Abstract
Low-fluoride (F) dentifrices (<600 µg F/g) are widely available worldwide, but evidence to recommend the use of such dentifrices, with either regular or improved formulations, is still lacking. Therefore, the aim of this study was to evaluate the anticaries potential of low-F dentifrices found in the Brazilian market, using a validated and tested pH-cycling model. Enamel blocks were selected by surface hardness (SH) and randomized into four treatment groups (n=12): non-F dentifrice (negative control), low-F dentifrice (500 μg F/g), low-F acidulated dentifrice (550 μg F/g) and 1,100 μg F/g dentifrice (positive control). The blocks were subjected to pH-cycling regimen for 8 days and were treated 2x/day with dentifrice slurries prepared in water (1:3, w/v). The pH of the slurries was checked, and only the acidulated one had low pH. After the pH cycling, SH was again determined and the percentage of surface hardness loss was calculated as indicator of demineralization. Loosely- and firmly-bound F concentrations in enamel were also determined. The 1,100 μg F/g dentifrice was more effective than the low-F ones to reduce enamel demineralization and was the only one that differed from the non-F (p<0.05). All F dentifrices formed higher concentration of loosely-bound F on enamel than the non-F (p<0.05), but the 1,100 μg F/g was the only one that differed from the non-F in the ability to form firmly-bound F. The findings suggest that the low-F dentifrices available in the Brazilian market, irrespective of their formulation, do not have anticaries potential.
Key Words:
fluorides; toothpaste; dental caries; demineralization
Resumo
Dentifrícios de baixa concentração de fluoreto (F) (< 600 µg F/g) estão amplamente disponíveis no mundo, mas ainda não há evidência para recomendar seu uso, quer seja em formulações regulares ou melhoradas. Assim, o objetivo deste estudo foi avaliar o potencial anticárie de dentifrícios de baixa concentração de fluoreto encontrados no mercado brasileiro, utilizando um modelo de ciclagens de pH validado e testado. Blocos de esmalte bovinos foram selecionados pela dureza de superfície e randomizados em quatro grupos (n=12): dentifrício sem fluoreto (controle negativo), dentifrício de baixa concentração de fluoreto (500 μg F/g), dentifrício acidulado de baixa concentração de fluoreto (550 μg F/g) e dentifrício de 1100 μg F/g (controle positivo). Os blocos foram submetidos ao regime de ciclagem de pH por 8 dias e tratados 2 x/dia com suspensões aquosas dos dentifrícios (1:3 p/v). O pH das suspensões foi checado, e apenas o acidulado tinha baixo pH. Após a ciclagem de pH, a dureza de superfície foi novamente determinada e a porcentagem de perda de dureza foi calculada como indicador de demineralização. As concentrações de fluoreto fracamente e firmemente ligado ao esmalte também foram determinadas. O dentifrício de 1.100 μg F/g foi mais efetivo do que os de baixa concentração na redução da desmineralização do esmalte e foi o único que diferiu significativamente do não fluoretado (p<0,05). Todos os dentifrícios fluoretados foram capazes de formar maiores concentrações de fluoreto fracamente ligado ao esmalte do que o não fluoretado (p<0,05), mas o de 1.100 μg F/g foi o único que diferiu do não fluoretado na capacidade de formar fluoreto firmemente ligado ao esmalte. Os resultados sugerem que dentifrícios de baixa concentração de fluoreto disponíveis no mercado brasileiro, independentemente da formulação, não têm potencial anticárie.
