Abstract
Psidium cattleianum (PC) has been displaying inhibitory effect against a variety of microorganisms, but this effect has not yet been tested against endodontic pathogens. The aim of this study was to evaluate the antimicrobial activity and biocompatibility of the aqueous (PCAE) and hydroethanolic (PCHE) extracts from Psidium cattleianum (PC) leaves. Minimum inhibitory concentration (MIC) and minimum lethal concentration (MLC) were determined using the microdilution broth method in order to analyze the antimicrobial effect against Enterococcus faecalis, Pseudomonas aeruginosa, Actinomyces israelii and Candida albicans in planktonic conditions. Biofilm assays were conducted only with the extracts that were able to determine the MLC for microorganisms in planktonic conditions. Immediate and late tissue reactions against PC extracts were evaluated using edemogenic test and histological analysis of subcutaneous implants in Wistar rats. The results showed that the MIC and MLC values ranged between 0.25 and 4 mg/mL. The MLC obtained for PCHE inhibited 100% growth of all the tested strains, except for C. albicans. PCAE had the same effect for E. faecalis and P. aeruginosa. Both PC extracts were able to eliminate E. faecalis biofilms and only the PCHE eliminated P. aeruginosa biofilms. The positive controls inhibited the growth of all tested strains in MIC and MLC essays, but no CHX tested concentrations were able to eliminate A. israelii biofilm. PCAE caused a discrete increase in the edema over time, while PCHE caused a higher initial edema, which decreased progressively. Both PCAE and PCHE extracts were biocompatible, but PCHE showed better results with slight levels of inflammation at 28 days. In conclusion, PCHE was biocompatible and presented better antimicrobial effect against important pathogens associated with persistent endodontic infections
Key Words:
dental pulp diseases; phytotherapeutic drugs; antimicrobial activity
Resumo
Psidium cattleianum (PC) tem apresentado atividade inibitória frente diversos microrganismos, entretanto esse efeito ainda não foi testado contra microrganismos de interesse endodôntico. O objetivo desse estudo foi avaliar a atividade antimicrobiana e a biocompatibilidade dos extratos aquoso (EAPC) e hidroetanólico (EHPC) das folhas de Psidium cattleianum. As concentrações inibitória mínima (CIM) e letal mínima (CLM) foram determinadas pelo método de microdiluição em caldo, com o objetivo de analisar o efeito antimicrobiano frente Enterococcus faecalis, Pseudomonas aeruginosa, Actinomyces israelii e Candida albicans em condições planctônicas. Os ensaios de biofilme foram realizados somente com os extratos em que se determinou a CLM frente os microrganismos em condições planctônicas. Respostas teciduais imediata e tardia frente aos extratos de Psidium cattleianum foram avaliadas por teste edemogênico e análise histológica de implantes subcutâneos em ratos Wistar. Os resultados mostraram que CIM e CLM variaram entre 0,25 e 4 mg/mL. As CLMs determinadas pelo EHPC inibiram 100% do crescimento de todas as cepas testadas, exceto Candida albicans. EAPC apresentou o mesmo efeito para E. faecalis e P. aeruginosa. Ambos os extratos de PC conseguiram eliminar o biofilme de E. faecalis, e somente o EHPC eliminou o biofilme de P. aeruginosa. Os controles positivos inibiram o crescimento de todos os microrganismos testados nos ensaios de CIM e CLM, mas nenhuma das concentrações de clorexidina testadas foi capaz de eliminar o biofilme de A. israelii. O EAPC provocou um discreto aumento de edema com o tempo, enquanto EHPC provocou um edema inicial severo, que diminuiu progressivamente. Ambos os extratos EAPC e EHPC foram biocompatíveis, entretanto, EHPC apresentou melhores resultados com baixos níveis de inflamação em 28 dias. Pode-se concluir que EHPC foi biocompatível e apresentou melhor efeito antimicrobiano frente importantes patógenos associados a infecções endodônticas persistentes.
