium hydroxide solution. On the other hand, when exposed to the alkaline culture broth, the yeasts were found to be viable at pHs 9.5 and 10.5 for up to 7 days. In conclusion, C. albicans can only be completely inhibited by direct contact with saturated calcium hydroxide aqueous solution after 48 h of exposure.
Candida albicans; oral cavity; calcium hydroxide; susceptibility
Candida albicans está presente na cavidade oral e em todo trato digestivo de humanos e outros animais, podendo estar relacionada a fracassos do tratamento endodôntico. O presente trabalho determinou a incidência de C. albicans na cavidade oral e a susceptibilidade das amostras isoladas frente a diferentes valores de pH e à solução aquosa saturada de hidróxido de cálcio em pH 12,5. Sessenta e cinco pacientes atendidos na Clínica de Endodontia da Universidade Sagrado Coração participaram da pesquisa. As amostras coletadas foram cultivadas em meios seletivos para C. albicans. As cepas isoladas foram testadas quanto a resistência ao pH alcalino e à solução saturada de hidróxido de cálcio. Frente às variáveis de tempo, a viabilidade das leveduras foi avaliada pelos métodos da cultura sobre ágar Sabouraud e de fluorescência pela técnica do diacetato de fluoresceína e brometo de etídio. Foram isoladas 30 linhagens de C. albicans coletadas da cavidade oral dos pacientes. Quando em contato com a solução de hidróxido de cálcio as leveduras foram totalmente inviabilizadas após 48 h de exposição. Quando expostas ao caldo de cultura alcalinizado as leveduras mantiveram-se viáveis em pH 9,5 e 10,5 por até 7 dias. Em conclusão, C. albicans pode ser completamente inibida pelo contato direto com solução aquosa saturada de hidróxido de cálcio após 48 h de exposição.
In vitro susceptibility of oral Candida albicans strains to different pH levels and calcium hydroxide saturated aqueous solution
Paulo Henrique WeckwerthI; Cristiane CarniettoI; Ana Carolina Villas Boas WeckwerthII; Marco Antonio Hungaro DuarteIII; Milton Carlos KugaIV; Rodrigo Ricci VivanV
IDepartment of Biological Sciences, Center of Health Sciences, USC - Sagrado Coração University, Bauru, SP, Brazil
IILauro de Souza Lima Hospital, Bauru, SP, Brazil
IIIDepartment of Operative Dentistry, Dental Materials and Endodontics, Bauru Dental School, USP - Univeristy of São Paulo, Bauru, SP, Brazil
IVDepartment of Restorative Dentistry, Araraquara Dental School, UNESP - Univ Estadual Paulista, Araraquara, SP, Brazil
VDepartment of Dentistry, Center of Health Sciences, USC - Sagrado Coração University, Bauru, SP, Brazil
Correspondence Correspondence: Dr. Paulo Henrique Weckwerth, Departamento de Ciências Biológicas, Centro de Ciências da Saúde, Universidade Sagrado Coração, Rua Irmã Arminda, 10-50, Jardim Brasil, 17011-160 Bauru, SP, Brasil. Tel.: +55-14-2107-7196. e-mail: phweck@terra.com.br
ABSTRACT
Candida albicans is present in the oral cavity and in the whole digestive tract of humans and other animals, being frequently related to endodontic treatment failure. The present study determined the incidence of C. albicans in the oral cavity and the susceptibility of isolates to different pH values and saturated calcium hydroxide aqueous solution at pH 12.5. Sixty-five patients attending the Endodontic Clinic at the Sagrado Coração University participated in the study. The collected samples were cultivated in selective media for C. albicans and the isolates were tested in terms of resistance to both alkaline pH and saturated aqueous solution of calcium hydroxide. In relation to time variables, yeast viability was assessed by the Sabouraud's agar culture and fluorescein diacetate and ethidium bromide fluorescent staining method. Results from the different pHs and experimental times, including those from different techniques measuring fungal viability, were compared using the chi-square and Fisher's exact tests (α=0.05). The yeasts became completely inviable after 48 h of contact with the calcium hydroxide solution. On the other hand, when exposed to the alkaline culture broth, the yeasts were found to be viable at pHs 9.5 and 10.5 for up to 7 days. In conclusion, C. albicans can only be completely inhibited by direct contact with saturated calcium hydroxide aqueous solution after 48 h of exposure.