Introduction
The importance of fluoride (F) dentifrices to the decline of dental caries that occurred in the last decades is clearly established 11. Bratthall, D; Hänsel-Petersson, G; Sundberg H. Reasons for the caries decline: what do the experts believe? Eur J Oral Sci 1996;104:416-422.,22. Cury, JA; Tenuta, LMA; Ribeiro, CCC; Paes Leme AF. The importance of fluoride dentifrices to the current dental caries prevalence in Brazil. Braz Dent J 2004;15:167-174.. On the other hand, their use has been pointed out as a risk factor for dental fluorosis 33. Mascarenhas AK. Risk factors for dental fluorosis: a review of the recent literature. Pediatr Dent 2000;22:269-277.. As an alternative to reduce the risk of fluorosis from dentifrices, low-F formulations (< 600 μg F/g) have been suggested. However, considering the best evidence available, they are not effective to satisfy the binomial anticaries effect by the use of F dentifrice with a lower risk of fluorosis 44. Santos, APP; Oliveira, BH; Nadanovsky P. Effects of low and standard fluoride toothpastes on caries and fluorosis: systematic review and meta-analysis. Caries Res 2013;47:382-390.. Moreover, systematic reviews focusing on caries prevention in permanent and deciduous teeth 55. Walsh, T; Worthington , HV; Glenny, AM; Appelbe, P; Marinho, VCC; Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010 (1): CD 007868.,66. Santos, APP; Nadanovsky, P; Oliveira BH. A systematic review and meta-analysis of the effects of fluoride toothpastes on the prevention of dental caries in the primary dentition of preschool children. Community Dent Oral Epidemiol 2013;41:1-12. showed that a dentifrice should have at least 1,000 μg F/g to provide significant anticaries effect.
Nevertheless, low-F dentifrices are available in many countries. Although in the United States they cannot be sold, because the American legislation 77. US Food and Drug Administration. CFR - Code of Federal Regulations. Title 21: Food and drugs. Washington (DC); 2015 [Apr 1]. Chapter I, Food and Drug Administration Department of Health and Human Services, Subchapter D, Drugs from human use (part 355): anticaries drugs products for over the counter human use. Available from: Available from: http://www.gpo.gov/fdsys/pkg/CFR-2015-title21-vol5/pdf/CFR-2015-title21-vol5-part355.pdf
2015
http://www.gpo.gov/fdsys/pkg/CFR-2015-ti...
requires a minimum amount of soluble F of at least 650 ppm F for sodium fluoride (NaF) or 800 ppm F for monofluorophosphate (MFP) formulations, the European 88. European Union. Statutory Instruments. Consumer Protection: The Cosmetic Products (Safety) Regulations 2008: nº 1284. London: Stationery Office; 2008 [cited 2015 Apr 30]. Available from: Available from: http://www.legislation.gov.uk/uksi/2008/1284/pdfs/uksi_20081284_en.pdf
http://www.legislation.gov.uk/uksi/2008/...
and Mercosur 99. MERCOSUL. MERCOSUL/GMC/RES nº 48/02. Regulamento técnico MERCOSUL sobre lista de substâncias que os produtos de higiene pessoal, cosméticos e perfumes não devem conter, exceto nas condições e com as restrições estabelecidas. Brasília (DF): Sistema de Informação do Comércio Exterior; 2002 {cited 2015 Apr 30}. Available from:Available from:http://www.sice.oas.org/trade/mrcsrs/resolutions/res4802p.asp
http://www.sice.oas.org/trade/mrcsrs/res...
regulations only state the maximum F concentration that a dentifrice must contain (1,500 μg F/g). Similarly, the Brazilian regulatory agency (ANVISA) 1010. Brazil, National Health Surveillance Agency (ANVISA) . Resolution 79, August 28, 2000 (in Portuguese). only requires that a dentifrice has a maximum of 1,500 μg F/g, without mentioning the concentration of soluble F or the effectiveness of the formulations based on the best available evidence 1111. Cury, JA; Caldarelli ,PG; Tenuta LMA. Necessity to review the Brazilian regulation about fluoride toothpastes. Rev Saúde Pública 2015; 49:1-7.. Low-F dentifrices with modified formulations have been developed to improve their anticaries effect with lower fluorosis risk 1212. Brighenti, FL; Delbem, ACB; Buzalaf, MAR; Oliveira, FAL; Ribeiro, DB; Sassaki KT. In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res 2006;40:239-244.,1313. Alves, KMRP; Pessan, JP; Brighenti, FL; Franco , KS; Oliveira, FAL; Buzalaf MAR et al. In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res 2007;41:263-267.,1414. Vilhena, FV; Olympio, KPK; Lauris, JRP; Delbem,, ACB; Buzalaf MAR. Low-fluoride acidic dentifrice: a randomized clinical trial in a fluoridated area. Caries Res 2010;44:478-484.,1515. Cardoso, CAB; Mangueira, DFB; Olympio, KPK; Magalhães, AC; Rios, D; Honório HM et al. The effect of pH and fluoride concentration of liquid dentifrices on caries progression. Clin Oral Invest 2014;18:761-767.. However, there is still no clinical data available that low-F dentifrices, irrespective of the formulation, are as effective as those with standard concentration (1,000-1,500 µg F/g) to reduce dentine caries without fluorosis risks. Therefore, the aim of this study was to assess the anticaries potential of low-F dentifrices available in the Brazilian market. The inhibition of enamel demineralization of two low-F dentifrices (500-550 μg F/g), with conventional or modified formulations, was compared with a standard dentifrice (1,100 μg F/g) using a validated pH-cycling model.