Introduction
The microbiota remaining after conventional endodontic treatment has been related to the maintenance or development of periapical diseases. The persistence of some microorganisms, like Enterococcus faecalis and Candida albicans in cases of endodontic treatment failures and Actinomyces israelii and Pseudomonas aeruginosa in refractory periapical lesions, associated with the complex anatomy of the root canal system require irrigants and dressings with a large spectrum of antimicrobial action for better disinfection 11 Sakamoto, M; Siqueira, JF Jr; Rôças, IN; Benno, Y. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol Immunol 2008;23:275-281.,22 Siqueira, JF Jr; Rôças, IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. Journal of Endodontics 2008;34:1291-1301.e3,33 Zhang, S; Wang, QQ; Zhang, CF; Soo, I. Identification of dominant pathogens in periapical lesions associated with persistent apical periodontitis. Chin J Dent Res 2010;13:115-121.. The most commonly used antimicrobial agents in endodontics are calcium hydroxide (CH) and chlorhexidine (CHX) 44 Mohammadi, Z; Dummer, PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J 2011;44:697-730.,55 Vaghela, DJ; Kandaswamy, D; Venkateshbabu, N; Jamini, N; Ganesh, A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: As intracanal medicaments against Enterococcus faecalis and Candida albicans: An in vitro study. J Cons Dent 2011;14:182-186.. Although most endodontic pathogens are effectively eliminated by CH, E. faecalis and C. albicans can be resistant, depending on the CH vehicles 66 Turk, BT; Sen, BH; Ozturk, T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:297-301.. CHX has a reasonably wide range of activity against aerobic and anaerobic bacteria, as well as Candida species. Several studies have reported a better antimicrobial activity for CHX when used as an endodontic dressing, compared with CH and the association of CHX and CH 77 Gomes, BP; Souza, SF; Ferraz, CC; Teixeira, FB; Zaia, AA; Valdrighi, L; et al.. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J 2003;36:267-275.,88 Gomes, BP; Vianna, ME; Sena, NT; Zaia, AA; Ferraz, CC; de Souza Filho, FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:544-550.,99 de Souza-Filho, FJ; Soares, A de J; Vianna, ME; Zaia, AA; Ferraz, CC; Gomes, BP. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J 2008;19:28-33.. Although CHX is also a potent antimicrobial irrigant, its capacity of tissue dissolution is reduced and when in contact with a common endodontic irrigant, sodium hypochlorite, it results in a “potentially toxic precipitate” 1010 Cintra, LT; Watanabe, S; Samuel, RO; da Silva Facundo, AC; de Azevedo Queiroz, IO; Dezan-Júnior, E; et al.. The use of NaOCl in combination with CHX produces cytotoxic product. Clin Oral Invest 2014;18:935-940..
Herbal medicines are emerging as potential antioxidant and antimicrobial agents for the treatment of human diseases 1111 de Souza, GC; Haas, AP; von Poser, GL; Schapoval, EE; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 2004;90:135-143.,1212 Biegelmeyer, R; Andrade, JM; Aboy, AL; Apel, MA; Dresch, RR; Marin, R; et al.. Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianum var. lucidum) strawberry guava fruit. J Food Sci 2011;76:C991-996. including those in oral cavity 1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.,1414 Brighenti, FL; Gaetti-Jardim, E Jr; Danelon, M; Evangelista, GV; Delbem, AC. Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ. Arch Oral Biol 2012;57:1034-1040.. Species of Psidium have been largely studied due to its antimicrobial 1111 de Souza, GC; Haas, AP; von Poser, GL; Schapoval, EE; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 2004;90:135-143.,1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.,1414 Brighenti, FL; Gaetti-Jardim, E Jr; Danelon, M; Evangelista, GV; Delbem, AC. Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ. Arch Oral Biol 2012;57:1034-1040.,1515 McCook-Russell, KP; Nair, MG; Facey, PC, Bowen-Forbes, CS. Nutritional and nutraceutical comparison of Jamaican Psidium cattleianum (strawberry guava) and Psidium guajava (common guava) fruits. Food Chem 2012;15;134:1069-1073., anti-inflammatory 1515 McCook-Russell, KP; Nair, MG; Facey, PC, Bowen-Forbes, CS. Nutritional and nutraceutical comparison of Jamaican Psidium cattleianum (strawberry guava) and Psidium guajava (common guava) fruits. Food Chem 2012;15;134:1069-1073., antioxidant and antiproliferative 1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154. properties. Psidium cattleianum (PC), commonly known as araçá or strawberry guava, belongs to the Myrtaceae family and is native from tropical America, although it may be cultivated in other countries because it tolerates a large diversity of climates 1212 Biegelmeyer, R; Andrade, JM; Aboy, AL; Apel, MA; Dresch, RR; Marin, R; et al.. Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianum var. lucidum) strawberry guava fruit. J Food Sci 2011;76:C991-996.. PC extracts have displayed inhibitory activity against oral microorganism 1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.. However, the antimicrobial effects of extracts from PC leaves have not yet been tested against pathogens related in endodontic infections. Thus, the aim of this study was to evaluate the biocompatibility and antimicrobial activity of the aqueous (PCAE) and hydroethanolic (PCHE) extracts obtained from PC leaves against persistent endodontic pathogens.
Materials and Methods
Plants Extracts
The leaves were collected in the Araçatuba School of Dentistry, UNESP, Araçatuba, SP, Brazil. The specimen, number HLF2006/7, was deposited at the Pharmacology and Research Herbarium, Phytotherapy Lab, São José do Rio Preto, SP, Brazil. The leaves were washed and dried at 37 °C for one week, when they become brittle and ground to a fine powder. Aqueous extract was obtained by decoction in deionized water (20 g/250 mL) for 5 min at 100 °C and at 55 °C for an additional 1 h. Hydroethanolic extract was obtained mixing 20 g of powdered leaves with 250 mL of 80% ethanol and shaking vigorously five times a day for 12 days 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.. Both solutions were then filter sterilized with 0.22 µm mixed cellulose ester membranes (EMD Millipore, Billerica, MA, USA). The hydroethanolic extract was concentrated under reduced pressures by a rotary evaporator (MA120/TH; Marconi Marconi Equipamentos para Laboratórios Ltda, Piracicaba, SP, Brazil) linked to a vacuum pump (TE-058; Tecnal Equipamentos Científicos; Piracicaba, SP, Brazil). The aqueous extract was incubated at 37 °C for 72 h. Both dried extracts were stored in dark bottles at -20 °C until use.