Key Words:Candida albicans, oral cavity, calcium hydroxide, susceptibility.
RESUMO
Candida albicans está presente na cavidade oral e em todo trato digestivo de humanos e outros animais, podendo estar relacionada a fracassos do tratamento endodôntico. O presente trabalho determinou a incidência de C. albicans na cavidade oral e a susceptibilidade das amostras isoladas frente a diferentes valores de pH e à solução aquosa saturada de hidróxido de cálcio em pH 12,5. Sessenta e cinco pacientes atendidos na Clínica de Endodontia da Universidade Sagrado Coração participaram da pesquisa. As amostras coletadas foram cultivadas em meios seletivos para C. albicans. As cepas isoladas foram testadas quanto a resistência ao pH alcalino e à solução saturada de hidróxido de cálcio. Frente às variáveis de tempo, a viabilidade das leveduras foi avaliada pelos métodos da cultura sobre ágar Sabouraud e de fluorescência pela técnica do diacetato de fluoresceína e brometo de etídio. Foram isoladas 30 linhagens de C. albicans coletadas da cavidade oral dos pacientes. Quando em contato com a solução de hidróxido de cálcio as leveduras foram totalmente inviabilizadas após 48 h de exposição. Quando expostas ao caldo de cultura alcalinizado as leveduras mantiveram-se viáveis em pH 9,5 e 10,5 por até 7 dias. Em conclusão, C. albicans pode ser completamente inibida pelo contato direto com solução aquosa saturada de hidróxido de cálcio após 48 h de exposição.
INTRODUCTION
Candida albicans is a commensal yeast of the human microbiota, which lives in the oral cavity, gastrointestinal and genitourinary tracts and plays an important role as an opportunistic pathogen (1). C. albicans causes common infections, such as stomatitis and female genital infections, but severe systemic infections can occur in immunocompromised patients (1). In terms of virulence, C. albicans has some pathogenic mechanisms allowing pathologies to develop more frequently. The most studied virulence determinants include proteinase production, such as collagenase, aminopeptidases, alkaline phosphatase, hyaluronidase and chondroitin sulphatase, all exerting some effect on extracellular matrix protein degradation (2).
Factors related to adherence to host cells as well as capacity for morphogenesis and biofilm formation are also important determinants of virulence in the species (3). Several studies have revealed the presence of fungi in endodontic infections. Sen et al. (4) used scanning electronic microscopy (SEM) to observe bacteria and fungi in root canals and dentinal tubules of teeth with endodontic infection, revealing the presence of fungi in 4 out of 10 teeth. Yeasts were identified by microscopic exploration of single cells, either spherical or oval, measuring 4-6 mm in diameter.
Another SEM study (5) examined the external surface of the apical root cement of teeth with periapical lesion, and identified yeasts in 25% of the cases, with filamentous hyphae having an adherence pattern in the bone resorption. Waltimo et al. (6) detected the presence of yeasts in microbiological cultures of samples from persistent infections in root canals. A total of 967 samples were analyzed, with 692 (72%) being positive for fungi and bacteria. Yeasts were detected in 47 (7%) of the positive cultures. The authors confirmed the presence of C. albicans, C. glabrata, C. guilliermondiiand C. inconspicua, with a higher prevalence of C. albicans over the other yeasts (80%).
Sen et al. (7) have demonstrated the growth pattern of C. albicans on root dentin of teeth infected in vitro with the yeast. Blastospores and fungal hyphae were observed on the root canal walls in all slices analyzed. In another study (8), the microbiota of teeth with periapical lesion resistant to endodontic treatment was examined in 54 root-filled teeth having or not radiographically visible periapical lesion. Twenty-four samples had positive microbiological cultures, with C. albicans being identified in two cases. A study using polymerase chain reaction (9) demonstrated that C. albicans is involved in infections of endodontic origin. The authors evaluated 24 samples of root canals and 19 samples of endodontic abscesses, and found C. albicans in 21% of the root canal samples, whereas the yeast was not detected in the abscess samples.