Material and Methods
Experimental Design
An in vitro pH-cycling model validated for the test of low-F dentifrices was used 1616. Queiroz, CS; Hara, AT; Paes Leme, AF; Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J 2008;19:21-27.. The experimental units were bovine enamel blocks selected by surface hardness (SH) values and randomized into four treatment groups (n = 12): non-F dentifrice (Cocoricó(r); Bitufo, Barueri, SP, Brazil) as a negative control, 500 μg F/g dentifrice (Oral-B Pro-Saúde Stages(r), Procter ?#61478; Gamble, Naucalpan, Mexico; importado por Procter ?#61478; Gamble do Brasil SA, Queimados, RJ, Brazil), 550 μg F/g acidulated dentifrice (Escovinha(r): Oralls, Dentalprev Ind. e Com. Ltda, Lorena, SP, Brazi) and 1,100 μg F/g dentifrice (Tandy(r); Colgate-Palmolive Industrial Ltda, São Bernardo do Campo, SP, Brazil), as a positive control. All dentifrices were bought in the Brazilian market and were within the expiration time. They were NaF/silica-based and F concentration was checked in all dentifrices through analysis with ion selective electrode 1717. Cury, JA; Oliveira, MJL; Martins, CC; Tenuta ,LMA; Paiva, SM. Available fluoride in toothpastes used by Brazilian children. Braz Dent J 2010;21:396-400.. The enamel blocks were subjected to daily pH cycles for 8 days; treatments with dentifrices slurries were made twice a day, before and after the demineralization cycle. The pH of the slurries and their fluoride concentration was checked immediately after preparation. After pH-cycling, the percentage of SH loss (%SHL) was calculated. Fluoride concentration in enamel (μg F/cm2) as loosely- and firmly-bound F was determined. F concentration in the de-remineralization solutions (μg F/mL) was also analyzed.
Enamel Block Preparation
Bovine enamel blocks (4 x 4 x 2 mm) were flattened, polished and baseline SH was determined using a FM-ARS microhardness tester (Future Technology Corp., Kanagawa, Japan) with a Knoop diamond indenter under a 50-g load for 5 s. Three indentations, spaced 100 µm from each other, were made on the central area of each block and used to calculate SH, whose values were averaged. Forty-eight enamel blocks with hardness of 306.7 ± 7.9 kg/mm2 were selected for this study.
Treatments and pH-Cycling Regimen
An adhesive tape was placed in the center of the enamel surface and the remaining surfaces of the block were coated with an acid-resistant varnish (Risqué(r), Barueri, SP, Brazil). After removal of the tape, an area of enamel of 8.0 mm2 (4 x 2 mm) was left to be exposed to the treatments.