Antimicrobial Properties of the Plant Extracts - Microbial Conditions
The following microbial strains used in the present study were kindly provided by the Oswaldo Cruz Foundation (FIOCRUZ, Rio de Janeiro, RJ, Brazil): E. faecalis (ATCC 51299), Actinomyces israelii (ATCC 12102), P. aeruginosa (ATCC 15442) and C. albicans (ATCC 26790). The purity of the strains was confirmed by the Gram method. Microbial suspensions were prepared from overnight grown culture in Brain Heart Infusion broth (Ref.M210/Lot.35340, Himedia, Mumbai, Maharashtra, India) for bacteria strains or Sabouraud Dextrose broth (Ref. 238230/Lot. 2082199, Difco, Bordeaux, France) for C. albicans and incubated at 37 °C for 24 h in a 5% CO₂ (HF212-UV Ultra Safe Incubator; Progen Scientific Ltd, London, UK). All microorganisms were incubated in these atmospheric conditions to simulate low oxygen concentration inside root canals. Growth curve assays were performed for each microorganism in order to determine the optical density at the mid-log phase [approximately 0.5 (5-10×10⁸ CFU/mL) for bacteria and 0.3 (5-10×10⁶ CFU/mL) for C. albicans at 550 nm] to be used in the next experiments. The absorbance was measured using a microplate reader (Eon Microplate Spectrophotometer; BioTek Instruments, Winooski, VT, USA) to assess the cell growth.
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Lethal Concentration (MLC)
MIC and MLC were determined by microdilution broth method, in 96-well microtiter plates, following the criteria previously described by Clinical and Laboratory Standard Institute M7-A9 and M27-A3. The final concentration of bacterial suspension in the wells was 5-9x105 CFU/mL and 2.5-5×10³ CFU/mL for C. albicans. Initially, the plant extracts were serially diluted in water and then the microbial suspensions (correctly adjusted for the concentrations), were inoculated in each well. The plates were incubated at 37 °C for 24 h in a 5% CO₂ atmosphere. Afterwards, 15 µL of 0.01% Resazurin (R7017; Sigma-Aldrich, St. Louis, MI, USA) was applied in each well and incubated for 4 h to promote oxidation-reduction and determine the cell viability by visually detected color change. Posteriorly, the wells corresponding to MIC (the least blue well) and at least three previous wells were homogenized, six times diluted and plated on Mueller-Hinton agar or Sabouraud Dextrose agar to determine the MLC. The plates were incubated at 37 °C for 24 h in a 5% CO₂ atmosphere. The colonies were counted and the number of viable bacteria was determined in CFU/mL. MLC was considered when the extracts/fractions killed over 50% to 100% of the tested microbial culture. Chlorhexidine digluconate (CHX) and Amphotericin B (AB) were used as positive controls. The negative controls were cultures without antimicrobial agents in their media. Assays were repeated three times for each microorganism, in three independent experiments.
Biofilm Formation in Polystyrene Microplates
Biofilm assays were conducted with the extracts that showed 100% growth inhibition on the MLC tests. These assays were conducting according to Budzynska et al. 1717 Budzynska, A; Wieckowska-Szakiel, M; Sadowska, B; Kalemba, D; Rózalska, B. Antibiofilm activity of selected plant essential oils and their major components. Pol J Microbiol 2011;60:35-41., with some modifications. Twenty microliters of each microorganism suspension (approximately 5-9×10⁶ CFU/mL) were inoculated in sterile U-shaped bottom 96-well polystyrene microplates containing 180 µL BHI for bacteria or Sabouraud Dextrose broth for Candida, both supplemented with 0.5% glucose. The plates were incubated at 37 °C in a 5% CO₂ atmosphere. After 48 h, the culture medium was removed and the wells were washed with sterile saline for subsequent addition of 200 µL of plant extract solution in each well. The concentrations used for these assays were 5 and 10 times higher than the MLC concentration. The microplates were incubated again in the same conditions for 24 h. All cultures in the wells were diluted six times and plated in Brain Heart Infusion agar or Sabouraud Dextrose agar and incubated for 24 h. After this, the colony forming units (CFU)/mL was determined. CHX (at 5× or 10× MLC concentrations) and AB (at 5× or 10× MLC concentrations) were used as positive controls and biofilm in water without antimicrobial agents, as negative control. Assays were repeated three times for each microorganism, in three independent experiments.