Siqueira et al. (10) have also investigated the pattern of root dentin colonization by several yeast species. Bovine teeth were contaminated with C. albicans, C. glabrata, C. guilliermondii, C. parapsilosis and Saccharomyces cerevisiae, and after 14 days, the sections were analyzed by SEM. In this study, C.albicans exhibited different patterns of dentin infection. The microorganisms lodged in the dentinal tubules might not be affected by chemomechanical preparation, which involves both physical action of instruments and chemical action by irrigants. It is well known that C. albicans has the ability to invade dentinal tubules; once inside them, the yeast can be protected from the lethal action of chemical agents because the curative substances are inactivated by the buffering capacity of dentin (11).
In addition to the capacity of invasion, C. albicans has been found to be resistant to some intracanal medications after direct contact (12). Considering the increasing interest for the role of fungi in endodonticinfections, secondary or persistent ones, and endodontic treatment failure as well as the recent reports on fungal resistance against commonly used intracanal medications, this study evaluated the in vitro susceptibility of oral C. albicans samples to different pH values and to saturated calcium hydroxide aqueous solution.
MATERIAL AND METHODS
Sixty-five patients attending the Dentistry Clinic of the Sagrado Coração University took part in the study after approval of the research protocol by the local ethics committee (Process #127/09).
Collection of Yeast Strains
Samples from all patients were collected by rubbing a sterile tongue depressor on the posterior third of the tongue dorsum or whitened regions of the oral cavity, according to protocol established by Sharp et al. (13). The wooden spatula was rinsed in a flask containing 10 mL of sterile saline, which was hermetically sealed when turbidity was noticed. The samples were readily taken to the microbiology laboratory for immediate processing.
Sample Screening
A drop of saline was delivered between the slides for microscopic exploration at ×40 magnification to evaluate the presence of blastoconidia, hyphae or pseudo-hyphae.
Sample Culture for Yeast Isolation
The samples were seeded by drop inoculation on the surface of Sabouraud's agar dextrose plates (Merck KGaA, Darmstadt, Germany), added with 5% chloramphenicol (14). The plates were incubated in mycological culture at 37ºC for 24-48 h. Colonies with morphological aspects of yeast were submitted to direct examination for confirmation of yeast isolates.
Presumptive Identification of C. albicans
Once the yeast isolates were confirmed, the colonies were seeded on the surface of CHROMAgar Candida plates (Difco Laboratories Inc., Detroit, MI, USA) for presumptive identification of C. albicans isolates (15). The seeded plates were incubated at 30ºC for 48 h. In this medium, the presumptive identification of C. albicans is determined by the presence of light-green colored colonies (15). The positive growth control was performed by using ATCC 10231 strain.
Identification of C. albicans strains
The light-green colonies isolated on the CHROMAgar Candida plates were submitted to confirmatory identification of C. albicans through techniques of germinative tube production and chlamydoconidia formation (15). ATCC 10231 strain was used as the positive control of germinative tube production and chlamydoconidia production. Yeasts confirmed as C. albicans were stored in Sabouraud's agar dextrose and then frozen until the moment of susceptibility tests. From the 65 samples collected, 30 C. albicans strains were identified (46.15%).
Activation of Stored C. albicans strains
Stored C. albicans oral isolates and referencestrain ATCC 10231 were activated in Sabouraud's agar dextrose plates and then incubated in mycological culture at 37ºC for 24-48 h. From these plates, colonies were replicated in tubes containing 4 mL of Sabouraud's dextrose broth (Merck KGaA) until total turbidity was noticed.
C. albicans Susceptibility to Different pHs
Aliquots of 200 mL of Sabouraud's dextrose broth were distributed into Erlenmeyer flasks, with pH adjusted with 6 M sodium hydroxide (NaOH) by using a pH meter at the following values: 9.5, 10.5, 11.5, and 12.5. After precise alkalinization, the broth was sterilized by cold filtration by using Millipore filter with 0.22 mm pore size and a vacuum tube. Next, aliquots of 4.95 mL of each broth with adjusted pH were distributed into sterilised Bakelite tubes, totalizing 124 tubes. All tubes were incubated at 37ºC overnight for sterility control.