The pH-cycling regimen 1616. Queiroz, CS; Hara, AT; Paes Leme, AF; Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J 2008;19:21-27. consisted of daily 4-h exposure to the demineralizing solution and approximately 20-h exposure to the remineralizing solution, at 37?#61679;C, for 8 days. Twice a day (before and after immersion in the demineralizing solution), the blocks were treated with a 1:3 (w/v) slurry of the dentifrices in purified water, for 5 min, under agitation (60 rpm), at room temperature, to simulate in vivo dentifrice exposure during toothbrushing 1818. Duke, SA; Forward GC. The conditions occurring in vivo when brushing with toothpastes. Br Dent J 1982;152:52-54.. Before and after the treatments, the blocks were washed with purified water for 20 s and dried with soft paper. The demineralizing solution (pH 5.0) consisted of a 0.05 mol/L acetate buffer, containing 1.28 mmol/L Ca, 0.74 mmol/L P and 0.03 μg F/mL. The remineralizing solution (pH 7.0) was 0.1 mol/L TRIS buffer, containing 1.5 mmol/L Ca, 0.9 mmol/L P, 150 mmol/L KCl and 0.05 μg F/mL. The proportion of de-remineralizing solutions per area of exposed enamel surface was 6.25 and 3.12 mL/mm2, respectively. After the 4th day, the de-remineralizing solutions were replaced by fresh batches. After the 8th day of the pH-cycling regimen, the blocks were kept in the remineralizing solutions for 24 h until analysis.
Soluble F concentration in dentifrices slurries was measured in triplicate, using an ion-selective electrode (Orion 96-09) and an ion analyzer (Orion EA-940), previously calibrated with F standards containing 0.5 to 32.0 µg F/mL according to a previously described method 1717. Cury, JA; Oliveira, MJL; Martins, CC; Tenuta ,LMA; Paiva, SM. Available fluoride in toothpastes used by Brazilian children. Braz Dent J 2010;21:396-400.. The results were expressed as µg F/mL. pH of the slurries was checked in four different samples of each dentifrice, immediately after the preparation of the slurries for use in the pH-cycling.
Enamel Demineralization Assessment
After the pH-cycling, the SH of the enamel blocks was measured again, as described above, and the %SHL was calculated [% SHL = 100 x (sound enamel hardness - hardness after pH-cycling) /sound enamel hardness]. SH is a validated technique to estimate mineral loss or gain by enamel because it reflects the demineralization degree of the enamel lesion 1919. Arends, J; Schuthof, J; Jongebloed WG. Microhardness Indentations on artificial white spot lesions. Caries Res 1979;13:290-297..
Determination of Enamel Loosely- and Firmly-Bound Fluoride
After surface hardness analysis, the area of the blocks, which were covered by acid resistant varnish, was cut out and the remaining section was longitudinally sectioned through the center. The cut surfaces were isolated with wax leaving only a 4-mm2 area (2 x 2 mm) of the enamel surface exposed for F enamel analysis.
Each enamel block was immersed in 0.15 mL of 1 M KOH for 24 h under agitation. After this period, the extract was buffered with an equal volume of TISAB II containing 1 M HCl. The concentration of loosely-bound F was determined in the extract using an ion-selective electrode (Orion 96-09; Orion Research, Inc., Boston, MA, USA) and an ion analyzer (Orion EA-940; Orion Research, Inc.) previously calibrated with F standards containing 0.1 to 8.0 µg F/mL. The results were expressed as µg F/cm2 of enamel area.
After loosely-bound fluoride extraction, the blocks were immersed in 0.25 mL of 0.5 M HCl for 30 s under agitation. After this period, the extracts containing the dissolved enamel layer were buffered with an equal volume of TISAB II modified with 20 g of NaOH/L. The concentration of firmly-bound fluoride was determined as described above, against standards containing 0.125 to 4.0 µg F/mL. The results were expressed as µg F/cm2 of enamel area.
Determination of Fluoride Concentration in the De- and Remineralizing Solutions
Fluoride concentration in the de-remineralizing solutions was checked immediately after preparation. After the pH-cycling, all the solutions in which the blocks were individually immersed were again measured for fluoride concentration. Solutions were buffered with TISAB III and F concentration determined using an ion-selective electrode (Orion 96-09) and an ion analyzer (Orion EA-940) previously calibrated with fluoride standards containing 0.025 to 0.4 µg F/mL.
Statistical Analysis
The equality of variances and a normal distribution of error were checked for all response variables. The %SHL, loosely- and firmly-bound F data were transformed to the log10. ANOVA was used for all analysis, followed by Tukey test. The SAS System 9.0 software (SAS Institute Inc., Cary, NC, USA) was used and the significance level was set at 5%.
