Abstracts
This study evaluated a whitening effect and the likely side effect (tooth sensitivity and pulp response) of human teeth subjected to different in-office bleaching (IOB) techniques and materials, mainly the presence of calcium in the IOB materials. A calcium-free (CF) and a calcium-containing (CC) 35% hydrogen peroxide (HP) gels were evaluated. The CF was refreshed every 15 minutes, three times (CF 3-15) or in a single 45-min application (CF 1-45) at one bleaching appointment. The CC was used only in a single 45-min application (CC 1-45). Each technique was applied in 5 mandibular incisors scheduled for extraction for different patients. In control group, no tooth bleaching was performed. The tooth colour (TC) and tooth sensitivity (TS) were recorded at baseline and after IOB. The teeth were extracted 2 days after the application of IOB and subjected to histological analysis. The data was submitted to appropriate statistical analysis (α=0.05). The changes of TC were similar between groups and statistically different from the control (p<0.05). However, TS of groups bleached with CF was statistically higher than that recorded for CC and the control (p<0.05). In CF 3-15 and CF 1-45 groups, the coronal pulp tissue exhibited partial necrosis associated with tertiary dentin deposition. In CC 1-45 group smaller area of necrosis occurred only in three bleached teeth in which tertiary dentin deposition was observed. The calcium-containing 35%HP gel could be preferable for in-office bleaching because it caused less tooth sensibility and pulp damage.
Tooth bleaching; hydrogen peroxide; materials testing; metabolism
O objetivo do estudo foi avaliar o efeito clareador e seus efeitos adversos (sensibilidade e resposta pulpar) de dentes humanos submetidos a diferentes técnicas e materiais para o clareamento em consultório (CLCO), principalmente a presença de cálcio nos materiais para CLCO. Um agente clareador a base de peróxido de hidrogênio (PH) a 35% sem cálcio (SC) e com cálcio (CC) foram avaliados. O agente clareador SC foi usado em duas técnicas de aplicação: o gel clareador foi reaplicado a cada 15 minutos, três vezes (SC 3-15) ou 1 x 45-min por aplicação (SC 1-45) em uma sessão clínica. O agente clareador CC foi usado apenas em 1 x 45-min por aplicação (CC 1-45). Cada técnica foi aplicada em 5 incisivos inferiores indicados para extração de pacientes diferentes. No grupo controle, o clareamento não foi realizado. O efeito clareador (EC) e a sensibilidade dental (SD) foram registrados inicialmente e após o CLCO. Os dentes foram extraídos após 2 dias da aplicação do CLCO e foram submetidos ao análise histológica. Os dados foram submetidos a análise estatística apropriada (α=0.05). As mudanças foram semelhantes entre os grupos e significativamente diferentes do controle (p<0.05). Entretanto, a SD nos grupos clareados com SC foi estatisticamente maior do que a registrada nos grupos CC e do grupo controle (p<0.05). Nos grupos SC 3-15 e SC CF 1-45, o tecido pulpar da região coronária exibia necrose parcial associada a deposição de dentina terciária. No grupo CC 1-45, pequenas áreas de necrose ocorreram somente em 3 dentes clareados, nos quais deposição de dentina terciária também foi observada. O gel CC de HP a 35%HP gel poderia ser preferível para a realização de CLCO devido ao fato de causar menos danos ao tecido pulpar
Introduction
Although clinical studies have shown that in-office bleaching can reach as satisfactory degree of whitening as the at-home bleaching as long as the materials are used for appropriate time (1)1. Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent 2005;26:11-20. in-office tooth bleaching shows a high risk of tooth sensitivity (TS). Approximately 55 to 100% of the patients experience bleaching-induced TS with a mild to severe intensity (22. Tay LY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc 2009;140:1245-1251. , 33. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596.).