All samples mixed with Sabouraud's dextrose broth were standardized until optical cell density reached the McFarland scale of 0.5, that is, 1.5 x 108 colony-forming units (CFUs) mL-1 in PBS buffer (pH 7.4). Fifty microliters of each yeast suspension were added to 4.95 mL of each broth at different pH values, thus obtaining a final suspension of 1.5 x 106 CFU mL-1. The tubes were incubated at 37ºC for 6, 12, 24, 48, and 72 h and then for 7 days. C. albicans susceptibility to the different pHs was assessed at each experimental time by mycological culture method and the fluorescein diacetate and ethidium bromide (FDA-EB) fluorescent staining method under fluorescence microscopy. Ten tubes were used as positive controls, that is, containing Sabouraud's dextrose broth at pH 5.6 (final pH) inoculated with yeasts and 10 tubes as negative controls at pH 5.6 without inoculation.
In the mycological culture method, at each experimental time, the test tubes were seeded on Sabouraud's agar dextrose plates and incubated at 37ºC for 48 h. The presence or absence of growth on the culture medium were confirmed.
The test tubes were also subjected to assessment of yeast susceptibility by the FDA-EB fluorescent staining method (16). The FDA solution was prepared in acetone at 5 mg/mL concentration and kept at -20ºC, whereas the EB solution was prepared in buffered saline solution at the same concentration, but kept at 4ºC. The solutions were diluted at a 1:100 ratio in buffered saline solution for use.
For assessment, 0.1 mL of the test tube content was placed into a 0.5 mL Eppendorf tube and then mixed with equal volumes of FDA and EB solutions prepared shortly before use. The tube was homogenized and incubated at 37ºC for 30 min. The mixture was observed using a Neubauer counting chamber and fluorescence microscopy.
Yeast presenting green-fluorescence were considered viable, whereas the orange-colored ones were considered inviable. The percentage of viability was determined by counting 100 yeast cells. The strain ATCC 10231 was used for control.
C. albicans Susceptibility to Saturated Calcium Hydroxide Aqueous Solution
The saturated calcium hydroxide aqueous solution was prepared by dissolving the calcium hydroxide powder (J.T. Baker®) in sterile distilled water at room temperature until a liquid phase and a solid phase was obtained. The supernatant liquid phase was adjusted at pH 12.5. Aliquots of this supernatant were fractionated into 4.95 mL volumes in Bakelite tubes, totalizing 31 tubes. Fifty microliters of standardized yeast suspension were added to each tube test.
The tubes were inoculated at 37ºC for 6, 12, 24, 48, and 72 h, and finally for 7 days, with yeast susceptibility being assessed at each experimental time by using mycological culture method and the FDA-EB fluorescent staining method under fluorescence microscopy.
Statistical Analysis
The results obtained from the different variables of pH and experimental time, including those from different techniques measuring fungal viability, were compared using the chi-square and Fisher's exact tests.Significance level was set at 5%.
RESULTS
C. albicans was identified in the oral cavity of 30 (46.15%) of the 65 patients subjected to sample collection.
Regarding the efficacy of saturated calcium hydroxide solution at pH 12.5 against C. albicans strains isolated from the oral cavity and the ATCC strain at different experimental times by the mycological culture method, a decline was observed in candida growth at 6, 12, and 24 h, with full inhibition only at 48 h after incubation (Fig. 1). There was statistically significant difference (p<0.05) between 6 h and the other times.
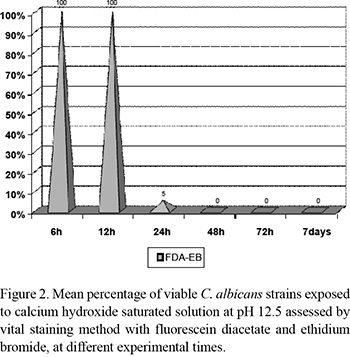
Using the vital staining method, it was observed that the strains were viable at 6 and 12 h after incubation, declining at 24 h and being completely inviable at 48 h (Fig. 2). Comparison between culture and staining methods revealed significant difference (p<0.05) at 6 and 12 h.
With regard to the efficacy of culture broth alkalinized with 6 M NaOH on the strains, which was assessed by the methods of mycological culture in Sabouraud agar and FDA-EB fluorescent staining, it was observed that pHs of 9.5 and 10.5 did not inhibit the strains at any experimental time. Culture recovery declined at pH 11.5 and was completely inhibited at pH 12.5 (Fig. 3). There was significant difference between pH 12.5 and other pH values.