Results
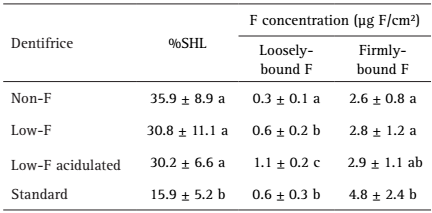
Fluoride concentration and pH of the dentifrices slurries are presented in Table 1.
After the pH-cycling regimen (Table 2), all groups presented a decrease of surface hardness, which was significantly lower for the positive control (p<0.05). Similarly, only blocks treated with the positive control presented significantly higher firmly-bound F concentration after the cycling than the negative control group (p<0.05). The low-F dentifrices did not differ significantly from the negative control regarding the %SHL and firmly-bound F concentration (p>0.05). The concentration of loosely-bound F was higher in all F groups when compared with the negative control (p<0.05), being the highest for the acidulated dentifrice.
All F groups were able to significantly increase F concentration in the de- and remineralizing solutions when compared with the negative control (p<0.05) (Table 3). This effect was higher for the group treated with the acidulated dentifrice.
Discussion
F present in dentifrices acts as a preventive-therapeutic agent 2020. Tenuta LMA, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpaste. Monogr Oral Sci 2013;23:108-124., but the currently available evidence suggests that they must have at least 1,000 μg soluble F/g to be able to significantly control caries in permanent 55. Walsh, T; Worthington , HV; Glenny, AM; Appelbe, P; Marinho, VCC; Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010 (1): CD 007868. and deciduous teeth 66. Santos, APP; Nadanovsky, P; Oliveira BH. A systematic review and meta-analysis of the effects of fluoride toothpastes on the prevention of dental caries in the primary dentition of preschool children. Community Dent Oral Epidemiol 2013;41:1-12.. However, the current Brazilian legislation 1010. Brazil, National Health Surveillance Agency (ANVISA) . Resolution 79, August 28, 2000 (in Portuguese). on fluoride dentifrices only determines the maximum F concentration that a dentifrice must contain, without specifying that it should be soluble or requiring a minimum concentration. This raises two concerns: 1. Depending on the dentifrice formulation, not all fluoride is soluble 1111. Cury, JA; Caldarelli ,PG; Tenuta LMA. Necessity to review the Brazilian regulation about fluoride toothpastes. Rev Saúde Pública 2015; 49:1-7.,1717. Cury, JA; Oliveira, MJL; Martins, CC; Tenuta ,LMA; Paiva, SM. Available fluoride in toothpastes used by Brazilian children. Braz Dent J 2010;21:396-400.,2121. Ricomini Filho AP, Tenuta LMA, Fernandes FSF, Calvo AFB, Kusano SC, Cury JA. Fluoride concentration in the top-selling Brazilian toothpastes purchased at different regions. Braz Dent J 2012;23:45-48., specially as dentifrices ages 2222. Cury JA , Vieira-Dantas ED, Tenuta LMA , Romão DA, Tabchoury CPM, Nóbrega DF, Velo MMAC, Pereira CM. Fluoride concentration in the most sold MFP/CaCO3-based Brazilian toothpastes at the expiration time. Rev APCD 2015;69:248-251. (in Portuguese)., suggesting that the concentration of soluble F should be considered in the legislation 1111. Cury, JA; Caldarelli ,PG; Tenuta LMA. Necessity to review the Brazilian regulation about fluoride toothpastes. Rev Saúde Pública 2015; 49:1-7.; 2. If low-F dentifrices are used, the concentration of F used does not reach the recommended 1,000 μg F/g. In addition, currently there is no evidence that using a low-F dentifrice reduces the risk of dental fluorosis 44. Santos, APP; Oliveira, BH; Nadanovsky P. Effects of low and standard fluoride toothpastes on caries and fluorosis: systematic review and meta-analysis. Caries Res 2013;47:382-390.. Therefore, considering the availability of low-F dentifrices in the Brazilian market, we aimed to test their anticaries potential.