Biologic Tissue Responses to PCAE and PCHE - Immediate Tissue Reaction (Edemogenic Test)
The animal experiment was approved by Animal Experimentation Ethics Committee of Araçatuba School of Dentistry (Process #2008-000166). Eighteen 4-6 month-old Wistar male rats weighting 250-280g were divided in 6 groups of 3 animals according to the experimental period (3 and 6 h) and treatment with hydroethanolic (PCHE) or aqueous (PCAE) extract, both with a 4.0 mg/mL concentration or saline (S) as control. Anesthetized animals received an intravenous injection of 2% Evan’s blue (0.1 mL/100 g of body weight) (Evan’s Blue; Difco Lab). After 30 min, 0.1 mL of the extracts or saline solution were injected at the dorsum region, near the tail and using the median line as a reference 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.. After 3 and 6 h the animals were euthanized by anesthetic overdose and a fragment of the tissue with a 23 mm diameter containing a blue halo in the center was removed. The tissue was fixed in 4 mL of formamide for 72 h at 45 °C and filtered for posterior analysis in a spectrophotometer at 630 nm.
Late Tissue Reaction (Histological Analysis)
Thirty 4-6 month-old Wistar male albino rats weighting 250-280g were divided in groups of 5 for each experimental group (PCAE, PCHE and S) and period of time (7 and 28 days). Sixty polyethylene tubes with 1.0 mm inner diameter, 1.6 mm external diameter and 10 mm length with a paper cone, were prepared and filled with the extracts (at 40 mg/mL) and saline and subcutaneously implanted according to Machado et al. 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.. After 7 and 28 days of implantation the animals were killed by anesthetic overdose and tubes/surrounding tissue removed and fixed in 10% formalin. Histological analysis was performed with a light microscope and original magnification of 40 x after hematoxylin-eosin staining. The quantitative analysis of inflammation was performed according to the number of inflammatory cells present and classified in the following scores 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.,1818 Fédération Dentaire International, Commission of Dental Materials, Instruments, Equipment and Therapeutics. Recommended standard practices for biological evaluation of dental materials. Int Dent J 1980;30:140-188.: 0: absent (absence of inflammatory cells), 1: low (<25 inflammatory cells), 2: moderate (25-125 inflammatory cells) and 3: severe (>125 inflammatory cells). For the thickness of fibrous capsule, the scores were: 0: thin (thickness <150 µm) and 1: thick (thickness >150 µm).
Statistical Analysis
Data from planktonic growth in MIC and MLC conditions were converted to logarithm scale (log10 (CFU+1) and the percentage (%) of microbial reduction was compared to normal growth (control group). Box-whisker plots were performed to represent the distribution of non-parametric data obtained in the biofilm assays. Mann-Whitney tests were applied to compare experimental groups and the ones with positive control for microbiological assays. Results of edemogenic test were analyzed by two-way ANOVA complemented by Tukey test (p<0.05). Data from histological study were analyzed by Mann-Whitney and Wilcoxon tests (p<0.05).
Results
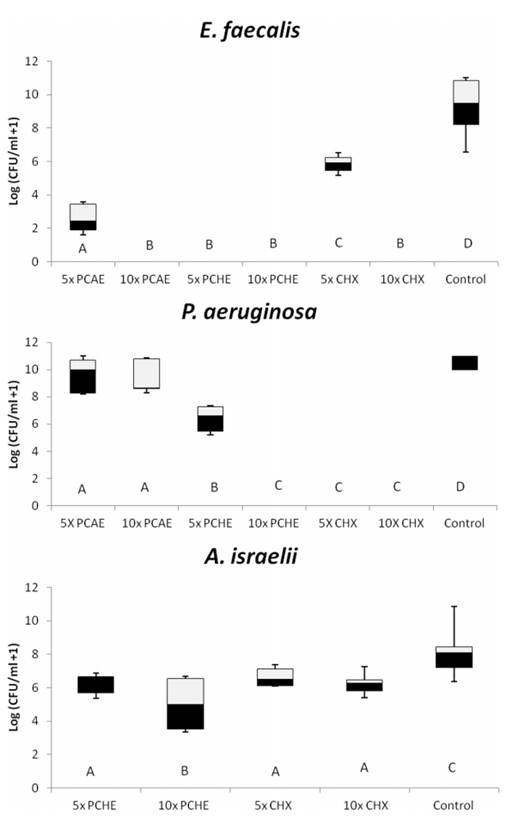
The MIC and MLC values in the present study ranged between 0.25 mg/mL and 4 mg/mL (Table 1). The results showed strong antibacterial activity of the Psidium cattleianum aqueous extract (PCAE) and Psidium cattleianum hydroethanolic extract (PCHE) against E. faecalis and A. israelii, and weak activity for P. aeruginosa in planktonic conditions. Both extracts were unable to inhibit C. albicans growth by the microdilution broth method. The percentages of microbial reduction in MIC and MLC for Psidium cattleianum extracts are in Figure 1A and B, respectively. The values of MIC inhibited completely the growth of E. faecalis and P. aeruginosa for PCAE and P. aeruginosa and A. israelii for PCHE. P. aeruginosa was the most sensitive microorganism and presented 100% growth reduction in presence of MIC obtained for both extracts. C. albicans was the most resistant strain for both tested substances and no extract had effect on its growth. MLC obtained for PCHE inhibited 100% growth of all the tested strains, except for C. albicans. The MLC for PCAE had the same effect for E. faecalis and P. aeruginosa. The positive controls (Chlorhexidine and Amphotericin B) inhibited the growth of all tested strains. Figure 2 shows box-whisker plots of the activity of the plant extracts against biofilms. PCHE (5´ and 10´ MLC), CHX and PCAE (10´ MLC) were able to completely eliminate the E. faecalis biofilm. The same was observed for PCHE (10´) and CHX (5´ and 10´ MLC) for P. aeruginosa. PCHE (10´ MLC) reduced the growth of A. israelii after 24 h of exposition. Both CHX (5´ and 10´ MLC) tested were not able to eliminate A. israelii biofilm.