The FDA-EB fluorescent staining method revealed strain viability at all experimental times for all pH values (Fig. 4), with no significant difference (p>0.05) among the pH values.
DISCUSSION
C. albicans is the most frequently isolated species in mouth and other anatomical sites of healthy individuals (17). In this study, this yeast was identifiedin 46.15% of the patients subjected to sample collection, corroborating the findings of other authors (17).
Several studies have demonstrated the presence of fungi, mainly yeast forms, in persistent periapical lesions (4-10) and primary root canal infections (18). Microbial control in the root canal system involves different steps during endodontic treatment. Chemomechanical preparation leads to a significant reduction of the microbial load (10), but the use of intracanal medication is necessary in case of persistent infections (19). These refractory microorganisms remain inside dentinal tubules and complex anatomical areas of difficult access to the action of instruments, irrigants and medications (19).
C. albicans has the ability to invade dentinal tubules (10) and remain protected from the action of alkaline endodontic medications because of the buffering effect of dentin (11).
The susceptibility of 30 oral C. albicans strains to saturated calcium hydroxide aqueous solution has been determined in the present study. After direct contact of the yeasts with the solution, the yeast viability was investigated by means of culture and vital staining methods. The mycological culture showed complete inhibition of yeasts only after 48 h of incubation, revealing high resistance of C. albicans strains to calcium hydroxide, as reported previously (12,13). On the other hand, the vital staining method revealed total viability at 6 and 12 h after incubation. Therefore, the C. albicans might be viable but not culturable because of the high pH resulting from the effect of calcium hydroxide on this yeast.
Microscopically, the yeasts had several morphological aspects. In addition to the blastospores, there was also formation of hyphae and pseudo-hyphae, including germinative tubes, probably due to the high calcium concentration. C. albicans produces the so-called self-regulating morphogenic substance which can regulate its growth in the form of hyphae and pseudo-hyphae (3).
C. albicans presents high levels of phospholipase and protease activities, all affecting the extracellular matrix degradation. This enzymatic activity might result in the release of mineral substances, such as calcium, allowing hyphae to penetrate into the dentinal tubules, as reported elsewhere (7).
The susceptibility pattern of microorganisms to calcium hydroxide and other substances used in Endodontics has been extensively studied. Antifungal action of 2% chlorhexidine, 6% sodium hypochlorite, hydrogen peroxide, and calcium hydroxide paste on ATCC strains of C. albicans has been demonstrated (20) despite the known resistance of these strains to saturated calcium hydroxide solution (20), which are also confirmatory of our findings.
Several studies (21) have shown the greater efficacy of calcium hydroxide pastes containing camphorated paramonochlorophenol. Nevertheless, inefficacy or reduced action when the paste is used without chlorhexidine has been reported (22).
A differential in the present study was the use of 30 C. albicans strains isolated from human oral cavity, whereas other studies used ATCC strains only (23,24). In fact, the C. albicans ATCC strains used in research studies can present different degrees of susceptibility to calcium hydroxide. Therefore, we believe that the results of the present study can reflect more consistently the resistance pattern of C. albicans to the stressing condition produced by saturated calcium hydroxide aqueous solution because different strains carry different patterns of genetically expressed virulence (2,3).
The strains used in the present study have been recently activated and, therefore, they were pure and in a phase of exponential growth, that is, they were not submitted to tests in the form of biofilm or polymicrobial cultures. A recent study showed that C. albicans resistance to saturated calcium hydroxide aqueous solution does not depend on the yeast being in planktonic growth or monomicrobial/polymicrobial biofilm (25).
It should be emphasized that the time required to eliminate C. albicans with calcium hydroxide in in vitro experiments cannot be directly extrapolated to clinical conditions because the microorganisms can be protected from the curative action of calcium hydroxide as a result of inactivation of medication by the dentin (11). Therefore, it seems clear that the C. albicans resistance to calcium hydroxide can be even higher in clinical situations.
With regard to the culture broths alkalinized with 6 M NaOH, used as comparative criterion for other pH values, it can noted that pHs 9.5 and 10.5 did not inhibit the strains at any experimental time. It was also observed that strains suffered some type of stressing condition at pH 11.5, with recovery decreasing as the exposure period increased. Total inhibition of C. albicans strains was observed only at pH 12.5, although the vital staining method showed that all strains were viable for all pH values, with no significant difference.