The results showed that only the standard F dentifrice (1,100 μg F/g) was able to significantly reduce mineral loss during the pH-cycling. This result is confirmed by the firmly-bound F concentration found after the pH-cycling, indicating the F that was incorporated in enamel as a result of the caries process 2020. Tenuta LMA, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpaste. Monogr Oral Sci 2013;23:108-124.. This finding suggests that the highest F concentration present in the standard dentifrice provided higher incorporation of fluorapatite in the enamel during the de-remineralization cycles. In this process, part of the dissolved minerals was replaced in the enamel in the form of a more stable mineral, slowing the mineral loss.
On the other hand, the loosely-bound F concentration found on enamel after the pH-cycling showed that the reduction in pH in the low-F dentifrice was able to enhance the formation of this material (calcium-fluoride like) on enamel. This result was expected given that the formation of calcium fluoride reservoirs on the enamel increases with lowering the pH of the fluoridated agent 2323. Saxegaard E, Rolla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res 1988;96:523-535.. In fact, previous study 2424. Negri HMD, Cury JA. Dose-response effect of a dentifrice formulation with low fluoride concentration - an in vitro study. Pesqui Odontol Bras 2002;16:361-365 (in Portuguese). confirmed that lowering the pH of a low-F dentifrice may enhance its reactivity with enamel to values similar to those of a standard F dentifrice.
Taking into account the solubility of calcium fluoride deposits formed on the dental structure 2525. Rolla G, Ogaard B. Studies on the solubility of calcium fluoride in human saliva. In: Factors relating to demineralisation and remineralisation of the teeth. Leach SA (ed). Oxford: IRL Press Ltd; 1986. p 45-50., the greater reactivity of the acidulated dentifrice resulted in higher F release for de-remineralizing solutions, according to the results obtained by Brighenti et al. 1212. Brighenti, FL; Delbem, ACB; Buzalaf, MAR; Oliveira, FAL; Ribeiro, DB; Sassaki KT. In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res 2006;40:239-244.. However, the higher reactivity of acidulated dentifrice and the subsequent higher fluoride concentration released to the solutions did not reduce the mineral loss. F released to the solutions was not sufficient to facilitate the incorporation of fluorapatite in enamel, limiting the anticaries potential of the acidulated low F dentifrice. These results differed from those obtained by Brighenti et al. 1212. Brighenti, FL; Delbem, ACB; Buzalaf, MAR; Oliveira, FAL; Ribeiro, DB; Sassaki KT. In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res 2006;40:239-244. and Alves et al., 1313. Alves, KMRP; Pessan, JP; Brighenti, FL; Franco , KS; Oliveira, FAL; Buzalaf MAR et al. In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res 2007;41:263-267., who showed that acidic formulations (550 μg F/g) had similar anticaries effect than neutral formulations (1,100 μg F/g) in reducing enamel demineralization. The discrepancy of results can be explained by the ratio volume of de-remineralizing solutions per area of enamel exposed. We used a 3 times higher ratio, as recommended 2626. Featherstone, JDB, O'Reilly, MM. Shariati, M, Brugler, S. Enhancement of remineralisation in vitro and in vivo. In: Factors relating to demineralisation and remineralisation of the teeth. Leach SA (ed). Oxford: IRL Press Ltd ; 1986. p 23-34., avoiding that F released from the loosely-bound reservoirs formed by the low-F acidulated dentifrices had accumulated in the pH-cycling solutions. In fact, the difference of fluoride concentration in de-remineralizing solutions for the different treatments is very small (Table 2). Therefore, the ratio volumes of de-remineralizing solutions per area of enamel avoid artifacts and allow a better simulation of the mouth open system. Thus, in order to simulate the continuous dilution promoted by saliva in a closed in vitro design, the volume of the solutions must be high enough to avoid accumulation of the anticaries agents being tested due to the closed in vitro model 2626. Featherstone, JDB, O'Reilly, MM. Shariati, M, Brugler, S. Enhancement of remineralisation in vitro and in vivo. In: Factors relating to demineralisation and remineralisation of the teeth. Leach SA (ed). Oxford: IRL Press Ltd ; 1986. p 23-34.. Indeed, our findings are supported by a previous study showing that loosely-bound F reservoirs are not able to explain how fluoride from dentifrice works to control caries 2727. Tenuta, LMA Zamataro, CB, Del, Bel Cury, AA, Tabchoury, CPM, Cury JA. Mechanism of fluoride dentifrice effect on enamel demineralization. Caries Res 2009;43:278-285..