The literature believes that the damage caused by hydrogen peroxide (HP) diffusion into the pulp during tooth bleaching ( 44. Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res 1993;72:931-38. , 55. Sulieman M, Addy M, MacDonald E, Rees JS. The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent 2004;32:295-299. ) is responsible for the TS (6)6. Markowitz K. Pretty painful: why does tooth bleaching hurt? Med Hypotheses 2010;74:835-40.. Within 5 to 15 min after application of bleaching gels, HP can be found in the pulp tissue, where it irritates nerves and produces a reversible pulpitis for at-home bleaching gels (7)7. Anderson DG, Chiego Jr DJ, Glickman GN, McCauley LK. A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod 1999;25:247-250.. However, it seems that more severe effects occur, when a higher number of bleaching sessions were performed or when pulps of lower central incisors after application of a 38% HP in-office bleaching gel ( 88. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:59-64. , 99. Roderjan DA, Stanislawczuk R, Hebling J, Souza Costa CA, Soares DB, Reis A, et al.. Histopathological features of dental pulp tissue from bleached mandibular incisors. J Mater Sci Engin B.2014;4:178-185. ). In addition, several current in vitro studies have demonstrated the occurrence of transenamel and transdentinal damage to cultured pulp cells after in-office HP application on enamel (1010. Coldebella CR, Ribeiro AP, Sacono NT, Trindade FZ, Hebling J, Costa CA. Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz Dent J. 2009;20:267-274. 1111. Soares DG, Basso FG, Hebling J, de Souza Costa CA. Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent. 2014;42:185-198. , 1212. Soares DG, Basso FG, Pontes EC, Garcia Lda F, Hebling J, de Souza Costa CA. Effective tooth-bleaching protocols capable of reducing H(2)O(2) diffusion through enamel and dentine. J Dent. 2014;42:351-358. , 1313. Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig 2015;19:673-680.).
Although the concentration of HP in in-office bleaching gels is similar among different brands, products differ one another as other components and additives are included in their formulation. For instance, there are products formulated with different desensitizing agents (1)1. Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent 2005;26:11-20.. Besides that, the pH of these products varies among product brands (14)14. Freire A, Archegas LR, de Souza EM, Vieira S. Effect of storage temperature on pH of in-office and at-home dental bleaching agentsActa Odontol Latinoam 2009;22:27-31. Non-study has so far addressed the effects of these variations on the pulp response of human teeth.
Variations in the product application may also have an impact on TS. In-office bleaching gels are usually applied on tooth surfaces, left undisturbed for a while (usually 15 min) and then the product is refreshed 3 to 4 times in each clinical appointment, depending on the bleaching gel brand. In an attempt to reduce in office-time and the costs associated with in-office bleaching gels, three studies evaluated the bleaching effectiveness and TS of a single 45-min application ( 33. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596. , 1313. Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig 2015;19:673-680. , 1515. Kossatz S, Martins GC, Loguercio AD, Reis A. Tooth sensitivity and bleaching effectiveness of a calcium-containing in-office bleaching gel. J Am Dent Assoc 2012;143:e81-e90. ). Since the diffusion of HP through dentin depends on the original concentration of this chemical component in the bleaching gel, the number of product applications and the length of time the gel is maintained in contact with the dentin ( 44. Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res 1993;72:931-38. , 55. Sulieman M, Addy M, MacDonald E, Rees JS. The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent 2004;32:295-299. ) this single-product application might reduce the amount of HP that reaches the pulp chamber and may also cause less damage to pulp cells than three 15-min applications, as well as, recently showed by Cintra et al. (16)16. Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E, et al.. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 2013;39:1576-1580. in dental pulps of rat incisors. However, to the extent of the author´s knowledge the effect of this technique on pulp tissue has not been addressed yet in human pulps. Therefore, the aim of this study was to evaluate the response of human pulps of mandibular anterior sound incisors subjected to in-office bleaching with different products and techniques.
Material and Methods
This clinical investigation was approved under protocol number 09171/10 by the Ethics Committee from the local University. Based on pre-established criteria, 17 participants with mandibular incisors scheduled to be extracted strictly indicated for orthodontic reasons were selected for this study.
All the participants had anterior teeth with initial shade A2 or darker as judged by comparison with a value-oriented shade guide (Vita Lumin; Vita Zahnfabrik, Bad Säckingen, Germany). Two weeks before the bleaching procedures, all the subjects received a dental screening and tooth prophylaxis. The diagnosis of pulp sensitivity was evaluated by a cold test application (Roeko-Endo-Frost; Coltène/Whaledent, Langenau, Germany). After 10 s of application, all patients should answer positively to this test indicating a few and not lingering pain response. After reading and receiving all necessary explanations including the experimental rationale, clinical procedures and possible risks, the selected patients signed a consent form explaining the research protocol, which was previously approved by the Ethics Committee.