Within the limits of the methodology used in the present study, it may be concluded that C. albicans can only be completely inhibited by direct contact with saturated calcium hydroxide aqueous solution after 48 h of exposure.
Received November 29, 2011
Accepted March 5, 2012
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007;20:133-163.
- 2.Haynes K. Virulence in Candida species. Trends in Microbiology 2001;9:591-596.
- 3.Calderone RA, Fonzi WA. Virulence factors of Candida albicans Trends in Microbiology 2001;9:327-335.
- 4.Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol 1995;11:6-9.
- 5.Lomçali G, Sen BH, Cankaya H. Scanning electron microscopic observations of apical root surfaces of teeth with apical periodontitis. Endod Dent Traumatol 1996;12:70-76.
- 6.Waltimo TM, Sirén EK, Torkko HL, Olsen I, Haapasalo MP. Fungi in therapy-resistant apical periodontitis. Int Endod J 1997;30:96-101.
- 7.Sen BH, Safavi KE, Spangberg LSW. Growth patterns of Candida albicans in relation to radicular dentin. Oral Surg Oral Med Oral Pathol 1997;84:68-73.
- 8.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol 1998;85:86-93.
- 9.Baumgartner JC, Watts CM, Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod 2000;26:695-698.
- 10.Siqueira JF Jr, Rôças IN, Lopes HP, Elias CN, de Uzeda M. Fungal infection of the radicular dentin. J Endod 2002;28:770-773.
- 11.Haapasalo HK, Sirén EK, Waltimo TM, Ørstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J 2000;33:126-131.
- 12.Waltimo TM, Sirén EK, Orstavik D, Haapasalo MP. Susceptibility of oral Candida species to calcium hydroxide in vitro Int Endod J 1999;32:94-98.
- 13.Waltimo TM, Orstavik D, Sirén EK, Haapasalo MP. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J 1999;32:421-429.
- 14.Yücesoy M, Esen N, Yulug N. Use of chromogenic tube and methyl blue-Sabouraud agar for the identification of Candida albicans strains. Kobe J Med Sci 2001;47:161-167.
- 15.Robles MG, Ausejo FU, Cavero JC. Manual de procedimientos y técnicas de laboratório para la identificación de los principales hongos oportunistas causantes de micoses humanas. Lima: Ministério de Salud, Instituto Nacional de Salud, 2007. 100p.
- 16.Corrêa B, Purchio A, Paula CR, Gambale W. Evaluation of a fluorescent method in the study of the viability of Candida albicans strains. Rev Microbiol 1987;18:258-263.
- 17.Ribeiro PM, Bacal F, Koga-Ito CY, Junqueira JC, Jorge AO. Presence of Candida spp in the oral cavity of heart transplantation patients. J Appl Oral Sci 2011;19:6-10.
- 18.Miranda TT, Vianna CR, Rodrigues L, Monteiro AS, Rosa CA, Corrêa Jr A. Diversity and frequency of yeasts from the dorsum of the tongue and necrotic root canals associated with primary apical periodontitis. Int Endod J 2009;42:839-844.
- 19.Nair PNR, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molar with primary apical periodontitis after "one visit" endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231-252.
- 20.Fergunson JW, Hatton JF, Gillespie MJ. Effectiveness of intra-canal irrigants and medications against the yeast Candida albicans J Endod 2002;28:68-71.
- 21.Siqueira JF Jr., Rôças IN, Lopes HP, Magalhães FA, de Uzeda M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J Endod 2003;29:501-504.
- 22.Turk BT, Sen BH, Ozturk T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:297-301.
- 23.Gomes BP, Ferraz CC, Vianna ME, Rosalen PL, Zaia AA, Teixeira FB, et al.. In vitro antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz Dent J 2002;13:155-161.
- 24.Vianna ME, Gomes BP, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ. In vitro evaluation of the susceptibility of endodontic pathogens to calcium hydroxide combined with different vehicles. Braz Dent J 2005;16:175-180.
- 25.Brändle N, Zehnder M, Weiger R, Waltimo T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J Endod 2008;34:579-582.
Publication Dates
-
Publication in this collection
16 July 2012 -
Date of issue
2012
History
-
Received
29 Nov 2011 -
Accepted
05 Mar 2012