The results of the present study emphasizes the importance of a minimum F concentration in dentifrices for a significant anticaries effect, since the higher reactivity of the low pH formulation was not able to reduce mineral loss when compared with the negative control. Although the acidulated dentifrice has released the highest concentration of fluoride to the de-remineralizing solutions, this increase was minimal showing no significant effect in preventing mineral loss compared to the effect provided by the fluoride concentration present in the positive control dentifrice. Additionally, it is important to consider that the dentifrice slurries were prepared with water, which maintains the low pH of the acidulated dentifrice slurries and consequently promotes higher reactivity with dental structure. However, when the acidulated formulation slurry was prepared using artificial saliva, the pH was higher than 6 (data not shown), suggesting that in vivo it would be even less effective to form loosely-bound F reservoirs on enamel.
In conclusion, the present in vitro study confirmed that a dentifrice must have at least 1,000 μg F/g to control caries, since none of the low-F dentifrices found in the Brazilian market were able to significantly reduce mineral loss.
Acknowledgements
This study was supported by FUNCAMP (Conv 4252). Authors acknowledge CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the Masters fellowship granted to the first author. The authors would like to thank Mr. Waldomiro Vieira Filho for the technical assistance. During the time that this study was conducted J.A.C. acted as consultant for Colgate Palmolive from Brazil, but the work done was not related to the present research.
References
-
1Bratthall, D; Hänsel-Petersson, G; Sundberg H. Reasons for the caries decline: what do the experts believe? Eur J Oral Sci 1996;104:416-422.
-
2Cury, JA; Tenuta, LMA; Ribeiro, CCC; Paes Leme AF. The importance of fluoride dentifrices to the current dental caries prevalence in Brazil. Braz Dent J 2004;15:167-174.
-
3Mascarenhas AK. Risk factors for dental fluorosis: a review of the recent literature. Pediatr Dent 2000;22:269-277.
-
4Santos, APP; Oliveira, BH; Nadanovsky P. Effects of low and standard fluoride toothpastes on caries and fluorosis: systematic review and meta-analysis. Caries Res 2013;47:382-390.
-
5Walsh, T; Worthington , HV; Glenny, AM; Appelbe, P; Marinho, VCC; Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010 (1): CD 007868.
-
6Santos, APP; Nadanovsky, P; Oliveira BH. A systematic review and meta-analysis of the effects of fluoride toothpastes on the prevention of dental caries in the primary dentition of preschool children. Community Dent Oral Epidemiol 2013;41:1-12.
-
7US Food and Drug Administration. CFR - Code of Federal Regulations. Title 21: Food and drugs. Washington (DC); 2015 [Apr 1]. Chapter I, Food and Drug Administration Department of Health and Human Services, Subchapter D, Drugs from human use (part 355): anticaries drugs products for over the counter human use. Available from: Available from: http://www.gpo.gov/fdsys/pkg/CFR-2015-title21-vol5/pdf/CFR-2015-title21-vol5-part355.pdf 2015
» http://www.gpo.gov/fdsys/pkg/CFR-2015-title21-vol5/pdf/CFR-2015-title21-vol5-part355.pdf -
8European Union. Statutory Instruments. Consumer Protection: The Cosmetic Products (Safety) Regulations 2008: nº 1284. London: Stationery Office; 2008 [cited 2015 Apr 30]. Available from: Available from: http://www.legislation.gov.uk/uksi/2008/1284/pdfs/uksi_20081284_en.pdf
» http://www.legislation.gov.uk/uksi/2008/1284/pdfs/uksi_20081284_en.pdf -
9MERCOSUL. MERCOSUL/GMC/RES nº 48/02. Regulamento técnico MERCOSUL sobre lista de substâncias que os produtos de higiene pessoal, cosméticos e perfumes não devem conter, exceto nas condições e com as restrições estabelecidas. Brasília (DF): Sistema de Informação do Comércio Exterior; 2002 {cited 2015 Apr 30}. Available from:Available from:http://www.sice.oas.org/trade/mrcsrs/resolutions/res4802p.asp
» http://www.sice.oas.org/trade/mrcsrs/resolutions/res4802p.asp -
10Brazil, National Health Surveillance Agency (ANVISA) . Resolution 79, August 28, 2000 (in Portuguese).