Inclusion and exclusion criteria. Participants included in this clinical study had good general and oral health. The participants should present at least one caries-free mandibular incisor without restorations on the labial surface with shade A2 or darker in need of extraction due to orthodontic reasons. Participants that had undergone tooth-whitening procedures, presenting anterior restorations, pregnant/lactating, with severe internal tooth discoloration (tetracycline stains, fluorosis, pulpless teeth), bruxism habits or any gross pathology in the mouth were excluded from the study. Participants using any continuous drug with anti-inflammatory and antioxidant action were also excluded. The participants were asked about the previous TS the week before the beginning of the bleaching therapy and those who reported any degree of spontaneous TS were not included in the study sample.
Study design. Participants were randomly divided into four groups according to control or the in-office bleaching therapies. After the prophylaxis procedures, the gingival tissue of the teeth to be bleached was isolated with a light-cured gingival barrier (Top Dam, FGM Dental Products, Joinville, SC, Brazil). A calcium-free and a calcium containing (Whiteness HP Maxx [CF], Whiteness HP Blue 35 [CC], FGM) 35% HP gels were applied on the buccal surface of the incisors according to the instructions briefly described following: for the groups CF (3-15) and (CF 1-45), three drops of phase 1 (hydrogen peroxide) was mixed with one drop of phase 2 (thickener) and a thin layer of gel was applied. The bleaching gel was left on the teeth surface for 15 min (CF 3-15) or 45 min (CF 1-45). For the CC 1-45, two syringes (hydrogen peroxide and thickener) were connected and mixed for several times. Following, the bleaching gel was left on the teeth surface for 45 min. At the end, for all groups, the bleaching gel was removed with an aspirator. All participants were instructed to brush their teeth regularly using a fluoridated toothpaste (Sorriso Fresh; Colgate-Palmolive, São Paulo, SP, Brazil).
Shade evaluation. The color was registered at baseline and two days after the bleaching session using a value-oriented shade guide (Vita Lumin, Vita Zahnfabrik). The shade guide's 16 tabs were arranged from highest (B1) to lowest (C4) value (B1*A1*B2*D2*A2*C1*C2*D4*A3*D3*B3* A3.5*B4*C3*A4*C4). Although this scale is not linear in the truest sense, we treated the changes as representing a continuous and approximately linear ranking for the purpose of analysis. The shade changes from the start of the active phase to the individual recall times were calculated by calculating the change in the number of shade guide units (Δ SGU) that occurred toward the lighter end of the value-oriented list of shade tabs. Only one calibrated and experienced evaluator recorded the shade of each subject's teeth at baseline and two days after the bleaching procedure. The measurement area of interest for shade matching was the middle third of the facial surface of teeth.
Tooth sensitivity evaluation. Two days after the procedure, the participants were asked if they had experienced any TS in the bleached tooth. No attempt was made to record the TS intensity but only if the participants experienced or not any degree of TS.
Clinical/Laboratorial procedures and Histological evaluation. This protocol followed a modified description of Costa et al. (8)8. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:59-64.. Two days after the bleaching procedure, the teeth were extracted under local anesthesia. The roots were immediately sectioned midway between the cement/enamel junction (CEJ) and the root tip with a high-speed handpiece under water spray. The teeth were stored for 48 h in formalin fixative solution at pH 7.2, decalcified in buffered Morse's solution, dehydrated, vacuum-infiltrated with wax paraffin and finally embedded in paraffin. Six-µm thick serial sections were cut ("820" Spencer Microtome, Carson, CA, USA), mounted on glass slides and stained with hematoxylin and eosin (H/E) and Masson Trichrome. Then, based upon previous in vivo studies (8), all sections obtained from those 17 teeth were submitted to evaluation by a calibrated examiner blinded to the groups. Using a light microscope (62774; Carl Zeiss, Oberköchen, Germany) the histopathologic events were assessed and classified in scores (0 to 3) for following items: inflammatory cell response, tissue disorganization and reactionary dentin formation (8).
