Abstracts
Different growth regulators combinations were tested on the production of anther callus in tomato cultivar IPA 5. Calli were induced on media supplemented with 1.0mgL-1 gibberellic acid (GA3), 0.05mgL-1 alpha-naphthaleneacetic acid (NAA) plus 0.1mgL-1 6-benzylaminopurine (BAP), or with 1.0mgL-1 BAP plus 1.0mgL-1 NAA. The medium containing 1.0mgL-1 BAP and 1.0mgL-1 NAA produced the highest calli frequency, and promoted plant regeneration by indirect organogenesis, when calli were transferred to 0.01mgL-1 BAP and 0.001mgL-1 NAA. Plants regenerated presented tetraploid cells and rare diploid cells. These tetraploid plants could be used as source for further obtainment of trisomic lines, for the purpose of genic localization studies and protein compounds analysis.
growth regulators; organogenesis; tetraploid cells
Diferentes combinações de reguladores de crescimento foram testadas na produção de calos a partir do cultivo de anteras de tomate cultivar IPA 5. Calos foram induzidos no meio suplementado com 1,0mgL-1 de ácido giberélico (GA3) + 0,05mgL-1 de alfa-ácido naftalenoacético (ANA) + 0,1mgL-1 de 6-benzilaminopurina (BAP), ou com 1,0mgL-1 de BAP + 1,0mgL-1 de ANA. O meio contendo 1,0mgL-1 de BAP e 1,0mgL-1 de ANA produziu a maior freqüência de calos e promoveu a regeneração de plantas através de organogênese indireta, quando os calos foram transferidos para 0,01mgL-1 de BAP e 0,001mgL-1 de ANA. As plantas regeneradas apresentaram células tetraplóides e, raramente, células diplóides. Estas plantas tetraplóides podem servir como fonte para posterior obtenção de linhagens trissômicas, para serem utilizadas em estudos de localização gênica e em análises de compostos protéicos.
reguladores de crescimento; organogênese; células tetraplóides
CALLUS INDUCTION AND PLANT REGENERATION OF TOMATO (Lycopersicon esculentum CV. IPA 5) VIA ANTHER CULTURE
INDUÇÃO DE CALOS E REGENERAÇÃO DE PLANTAS A PARTIR DO CULTIVO IN VITRO DE ANTERAS DE TOMATE (Lycopersicon esculentum CV. IPA 5)
Ana Christina Rabello Brasileiro1 1 Engenheiro Agrônomo, Mestre, Professor Substituto, Departamento de Biologia, Universidade Federal Rural de Pernambuco (UFRPE), 52171-950, Recife, PE. E-mail: vidal@elogica.com.br. Autor para correspondência. Lilia Willadino2 1 Engenheiro Agrônomo, Mestre, Professor Substituto, Departamento de Biologia, Universidade Federal Rural de Pernambuco (UFRPE), 52171-950, Recife, PE. E-mail: vidal@elogica.com.br. Autor para correspondência. Gianna Griz Carvalheira3 1 Engenheiro Agrônomo, Mestre, Professor Substituto, Departamento de Biologia, Universidade Federal Rural de Pernambuco (UFRPE), 52171-950, Recife, PE. E-mail: vidal@elogica.com.br. Autor para correspondência. Marcelo Guerra4 1 Engenheiro Agrônomo, Mestre, Professor Substituto, Departamento de Biologia, Universidade Federal Rural de Pernambuco (UFRPE), 52171-950, Recife, PE. E-mail: vidal@elogica.com.br. Autor para correspondência.
SUMMARY
Different growth regulators combinations were tested on the production of anther callus in tomato cultivar IPA 5. Calli were induced on media supplemented with 1.0mgL-1 gibberellic acid (GA3), 0.05mgL-1a-naphthaleneacetic acid (NAA) plus 0.1mgL-1 6-benzylaminopurine (BAP), or with 1.0mgL-1 BAP plus 1.0mgL-1 NAA. The medium containing 1.0mgL-1 BAP and 1.0mgL-1 NAA produced the highest calli frequency, and promoted plant regeneration by indirect organogenesis, when calli were transferred to 0.01mgL-1 BAP and 0.001mgL-1 NAA. Plants regenerated presented tetraploid cells and rare diploid cells. These tetraploid plants could be used as source for further obtainment of trisomic lines, for the purpose of genic localization studies and protein compounds analysis.
Key words: growth regulators, organogenesis, tetraploid cells.