Minimum inhibitory concentration (MIC) and minimal lethal concentration (MLC) of Psidium cattleianum extracts
A: Percentage (%) of microbial reduction compared to normal growth under minimum inhibitory concentration (MIC) of Psidium cattleianum extracts. B: Percentage (%) of microbial reduction compared to normal growth under minimum lethal concentration (MLC) of Psidium cattleianum extracts. Data were expressed in mean of log (CFU+1). Control for bacteria - CHX, Control for Candida albicans - AB. * - Statistical difference among the groups according to Mann-Whitney tests).
Box-whisker plots of the activity of the Psidium cattleianum extracts against biofilm of pathogenic microorganisms. Different capital letters show statistical difference among the groups and control (H2O), according to Mann-Whitney tests. Bars indicate minimum and maximum values. Black and white boxes indicate lower and upper quartiles, respectively. Line in the middle of boxes is median.
Figure 3 shows the results of edemogenic test. Saline and PCAE caused a discrete increase of edema over time while PCHE caused a higher initial edema that decreased gradually. Statistically significant differences were observed for edema between saline and PCHE (p<0.05). The medium scores of inflammatory cell counting after histological analysis are in Table 2. The saline group at 7 days presented the highest number of inflammatory cells and it was statistically different from other groups (p<0.05). The results from PCHE at 7 days presented the second worst result, differing statistically from PCHE at 28 days and saline at 7 days groups (p<0.05). PCHE at 28 days and saline 28 days presented the lowest numbers of inflammatory cells, but these values were not statistically significant different from PCAE (p>0.05). The thickness of fibrous capsule was statistically different between saline and the other groups, at 7 days. The qualitative evaluation of tissue response to both extracts (PCAE and PCHE) at 7 days showed a thick fibrous capsule with connective tissue in repair. There were numerous new fibroblasts and disorganized collagen fibers oriented parallel to the implant. A layer of macrophages can be observed near the implant, and some cells were dispersed in the repair tissue. There are lymphocytes and mononucleated cells, characterizing a chronic inflammation with the presence of angiogenesis (Figs. 4A and 4C). The saline group at 7 days presented more aggressive inflammatory response. The area of tissue reaction was higher and the fibrous capsule was thicker with macrophages, lymphocytes, mononucleated cells and new fibroblasts. Collagen fibers were distributed in a complex position (Fig. 4E). The PCAE and saline groups at 28 days presented a great reduction of inflammatory reaction and a higher tissue organization. The fibrous capsule was thinner with organized collagen fibers parallel to the implant. The number of inflammatory cells was reduced. There were few vessels with small diameter in the connective tissue (Figs. 4B and 4F). The hydroethanolic group (PCHE) at 28 days presented a more homogenous repair than the other groups. The fibrous capsule was thin and condensed and the collagen fibers were thicker and organized. The fibroblasts showed an elongated and thin nucleus. The macrophages around the implant presented a slight diameter indicating attenuation on the phagocytic activity. Occasional plasma cells may be observed between the implant and the macrophages, suggesting a humoral response to PCHE (Fig. 4D).
Means (standard deviations) values of edema on both periods of time for the PCAE, PCHE and control groups. *Statistical difference between PCHE and the other groups, according to ANOVA/Tukey tests.
Representative images of histological findings in the area of tubes opening. Psidium cattleianum aqueous extract (PCAE) group: A: Day 7, moderate inflammatory cell infiltrate and thick fibrous capsule. B: Day 28, thin fibrous capsule and moderate inflammatory cell infiltrate. Psidium cattleianum hydroethanolic extract (PCHE) group: C: Day 7, thick fibrous capsule and moderate inflammatory cell infiltrate with the presence of lymphocytes and macrophages. D: Day 28, fibrous capsule around the tube was thinner with few chronic inflammatory cells. Saline group (control): E: Day 7, thick fibrous capsule and highest inflammatory cell infiltrate. F: Day 28, reduction of inflammatory cells and fibrous capsule near the tube. HE: Original magnification: 100x.