Statistical analysis. The overall median of D SGU was calculated and compared with the non-parametric Kruskall-Wallis test and Mann-Whitney U-test. The absolute risk of TS among groups was evaluated by Fisher's exact test. Regarding the pulp response, three established histopathological events were evaluated (8)8. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:59-64. in such way that 3 scores per tooth were considered for statistical analysis. The overall comparison of the groups was performed with the non-parametric Kruskal-Wallis statistical analysis. The comparisons between groups were performed using the Mann-Whitney U-test. All tests were performed with appropriately computed critical values (α=0.05) in the MedCalc software (MedCalc Version 11.3.8.0, 2010, Mariakerke, Belgium).
Results
The age of the participants included in this study varied between 12 and 26 years old (mean 18.2 ± 4.9). The means and standard deviations of color measurement are shown in Table 1. The D SGU of all bleaching techniques were statistically similar (p>0.58) and different to the non-bleached control group (p≤0.003).
A statistically higher absolute risk of TS was observed for the groups bleached with CF, regardless of the application mode (Table 1). The CC 1-45 group was statistically different from the others experimental groups (p=0.04) and similar to the control group (p=0.76).
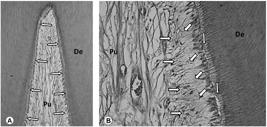
The scores for every criterion determined by the histological assessment of the specimens according to groups are shown in Table 2. Overall, the histological features showed that the pulp tissue observed in control group was quite different from the experimental groups in which the teeth were bleached (p=0.0003). Teeth from the control group showed pulp tissue with normal histological features, which were characterized by the presence of continuous odontoblast layer as well as defined cell-free zone and cell-rich zone. No inflammatory pulp reaction or tissue disorganization was observed (Figs. 1A and 1B).
Intact control. A: General view of the coronary pulp chamber (Pu). A thin layer of predentin is present between the continuous odontoblast layer (arrows) and the tubular dentin (De). H/E, 64×. B: Detail of figure 1A. Note the tubular dentin (De), predentin (vertical arrow) underlined by a continuous odontoblast layer, the cell-free zone (oblique arrows), cell-rich zone (horizontal arrows) and the central zone of the pulp tissue (Pu) with normal histological characteristics. H/E, 250×.
Four and five human incisors bleached with CF 3-15 and CF 1-45, respectively, exhibited large zone of coagulation necrosis in the coronary pulp tissue associated to deposition of tertiary dentin (Figs. 2A-D). In one and two of those five teeth bleached with CF 3-15 and CF 1-45 respectively, the reparative dentin adjacent to the necrotic tissue and the reactionary dentin observed in the lateral walls of the coronary pulp chamber was continuous to that deposited in the root pulp chamber (Figs. 2A-D). In nine of those ten teeth bleached with a gel CF, a mild inflammatory response mediated by mononuclear cells among dilated and congested blood vessels was observed (Figure 2A to D).
CF 3-15. A: Note the large necrosis (Ne) in the coronary pulp chamber. H/E, 32×. B - Subjacent to the necrotic zone heterogeneous reparative dentin is observed (arrows). H/E, 64×. C: Detail of figure 2B. Note the reparative dentin (vertical arrows) as well as the layer of reactionary dentin deposited in the lateral walls of pulp chamber (horizontal arrows). H/E, 125×. D: Detail of figure 2C. The pulp tissue (Pu) exhibits discrete inflammatory response mediated by mononuclear cells among a number of congested blood vessels (arrows). H/E, 250×. (De = dentin). As all histological features were very similar among CF 3-15 and CF 1-45, Only figures related to CF 3-15 were published.
In four out of those 5 teeth bleached with CC 1-45, the mild inflammatory pulp response was mediated by mononuclear cells. In this CC 1-45 group, two teeth did not present necrosis; however, a moderate deposition of reactionary dentin which was underlined by discontinuous odontoblast layer was observed around the coronary and root pulp tissue and in the upper part of the pulpal horn (Figs. 3A-D).