RESUMO
Diferentes combinações de reguladores de crescimento foram testadas na produção de calos a partir do cultivo de anteras de tomate cultivar IPA 5. Calos foram induzidos no meio suplementado com 1,0mgL-1 de ácido giberélico (GA3) + 0,05mgL-1 de a-ácido naftalenoacético (ANA) + 0,1mgL-1 de 6-benzilaminopurina (BAP), ou com 1,0mgL-1 de BAP + 1,0mgL-1 de ANA. O meio contendo 1,0mgL-1 de BAP e 1,0mgL-1 de ANA produziu a maior freqüência de calos e promoveu a regeneração de plantas através de organogênese indireta, quando os calos foram transferidos para 0,01mgL-1 de BAP e 0,001mgL-1 de ANA. As plantas regeneradas apresentaram células tetraplóides e, raramente, células diplóides. Estas plantas tetraplóides podem servir como fonte para posterior obtenção de linhagens trissômicas, para serem utilizadas em estudos de localização gênica e em análises de compostos protéicos.
Palavras-chave: reguladores de crescimento, organogênese, células tetraplóides.
INTRODUCTION
Plant tissue culture techniques are recognized as useful instruments in crop improvement. Among these techniques, in vitro anther culture stands out and is an increasingly powerful tool when integrated into breeding programs (HU& ZENG, 1984). This technique allows the acceleration of plant breeding by providing homozygous doubled haploids within a comparatively short time (NURHIDAYAH et al., 1996). In addition, obtaining haploid plants from segregant generations facilitates genetic analysis, eliminating the complexity of the heterozygous state (MORAES-FERNANDES, 1990).
Several studies have been reported with tomato anther culture. However, the function carried out by factors such as microspore developmental stages (GULSHAN et al., 1981; SUMMERS et al., 1992), dark-light regimes (JARAMILLO& SUMMERS, 1991), and growth regulators (GRESSHOFF& DOY, 1972; ROGOZINSKA & SKUTNIK, 1974) are still unclear.
SHARPet al. (1971) are among the pioneers of in vitro tomato anther culture. They observed that exogenous growth regulators have no obvious effect on the induction of calli proliferation. On the other hand, GRESSHOFF & DOY (1972) observed that differentiation of haploid calli of L. esculentum can be controlled by hormones added to a defined medium. Other authors also noticed different responses to distinct dosages of growth regulators on the formation of androgenic calli (DAO & SHAMINA, 1978; GULSHAN et al., 1981). In a work by Ziv et al. (1982), doubled haploid plants were regenerated from non-allelic male-sterile mutants (ms 1035), using a medium containing 0.5mgL-1 indole-3-acetic acid (IAA) and 0.25mgL-1 zeatin. Although, the regeneration of tomato plants from microspores has been strongly dependent on this mutant genotype.
The purpose of this work was to study the effect of different growth regulators on in vitro induction and growth of tomato anther calli, and their later differentiation into plants.
MATERIALS AND METHODS
Tomato plants of cultivar IPA 5 (2n=24) were grown in a greenhouse, watered daily and fertilized once a week. Flower buds 5 to 6mm long were harvested. The developmental stage of anthers varied from the beginning of meiosis to tetrad. For cytological analysis, the anthers were fixed in Carnoy (3:1) and stained in 1% (w/v) hematoxylin as described by FUJII & GUERRA (1998).
The buds harvested in the morning were conditioned in a moist chamber and submitted to a cold pretreatment at 5°C for 6h, in the dark. Later, they were surface sterilized in 70% (v/v) ethanol, for 15s, immersed in a 0.5% (v/v) sodium hypochlorite solution for 5min and then rinsed three times in sterile distilled water. Anthers were removed aseptically and plated on five callus induction media varying the growth regulators composition (table 1). Basic medium, according to CAPPADOCIA & SREE RAMULU (1980), containing MURASHIGE & SKOOG (1962) salts (MS), myo-inositol, glycine and vitamins of NITSCH (1969) medium, 20% (w/v) sucrose and 0.7% (w/v) agar (pH 5.8) was used for all treatments. The (10cm) Petri dishes used for culture were maintained in the darkness in a growth-chamber at 26 ± 1°C for one week and, afterwards, exposed to 16h light photoperiod (90 110µmolm-2s-1) provided by fluorescent illumination. The frequency of anthers producing calli was recorded after 30 and 60 days.