Medians (minimum-maximum) of scores for inflammatory cell infiltrate and thickness of fibrous capsule after 7 and 28 days of extract exposure
Discussion
The complexity of root canals, the limitations of the chemomechanical preparation and the vulnerability of each species involved in the infection are well recognized factors related to the failure of an endodontic treatment 88 Gomes, BP; Vianna, ME; Sena, NT; Zaia, AA; Ferraz, CC; de Souza Filho, FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:544-550.. In alternative to solve this problem, several authors have been studying medicinal plants to improve the endodontic treatment 1919 Dezan Jr, E; Sangalli, J; Gomes-Filho, JE; Gaetti-Jardim Jr, E. Psidium cattleianum plus Ca(OH)2 antimicrobial efficacy against Enterococcus faecalis. IADR; 15-16,2010, J Dent Res; 2010;89, Special Issue B.,2121 Ferreira, FB; Torres, SA; Rosa, OP; Ferreira, CM; Garcia, RB; Marcucci, MC; et al.. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:709-716.,2222 Abbaszadegan, A; Dadolahi, S; Gholami, A; Moein, MR; Hamedani, S; Ghasemi, Y; et al.. Antimicrobial and cytotoxic activity of Cinnamomum zeylanicum, calcium hydroxide, and triple antibiotic paste as root canal dressing materials. J Contemp Dent Pract 2016;1;17:105-113. . In the present study, the objective was to analyze the antimicrobial activity of Psidium cattleianum extracts against microorganisms involved in the persistent endodontic infection, apart from analyzing their immediate and late biologic responses.
The antimicrobial properties of PC have been recently studied 1111 de Souza, GC; Haas, AP; von Poser, GL; Schapoval, EE; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 2004;90:135-143.,1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.,1414 Brighenti, FL; Gaetti-Jardim, E Jr; Danelon, M; Evangelista, GV; Delbem, AC. Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ. Arch Oral Biol 2012;57:1034-1040.,1515 McCook-Russell, KP; Nair, MG; Facey, PC, Bowen-Forbes, CS. Nutritional and nutraceutical comparison of Jamaican Psidium cattleianum (strawberry guava) and Psidium guajava (common guava) fruits. Food Chem 2012;15;134:1069-1073., but the direct comparison of the obtained results with the literature is difficult, since different methodologies and microorganisms were used in these investigations, only a parallel analysis was done. Similar to this study results, de Souza et al. 99 de Souza-Filho, FJ; Soares, A de J; Vianna, ME; Zaia, AA; Ferraz, CC; Gomes, BP. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J 2008;19:28-33. did not observe an effect against C. albicans in any of the 18 tested extracts, including the PC extract 1111 de Souza, GC; Haas, AP; von Poser, GL; Schapoval, EE; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 2004;90:135-143.. PCAE showed antibacterial effect at high concentration in a reduced exposure time (5 min) or at a low concentration in 60 min against the Streptococcus mutans biofilm 1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.. After treatment with PCAE, in an in situ biofilm assay, total anaerobic microorganism, total streptococci and S. mutans counts decreased when compared with water or antiseptic treatments 1414 Brighenti, FL; Gaetti-Jardim, E Jr; Danelon, M; Evangelista, GV; Delbem, AC. Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ. Arch Oral Biol 2012;57:1034-1040.. In the present study, the PCAE also presented antibacterial effect against the E. faecalis and P. aeruginosa biofilms and eliminated the E. faecalis biofilm at higher concentrations (10×MLC). In order to reduce the time for calcium hydroxide activity as an endodontic dressing, PCHE was used as a vehicle for calcium hydroxide in a biofilm assay. The tested formulation eliminated E. faecalis in 24 h 1919 Dezan Jr, E; Sangalli, J; Gomes-Filho, JE; Gaetti-Jardim Jr, E. Psidium cattleianum plus Ca(OH)2 antimicrobial efficacy against Enterococcus faecalis. IADR; 15-16,2010, J Dent Res; 2010;89, Special Issue B.. This study results also showed E. faecalis elimination when exposed to PCHE (5× and 10× MLC), suggesting that only the presence of the PC extract produced bactericidal effect.
The large range between the MIC and MLC values were observed in the results for PCAE extract - MIC (0.25 mg/mL) and MLC (>4 mg/mL) against A. israelii. The same was also reported in studies using antibiotics and propolis against the same bacterium 2020 Barnard, D; Davies, J; Figdor, D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J 1996;29:320-326.,2121 Ferreira, FB; Torres, SA; Rosa, OP; Ferreira, CM; Garcia, RB; Marcucci, MC; et al.. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:709-716.. This suggested that the slow rate of A. israelii elimination is due to the genotypic tolerance of the species 2020 Barnard, D; Davies, J; Figdor, D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J 1996;29:320-326..