-
11Cury, JA; Caldarelli ,PG; Tenuta LMA. Necessity to review the Brazilian regulation about fluoride toothpastes. Rev Saúde Pública 2015; 49:1-7.
-
12Brighenti, FL; Delbem, ACB; Buzalaf, MAR; Oliveira, FAL; Ribeiro, DB; Sassaki KT. In vitro evaluation of acidified toothpastes with low fluoride content. Caries Res 2006;40:239-244.
-
13Alves, KMRP; Pessan, JP; Brighenti, FL; Franco , KS; Oliveira, FAL; Buzalaf MAR et al. In vitro evaluation of the effectiveness of acidic fluoride dentifrices. Caries Res 2007;41:263-267.
-
14Vilhena, FV; Olympio, KPK; Lauris, JRP; Delbem,, ACB; Buzalaf MAR. Low-fluoride acidic dentifrice: a randomized clinical trial in a fluoridated area. Caries Res 2010;44:478-484.
-
15Cardoso, CAB; Mangueira, DFB; Olympio, KPK; Magalhães, AC; Rios, D; Honório HM et al. The effect of pH and fluoride concentration of liquid dentifrices on caries progression. Clin Oral Invest 2014;18:761-767.
-
16Queiroz, CS; Hara, AT; Paes Leme, AF; Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J 2008;19:21-27.
-
17Cury, JA; Oliveira, MJL; Martins, CC; Tenuta ,LMA; Paiva, SM. Available fluoride in toothpastes used by Brazilian children. Braz Dent J 2010;21:396-400.
-
18Duke, SA; Forward GC. The conditions occurring in vivo when brushing with toothpastes. Br Dent J 1982;152:52-54.
-
19Arends, J; Schuthof, J; Jongebloed WG. Microhardness Indentations on artificial white spot lesions. Caries Res 1979;13:290-297.
-
20Tenuta LMA, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpaste. Monogr Oral Sci 2013;23:108-124.
-
21Ricomini Filho AP, Tenuta LMA, Fernandes FSF, Calvo AFB, Kusano SC, Cury JA. Fluoride concentration in the top-selling Brazilian toothpastes purchased at different regions. Braz Dent J 2012;23:45-48.
-
22Cury JA , Vieira-Dantas ED, Tenuta LMA , Romão DA, Tabchoury CPM, Nóbrega DF, Velo MMAC, Pereira CM. Fluoride concentration in the most sold MFP/CaCO3-based Brazilian toothpastes at the expiration time. Rev APCD 2015;69:248-251. (in Portuguese).
-
23Saxegaard E, Rolla G. Fluoride acquisition on and in human enamel during topical application in vitro Scand J Dent Res 1988;96:523-535.
-
24Negri HMD, Cury JA. Dose-response effect of a dentifrice formulation with low fluoride concentration - an in vitro study. Pesqui Odontol Bras 2002;16:361-365 (in Portuguese).
-
25Rolla G, Ogaard B. Studies on the solubility of calcium fluoride in human saliva. In: Factors relating to demineralisation and remineralisation of the teeth. Leach SA (ed). Oxford: IRL Press Ltd; 1986. p 45-50.
-
26Featherstone, JDB, O'Reilly, MM. Shariati, M, Brugler, S. Enhancement of remineralisation in vitro and in vivo In: Factors relating to demineralisation and remineralisation of the teeth. Leach SA (ed). Oxford: IRL Press Ltd ; 1986. p 23-34.
-
27Tenuta, LMA Zamataro, CB, Del, Bel Cury, AA, Tabchoury, CPM, Cury JA. Mechanism of fluoride dentifrice effect on enamel demineralization. Caries Res 2009;43:278-285.
Publication Dates
-
Publication in this collection
May-Jun 2016
History
-
Received
22 Dec 2015 -
Accepted
03 May 2016