The calli produced were transferred to a regeneration medium composed of the same basal medium supplemented with 0.01mgL-1 6-benzylaminopurine (BAP) and 0.001mgL-1a-naphthaleneacetic acid (NAA). The calli with organogenic characteristics were partially fixed in formolin/ glacial acetic acid/ 50% ethanol (5:5:90 v/v/v), for posterior histological analysis, as described by JOHANSEN (1940). In order to identify the ploidy level, root tips of regenerated plants were pretreated with 0.002M 8-hydroxyquinoline for 24h at 10°C, fixed in Carnoy (3:1), hydrolyzed in 5N HCl for 20min, squashed in 45% (v/v) acetic acid and stained with 2% (v/v) Giemsa (GUERRA, 1983).
The experiment was entirely randomized, with five treatments (six replications per treatment), where the experimental unit consisted of a Petri dish containing 25 anthers, performing 150 anthers per treatment. Statistical analyses were carried out by the ANOVA and Tukey test, at a 1% probability level, using  transformation.
transformation.
RESULTS AND DISCUSSION
Calli formation was observed exclusively in media containing both auxins and cytokinins (1.0mgL-1 GA3 plus 0.05mgL-1 BAP plus 0.1mgL-1 NAA, medium one; 1.0mgL-1 BAP plus 1.0mgL-1 NAA, medium two). A significant increment in callus production was verified as time progressed, for both media (table 2). Medium two presented the highest frequency of calli formation: 16% after 30 days and 67% after 60 days.
For some members of Solanaceae family, the inclusion in the culture medium of an auxin alone, or in combination with a cytokinin, frequently induces the formation of pollen calli (CANHOTO et al., 1990). The importance of this hormonal balance in tomato anther culture has been described by several authors. GULSHAN et al. (1981) verified that the medium containing 2.0mgL-1 NAA and 1.0mgL-1 kinetin was the most efficient for callus formation. This same auxin/ cytokinin (2/1) proportion, used in the present experiment (0.1mgL-1 NAA plus 0.05mgL-1 BAP) increased callus production significantly, but the highest frequency was observed in a medium supplemented with a 1/1 mix of auxin/cytokinin. This result is in agreement with ROGOZINSKA & SKUTINK (1974), who used both NAA and BAP at a concentration of 1.0mgL-1.
In this experiment, auxin in the form of 2,4 dichlorophenoxyacetic acid (2,4 D) (2.0mgL-1)did not stimulate callus induction. However, HENDER (1973) verified better callus development when cultivating tomato anthers in this same concentration of 2,4 D. The presence of 2,3,5 triiodobenzoic acid (TIBA) in the culture (medium four and five) did not propitiate either calli production, although this anti-auxin has been used to obtain doubled haploid maize (WILLADINO et al., 1995).
In the medium supplemented with 1.0mgL-1 GA3 plus 0.05mgL-1 BAP plus 0.1mgL-1 NAA, a darkening of the anthers surface was observed, although green calli were produced in about 21% of them. GRESSHOFF & DOY (1972) also observed a similar process of calli formation, but these calli presented a pale yellow color. Green calli were also produced on the medium supplemented with 1.0mgL-1 BAP and 1.0mgL-1 NAA.
Histological analysis showed that some of these calli presented tracheids, vessel elements and phloem cells initiation. Such structures were verified by GULSHAN et al. (1981) in tomato anther callus and the initiation of vessel elements was favoured by addition of NAA plus kinetin and that of the phloem in the presence of 2,4 D plus BAP.
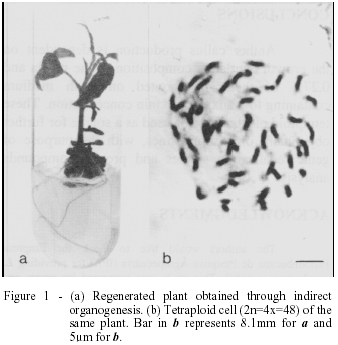
The calli that presented development of vascular elements were subcultured on a medium containing 0.01mgL-1 BAP plus 0.001mgL-1 NAA, showing later organogenic potential. After five months, plant regeneration had occurred on 0,27% of the calli obtained (figure 1a). Cytological analysis showed that these plants presented tetraploid cells and rare diploid cells (figure 1b). Such differences on ploidy level can be induced by components of the culture medium, time of culture, explant source, and the plant genotype (KARP, 1988). This instability was observed by KORNNEEF et al. (1989) in cells of plants regenerated from leaves of haploid genotypes of tomato. Of the 149 metaphase cells of root tips that were analyzed 41% were haploid, 58% diploid and 1% tetraploid. ANCORA et al. (1977), studying genotypes L. peruvianum, also observed that cells from one anther callus can differ in ploidy level.