P. aeruginosa was resistant to PC hexane, ethyl acetate and methanol fruit extracts by disc diffusion 1515 McCook-Russell, KP; Nair, MG; Facey, PC, Bowen-Forbes, CS. Nutritional and nutraceutical comparison of Jamaican Psidium cattleianum (strawberry guava) and Psidium guajava (common guava) fruits. Food Chem 2012;15;134:1069-1073.. In the present study, although others solvents and other tree parts were employed to obtain the PC extracts may observed the effect of PC extracts for both solvents against P. aeruginosa. Different results can be observed in other studies with plant material. This can occur due to the different methods of extraction employed and the different solvents used for obtaining the crude extracts 2323 Passari, LM; Scarminio, IS; Bruns, RE. Experimental designs characterizing seasonal variations and solvent effects on the quantities of coumarin and related metabolites from Mikania laevigata. Anal Chim Acta 2014;22;821:89-96. . Furthermore, the presence and concentration of the compounds may be different in each part of the tree 2424 Tortoriello, J; Meckes-Fischer, M; Villarreal, ML; Berlin, B; Berlin, E. Spasmolytic activity of medicinal plants used to treat gastrointestinal and respiratory diseases in the Highland of Chiapas. Phytomedicine1995;2:57-66. .
The presence of the phenolic compounds - three flavonoids (kaempferol, quercetin and cyaniding) and tannin (ellagic acid) - have been related as the factor responsible for the PC antibacterial activity 1313 Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.. Flavonoids are secondary metabolites naturally synthesized by plants, capable of penetrating into bacterial cells due to their lipophilic characteristic 2525 Martini, ND; Katerere, DR; Eloff, JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol 2004;93:207-12. . Quercetin-5.3-dimethylether from Combretum erythrophyllum displayed MICs values of 0.05 and 0.1 mg/mL for E. faecalis and P. aeruginosa, respectively 2525 Martini, ND; Katerere, DR; Eloff, JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol 2004;93:207-12. . Tannins can be toxic to filamentous fungi, yeasts and bacteria due to their ability for inactivating microbial adhesins, enzymes and cell envelopes that transport proteins 2626 Cowan, MM Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564-582. .
The present study demonstrated that the extracts are safe for use in biological tissues causing benefits to biologic response during repair. During the studied period, the wound healing was in an advanced stage presenting reduced levels of inflammation for PCAE and PCHE groups. These results were different from another study that followed the same methodology using Myracrodruon urundeuva aqueous and ethanolic extracts 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.. The Myracrodruon urundeuva ethanolic extract demonstrated persistency of inflammation until the 28th day 1616 Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8..
The edemogenic test showed a higher edema in the PCHE group at 3 h, which probably occurred due to the ethanol. However, this edema reduced progressively. The morphological analysis showed repair progress over time proving that the implants did not interfere with natural repair progression. Both extracts induced better tissue responses than control group (saline) suggesting a beneficial effect of these extracts for inflammation control. This was observed at 7 days when there were moderate inflammatory responses mainly due to the surgical trauma to tissues. Besides, the inflammatory tissue reactions caused by PCAE and PCHE extracts were lower than those caused by saline (control). At 28 days there was a regression of inflammatory process in all groups, but PCHE showed better results with slight levels of inflammation.
This present study has some limitations. First of all, the complete neutralization of antimicrobial agents was not confirmed before plating. Two common methods are used for inhibition of residual biocides: dilution and chemical neutralization. In this study was used the dilution of the antimicrobial agents, 6 times before plating. The choice was to not use chemical neutralization of biocides because of the toxicity displayed by several types of neutralizers. The use of neutralizers could not distinguish between neutralization of the biocide versus recovery of organisms injured by sub-lethal exposure to the biocide 2727 Sutton, SV; Proud, DW; Rachui, S; Brannan, DK. Validation of microbial recovery from disinfectants. PDA J Pharm Sci Technol 2002;5:255-266.. In the present study, was used a population of organisms without exposure to antimicrobial agents as a growth control. However, studies have appointed for the importance of the complete neutralization of biocides, like chlorhexidine, because the accuracy of a biocidal assay and low levels of residuals would lead to exaggerated results of microbicidal activity 2727 Sutton, SV; Proud, DW; Rachui, S; Brannan, DK. Validation of microbial recovery from disinfectants. PDA J Pharm Sci Technol 2002;5:255-266.. Another limitation of this study is absence of dentin as a substrate for bacterial growth. This is the first study that found antimicrobial activity for the Psidium cattleianum extracts against endodontic pathogens and new studies are required to confirm this property simulating the canal root environment.
In conclusion, PCHE was biocompatible and presented good antimicrobial effect against important pathogens associated with persistent endodontic infections.
Acknowledgements
The microbial strains were provided by Oswaldo Cruz Foundation (FIOCRUZ). This study was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) from Brazil.
References
-
1Sakamoto, M; Siqueira, JF Jr; Rôças, IN; Benno, Y. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol Immunol 2008;23:275-281.
-
2Siqueira, JF Jr; Rôças, IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. Journal of Endodontics 2008;34:1291-1301.e3
-
3Zhang, S; Wang, QQ; Zhang, CF; Soo, I. Identification of dominant pathogens in periapical lesions associated with persistent apical periodontitis. Chin J Dent Res 2010;13:115-121.