CC 1-45. A: General view of the coronary pulp tissue. Note the deposition of reactionary dentin mainly in the tip of pulp horn (arrows). Masson's Trichrome, 32×. B: Detail of figure 4A. Note the tubular reactionary dentin (RD) deposited by the odontoblasts (arrows). Masson's Trichrome, 64×. C: Detail of Figure 4A. A thin layer of reactionary dentin is also observed in the lateral walls of the pulp chamber (arrows). Masson's Trichrome, 125×. D: Root pulp tissue. Note the continuous odontoblast layer (horizontal arrows) underlying the tubular reactionary dentin (vertical arrows). The central part of the root pulp tissue exhibits dense collagen fibers and fibroblasts parallel to blood vessels. Masson's trichrome, 250×. (De = dentin).
Discussion
Although the main goal of this study was to measure the human pulpal responses to different bleaching techniques, the bleaching efficacy and TS was also assessed to verify if the bleaching agent was effective in producing a whitening effect and the likely side effect of the protocols evaluated.
One randomized clinical trial compared the bleaching efficacy (BE) and TS of a three 15-min application vs. a single 15-min application of a 35% HP (3)3. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596.. This study showed that a single 45-min application were effective in terms of bleaching, however with a slightly lower bleaching speed and a slightly higher TS ( 33. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596. ). Although in the present study no significant differences in terms of color change and TS was detected for the two application modes, this study was not planned for the analysis of clinical findings but for the histopathological investigation of human dental pulps. This topic is controversial and more research need to be done to clarify this finding. However, the BE and TS in the present study was only performed in one session and this was different in comparison with previous study ( 33. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596. ).
The histological response of the calcium-free bleaching gel (CF) submitted to both application modes showed sites of pulp necrosis and the presence of a mild inflammatory response mediated by mononuclear cells and congested blood vessels as already demonstrated in an earlier study (8)8. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:59-64.. It has been demonstrated HP and the products of its degradation, such as hydroxyl (OH-) ions may act as free radicals to cause oxidative stress in the pulp cells (17)17. Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod 2004;30:45-50.. The increase of reactive oxygen specimen levels cause deleterious effects to several cell components, like mutagenesis, carcinogenesis, cell membrane damage by peroxidation lipid, and protein fragmentation (18)18. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 2002;192:1-15., which may reduce cell proliferation and result in cell necrosis or apoptosis (1010. Coldebella CR, Ribeiro AP, Sacono NT, Trindade FZ, Hebling J, Costa CA. Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz Dent J. 2009;20:267-274. , 1919. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med 2000;28:463-499.).
Contrary to our previous expectation, no significant difference was observed between the two modes of HP application and thus the histopatological signs of dental pulps do not explain the slightly higher TS of the single 45-min application mode reported in earlier clinical trial (3)3. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596.. Although the damage caused by pulp cells were similar it is still unknown if the expression of inflammatory mediators that leads to pain and symptoms of neurogenic inflammation such as bradykinin (20)20. Lepinski AM, Hargreaves KM, Goodis HE, Bowles WR. Bradykinin levels in dental pulp by microdialysis. J Endod 2000; 26: 744-7. and the substance-P (21)21. Caviedes-Bucheli J, Ariza-Garcia G, Restrepo-Mendez S, Ríos-Osorio N, Lombana N, Muñoz HR. The effect of tooth bleaching on substance P expression in human dental pulp. J Endod 2008;34:1462-1465. have different expressions under these two application modes. Therefore, imunohistochemical studies should be further conducted in order to investigate the expression of inflammatory mediators in pulp tissues of teeth submitted to different bleaching protocols.
When it comes to the comparison of the different bleaching products, no statistical difference between the CF and CC products were observed, which seems that the HP concentration, which was the same in all experimental groups, may play the most important role on the damage caused to the human pulp tissue. However, it is worth mentioning that the scores employed for categorization of the histopatological events were not discriminative enough to detect some subtle differences that could be observed during the histopatological analysis what resulted in statistically similar results. Some important differences could be seen in the pulps of teeth bleached with CC product. For instance, 60% of the teeth bleached with CC 1-45 exhibited signs of pulp necrosis, while 90% of the teeth bleached with CF showed pulp necrosis. The features of these necrosis sites were also different. While in the former only narrow and superficial areas of necrosis were observed, larger areas of necrosis involving the whole pulp horns were seen for the latter product. This might indicate that some features of the CC gel might have prevented more detrimental effects on the pulp tissue.