Besides the microspores, growth of somatic tissue (filament, connective, or anther wall) could be induced ( WENZEL & FOROUGHI-WEHR, 1984). Levenko et al. (1977) verified that anther wall cells took part in the formation of tomato mixoploid calli. CAPPADOCIA & SREE RAMULU (1980) and ZAMIR et al. (1980; 1981) obtained plants derived from anther culture. However they were originated from somatic cells.
In anther culture, there are two pathways for plant regeneration: direct or indirect androgenesis (CANHOTO et al., 1990). Most of the plants regenerated through direct embryogenesis are haploids, while plants obtained from calli are generally diploids (CONSTANTIN, 1981).
In the present study we described the induction of anther organogenic calli, and regeneration of tomato tetraploid plants. In Oenothera hookeri De Vries, diploid plants were also obtained through indirect androgenesis (MARTINEZ & HALAC, 1995). In Solanaceae family, as in Datura metel (IYER & RAINA, 1972), both kinds of androgenesis, direct (embryos formation) or indirect (embryos or shoots formation), may be observed (OGURA, 1990). It appears that the type of androgenesis followed is to some extent determined by the culture medium composition and by the endogenous levels of growth regulators prevailing within anther, varying the magnitude of these influences with the species considered (CANHOTO et al., 1990). Further studies are necessary to understand the pathway by which environmental and genetical factors induce shooting from callus tissue (MARTINEZ & HALAC, 1995).
CONCLUSIONS
Anther callus production is dependent on the growth regulators composition of the media and 0,27% of the calli shooted on fresh medium containing low auxin-cytokinin concentration. These tetraploid plants could be used as a source for further obtainment of trisomic lines, with the purpose of genic localization studies and protein compounds analysis.
ACKNOWLEDGMENTS
The authors would like to thank the Empresa Pernambucana de Pesquisa Agropecuária (IPA) for providing L. esculentum material. This study was supported by grants and fellowships from Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Banco do Nordeste do Brasil (BNB).
2 Biólogo, Doutor, Professor Adjunto, Departamento de Biologia, UFRPE. E-mail: lilia@truenet.com.br.
3 Biólogo, Mestre, Professor Assistente, Departamento de Biologia, UFRPE. E-mail: carvalheira@netpe.com.br.
4 Biólogo, PhD., Professor Adjunto, Departamento de Botânica, Universidade Federal de Pernambuco, 50732-970, Recife, PE. E-mail: mguerra@npd.ufpe.br.
Recebido para publicação em 21.10.98. Aprovado em 27.01.99
- ANCORA, G., SREE RAMULU, K., DEVREUX, M. In vitro culture of anthers and stem internodes of Lycopersicon peruvianum: nuclear DNA determination in calli and cytological analysis of regenerated plants. Zeitschrift fuer Pflanzenphysiology, Stuttgart, v. 82, p. 377- 388, 1977.
- CANHOTO, J.M., LUDOVINA, M., GUIMARÃES, S., et al In vitro induction of haploid, diploid and triploid plantlets by anther culture of Iochroma warscewiczii Regel. Plant Cell, Tissue and Organ Culture, Dordrecht, v. 21, p. 171- 177, 1990.
- CAPPADOCIA, M., SREE RAMULU, K. Plant regeneration from in vitro cultures of anthers and stem internodes in an interspecific hybrid, Lycopersicon esculentum x L. peruvianum Plant Science Letters, Limerick, v. 20, p. 157- 166, 1980.
- CONSTANTIN, M.J. Chromosome instability in cell and tissue cultures and regenerated plants. Environmental and Experimental Botany, Elmsford, v. 21, p. 359- 368, 1981.
- DAO, N.T., SHAMINA, Z.B. Cultivation of isolated tomato anthers. Soviet Plant Physiology, New York, v. 25, p. 120- 126, 1978.
- FUJII, M.T., GUERRA, M. Improving the hematoxylin staining method for algal cytogenetics. Biotechnique & Histochemistry, Louisville, v. 73, n. 2, p. 7881, 1998.
- GRESSHOFF, P.M., DOY, C.H. Development and differentiation of haploid Lycopersicon esculentum (tomato). Planta, Berlin, v. 107, p. 161170, 1972.
- GUERRA, M. dos S. O uso de Giemsa na citogenética vegetal - comparação entre a coloração simples e o bandeamento. Ciência e Cultura, São Paulo, v. 35, p. 190193, 1983.