-
4Mohammadi, Z; Dummer, PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J 2011;44:697-730.
-
5Vaghela, DJ; Kandaswamy, D; Venkateshbabu, N; Jamini, N; Ganesh, A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: As intracanal medicaments against Enterococcus faecalis and Candida albicans: An in vitro study. J Cons Dent 2011;14:182-186.
-
6Turk, BT; Sen, BH; Ozturk, T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:297-301.
-
7Gomes, BP; Souza, SF; Ferraz, CC; Teixeira, FB; Zaia, AA; Valdrighi, L; et al.. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro Int Endod J 2003;36:267-275.
-
8Gomes, BP; Vianna, ME; Sena, NT; Zaia, AA; Ferraz, CC; de Souza Filho, FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:544-550.
-
9de Souza-Filho, FJ; Soares, A de J; Vianna, ME; Zaia, AA; Ferraz, CC; Gomes, BP. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J 2008;19:28-33.
-
10Cintra, LT; Watanabe, S; Samuel, RO; da Silva Facundo, AC; de Azevedo Queiroz, IO; Dezan-Júnior, E; et al.. The use of NaOCl in combination with CHX produces cytotoxic product. Clin Oral Invest 2014;18:935-940.
-
11de Souza, GC; Haas, AP; von Poser, GL; Schapoval, EE; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J Ethnopharmacol 2004;90:135-143.
-
12Biegelmeyer, R; Andrade, JM; Aboy, AL; Apel, MA; Dresch, RR; Marin, R; et al.. Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianum var. lucidum) strawberry guava fruit. J Food Sci 2011;76:C991-996.
-
13Brighenti, FL; Luppens, SB; Delbem, AC; Deng, DM; Hoogenkamp, MA; Gaetti-Jardim, E Jr; et al.. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res 2008;42:148-154.
-
14Brighenti, FL; Gaetti-Jardim, E Jr; Danelon, M; Evangelista, GV; Delbem, AC. Effect of Psidium cattleianum leaf extract on enamel demineralisation and dental biofilm composition in situ Arch Oral Biol 2012;57:1034-1040.
-
15McCook-Russell, KP; Nair, MG; Facey, PC, Bowen-Forbes, CS. Nutritional and nutraceutical comparison of Jamaican Psidium cattleianum (strawberry guava) and Psidium guajava (common guava) fruits. Food Chem 2012;15;134:1069-1073.
-
16Machado, AC; Dezan Junior, E; Gomes-Filho, JE; Cintra, LT; Ruviére, DB, Zoccal, R; et al.. Evaluation of tissue reaction to aroeira (Myracrodruon urundeuva) extracts: a histologic and edemogenic study. J Appl Oral Sci 2012; 20:414-8.
-
17Budzynska, A; Wieckowska-Szakiel, M; Sadowska, B; Kalemba, D; Rózalska, B. Antibiofilm activity of selected plant essential oils and their major components. Pol J Microbiol 2011;60:35-41.
-
18Fédération Dentaire International, Commission of Dental Materials, Instruments, Equipment and Therapeutics. Recommended standard practices for biological evaluation of dental materials. Int Dent J 1980;30:140-188.
-
19Dezan Jr, E; Sangalli, J; Gomes-Filho, JE; Gaetti-Jardim Jr, E. Psidium cattleianum plus Ca(OH)2 antimicrobial efficacy against Enterococcus faecalis IADR; 15-16,2010, J Dent Res; 2010;89, Special Issue B.
-
20Barnard, D; Davies, J; Figdor, D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J 1996;29:320-326.
-
21Ferreira, FB; Torres, SA; Rosa, OP; Ferreira, CM; Garcia, RB; Marcucci, MC; et al.. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:709-716.
-
22Abbaszadegan, A; Dadolahi, S; Gholami, A; Moein, MR; Hamedani, S; Ghasemi, Y; et al.. Antimicrobial and cytotoxic activity of Cinnamomum zeylanicum, calcium hydroxide, and triple antibiotic paste as root canal dressing materials. J Contemp Dent Pract 2016;1;17:105-113.
-
23Passari, LM; Scarminio, IS; Bruns, RE. Experimental designs characterizing seasonal variations and solvent effects on the quantities of coumarin and related metabolites from Mikania laevigata Anal Chim Acta 2014;22;821:89-96.
-
24Tortoriello, J; Meckes-Fischer, M; Villarreal, ML; Berlin, B; Berlin, E. Spasmolytic activity of medicinal plants used to treat gastrointestinal and respiratory diseases in the Highland of Chiapas. Phytomedicine1995;2:57-66.
-
25Martini, ND; Katerere, DR; Eloff, JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol 2004;93:207-12.
-
26Cowan, MM Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564-582.
-
27Sutton, SV; Proud, DW; Rachui, S; Brannan, DK. Validation of microbial recovery from disinfectants. PDA J Pharm Sci Technol 2002;5:255-266.
Publication Dates
-
Publication in this collection
May-Jun 2017
History
-
Received
13 Nov 2016 -
Accepted
04 Mar 2017