One important difference between the CC and CF products is that the former contains 2% calcium gluconate that was added to the bleaching gel with the aim to prevent enamel demineralization and manufacturer´s claims that the addition of this product might have played a role on the lower tooth sensitivity reported by the calcium-containing products. Perhaps, the 2% of calcium gluconate dissolved into the HP gel was able to decrease dentin permeability and block enamel surface defects, similarly to what is believed to occur clinically (15)15. Kossatz S, Martins GC, Loguercio AD, Reis A. Tooth sensitivity and bleaching effectiveness of a calcium-containing in-office bleaching gel. J Am Dent Assoc 2012;143:e81-e90.. This might reduce the rate of HP penetration in the pulp tissue as well as provide more time for the pulp cells to produce enough peroxidades, catalases (22)22. Bowles WH, Burns Jr H Catalase/peroxidase activity in dental pulp. J Endod 1992;18:527-34. and oxygenases (7)7. Anderson DG, Chiego Jr DJ, Glickman GN, McCauley LK. A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod 1999;25:247-250. to protect the connective pulp tissue from the immediate damage caused by HP.
Another important difference is that CC product keeps the pH high and stable (pH = 8.0 - 9.0) throughout the bleaching procedure, which allows them to be used in a single 45-minute application protocol. Most of the in-office bleaching gels are delivered in low pH (2.4 to 6.5) (14)14. Freire A, Archegas LR, de Souza EM, Vieira S. Effect of storage temperature on pH of in-office and at-home dental bleaching agentsActa Odontol Latinoam 2009;22:27-31 to allow them to be stored for prolonged periods (23)23. Chen JH, Xu JW, Shing CX. Decomposition rate of hydrogen peroxide bleaching agents under various chemical and physical conditions J Prosthet Dent 1993;69:46-48.. The CF gel has a pH of 6-7 immediately after mixing and may reach a pH of 5 after 45 min in contact with the dental structure (24)24. Marson FC, Sensi LG, Reis R. New concept for the in-office bleaching technique. Rev Dent Press Estet 2008;5:55-66..
The decomposition kinetics and the by-products produced by HP depend on the pH of the media, which may play a role on the prevalence and intensity of TS. In an acidic solution, higher concentrations of hydroxyl anions (OH-) are produced; however in an alkaline media there is a higher concentration of perhydroxyl ions (HOO-) (25)25. Sun G. The role of lasers in cosmetic dentistry Dent Clin North Am 2000;44:831-50.. These differences may explain the lower pulpal damage caused by CC 1-45 and the lower TS rates reported in a clinical trial for this product (13)13. Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig 2015;19:673-680.. This also may explain why a single 45-min application of CF product led to higher TS rates in earlier clinical trial (3)3. Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596..
From a clinical standpoint, the results of this study should be interpreted with caution. Whether or not the pulp damage caused by higher concentration of HP is reversible is yet to be addressed. Besides that, to the extent of the author´s knowledge, no clinical study has reported pulp death after in-office bleaching and thus further studies evaluating pulp vitality at long-term after in-office bleaching should be conducted. In addition, future studies should be encouraged in order to elucidate if calcium-containing products, as employed in this study, but in lower HP concentrations produce the same damage to the pulp tissue.
One limitation of this in vivo study is that the participants were young. It is known that teeth from young patients present wider dentinal tubules and thinner dentin layer as none of little secondary dentin has been deposited. Therefore, it is expected that higher amount of HP or other reactive oxygen species may diffuse faster through enamel and dentin to cause damage to the pulp tissue. In this way, a recently paper published by Roderjan et al. (9)9. Roderjan DA, Stanislawczuk R, Hebling J, Souza Costa CA, Soares DB, Reis A, et al.. Histopathological features of dental pulp tissue from bleached mandibular incisors. J Mater Sci Engin B.2014;4:178-185. showed that, regardless of the age of patients, the application of a 35%-HP bleaching gel to mandibular incisors causes pulp damage; however, superficial necrosis was more prevalent in young teeth
Based on the results of this clinical study, it can be concluded that the calcium-free (Whiteness HP Maxx) 35% HP gel applied on enamel surface of lower human incisors cause intense pulp damage regardless of the mode of application. Lower pulp damage was produced by the calcium-containing (Whiteness HP Blue) 35% HP gel that also caused less tooth sensitivity than that observed for calcium-free 35% HP gel.
Acknowledgements
This study was performed by Douglas Augusto Roderjan as partial fulfillment of his PhD's degree at the State University of Ponta Grossa (UEPG), Ponta Grossa, PR, Brazil. The authors would like to thanks FGM Dental Products for the generous donation of all products used in this study. This study was partially supported by National Council for Scientific and Technological Development under grants 301937/2009-5; 301891/2010-9; and 303599/2014-6.
-
1Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent 2005;26:11-20.
-
2Tay LY, Kose C, Loguercio AD, Reis A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J Am Dent Assoc 2009;140:1245-1251.
-
3Reis A, Tay LY, Herrera DR, Kossatz S,. Loguercio AD Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 2011;36:590-596.
-
4Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res 1993;72:931-38.
-
5Sulieman M, Addy M, MacDonald E, Rees JS. The effect of hydrogen peroxide concentration on the outcome of tooth whitening: an in vitro study. J Dent 2004;32:295-299.
-
6Markowitz K. Pretty painful: why does tooth bleaching hurt? Med Hypotheses 2010;74:835-40.
-
7Anderson DG, Chiego Jr DJ, Glickman GN, McCauley LK. A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod 1999;25:247-250.
-
8Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:59-64.
-
9Roderjan DA, Stanislawczuk R, Hebling J, Souza Costa CA, Soares DB, Reis A, et al.. Histopathological features of dental pulp tissue from bleached mandibular incisors. J Mater Sci Engin B.2014;4:178-185.
-
10Coldebella CR, Ribeiro AP, Sacono NT, Trindade FZ, Hebling J, Costa CA. Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz Dent J. 2009;20:267-274.
-
11Soares DG, Basso FG, Hebling J, de Souza Costa CA. Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent. 2014;42:185-198.
-
12Soares DG, Basso FG, Pontes EC, Garcia Lda F, Hebling J, de Souza Costa CA. Effective tooth-bleaching protocols capable of reducing H(2)O(2) diffusion through enamel and dentine. J Dent. 2014;42:351-358.
-
13Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig 2015;19:673-680.
-
14Freire A, Archegas LR, de Souza EM, Vieira S. Effect of storage temperature on pH of in-office and at-home dental bleaching agentsActa Odontol Latinoam 2009;22:27-31
-
15Kossatz S, Martins GC, Loguercio AD, Reis A. Tooth sensitivity and bleaching effectiveness of a calcium-containing in-office bleaching gel. J Am Dent Assoc 2012;143:e81-e90.
-
16Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E, et al.. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 2013;39:1576-1580.
-
17Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod 2004;30:45-50.
-
18Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 2002;192:1-15.
-
19Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med 2000;28:463-499.
-
20Lepinski AM, Hargreaves KM, Goodis HE, Bowles WR. Bradykinin levels in dental pulp by microdialysis. J Endod 2000; 26: 744-7.
-
21Caviedes-Bucheli J, Ariza-Garcia G, Restrepo-Mendez S, Ríos-Osorio N, Lombana N, Muñoz HR. The effect of tooth bleaching on substance P expression in human dental pulp. J Endod 2008;34:1462-1465.
-
22Bowles WH, Burns Jr H Catalase/peroxidase activity in dental pulp. J Endod 1992;18:527-34.
-
23Chen JH, Xu JW, Shing CX. Decomposition rate of hydrogen peroxide bleaching agents under various chemical and physical conditions J Prosthet Dent 1993;69:46-48.
-
24Marson FC, Sensi LG, Reis R. New concept for the in-office bleaching technique. Rev Dent Press Estet 2008;5:55-66.
-
25Sun G. The role of lasers in cosmetic dentistry Dent Clin North Am 2000;44:831-50.
Publication Dates
-
Publication in this collection
May-Jun 2015
History
-
Received
26 June 2014 -
Accepted
22 Sept 2015