- GULSHAN, T.M., VARGHESE, SHARMA, D.R. Studies on anther cultures of tomato Lycopersicon esculentum Mill. Biologia Plantarum, Prague, v. 23, n. 6, p. 414- 420, 1981.
- HENDER, A.B. Anther culture in the cultivated tomato, Lycopersicon esculentum Annual Report Glasshouse Crops Research Institute, p. 130133, 1973.
- HU, H., ZENG, J.Z. Development of new varieties via anther culture. In: AMMIRATO, P.V., EVANS, D.A., SHARP, W.R., et al. (eds). Handbook of Plant Cell Culture New York: Macmillan, 1984. v. 3. p. 6590.
- IYER, R.D., RAINA, S.K. The early ontogeny of embryoids and callus from pollen and subsequent organogenesis in anther cultures of Datura metel and rice. Planta, Berlin, v. 104, p. 146156, 1972.
- JARAMILLO, J., SUMMERS, W.L. Dark-light treatments influence induction of tomato anther callus. HortScience, Alexandria, v. 26, n. 7, p. 915916, 1991.
- JOHANSEN, W.A. Plant microtechnique. New York: MacGraw Hill, 1940. 523 p.
- KARP, A. Origins and causes of chromosome instability in plant tissue culture and regeneration. In: BRANDHAM, P.E. (ed). Kew Chromosome Conference III, HMSO. Kew: Royal Botanic Gardens, 1988. p. 185- 192.
- KOORNNEEF, M., DIEPEN J. van, HANHART, C.J., et al. Chromosomal instability in cell and tissue cultures of tomato haploids and diploids. Euphytica, Dordrecht, v. 43, n. 1/2, p. 179- 186, 1989.
- LEVENKO, B.A., KUNAKH, V.A., YURKOVA, G.N. Studies on callus tissue from anthers. I. Tomato. Phytomorphology, Delhi, v. 27, n. 4, p. 377- 383, 1977.
- MARTINEZ, L.D, HALAC, I.N. de. Organogenesis of anther-derived calluses in long-term cultures of Oenothera hookeri de Vries. Plant Cell, Tissue and Organ Culture, Dordrecht, v. 42, p. 91- 96, 1995.
- MORAES-FERNANDES, M.I.B de. Obtenção de plantas haplóides através da cultura da anteras. In: TORRES, A.C., CALDAS, L.S (eds). Técnicas e aplicações da cultura de tecidos de plantas Brasília: EMBRAPA, Centro Nacional de Pesquisas de Hortaliças, 1990. p. 311- 332.
- MURASHIGE, T., SKOOG, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, Copenhagen, v. 15, p. 473497, 1962.
- NITSCH, J.P. Experimental androgenesis in Nicotiana. Phytomorphology, Delhi, v. 19, p. 389404, 1969.
- NURHIDAYAH, T., HORN, R., RÖCHER, T., et al. High regeneration rates in anther culture of interspecific sunflower hybrids. Plant Cell Reports, Berlin, v. 16, p. 167173, 1996.
- OGURA, H. Chromosome variation in plant tissue culture. In: BAJAJ, Y.P.S. Somaclonal variation in crop improvement I Berlin: Springer-Verlag, 1990, p. 4984. Biotechnology in Agriculture and Forestry, 11.
- ROGOZINSKA, J.H., SKUTNIK, L. Effects of auxins and cytokinins on tomato callus from anthers. Acta Societatis Botanicorum Poloniae, Warsaw, v. 43, n. 1, p. 8192, 1974.
- SHARP, W.R., DOUGALL, D.K., PADDOCK, E.F. Haploids plantlets and callus from immature pollen grains of Nicotianaand Lycopersicon Bulletin of the Torrey Botanical Club, New York, v. 98, p. 219- 222, 1971.
- SUMMERS, W.L., JARAMILLO, J., BAILEY, T. Microspore developmental stage and anther length influence the induction of tomato anther callus. HortScience, Alexandria, v. 27, p. 838840, 1992.
- WENZEL, G., FOROUGHI-WEHR, B. Anther culture of cereals and grasses. In: VASIL, I. K. (ed) Cell culture and somatic cell genetics of plants. London: Academic Press, 1984. v. 1, p. 311327.
- WILLADINO, L, CAMARA, T.R., SANTOS, M.A., et al. Obtenção de uma linhagem de milho tolerante ao estresse salino mediante a cultura de anteras. Pesquisa Agropecuária Brasileira, Brasília, v. 30, n. 11, p. 13131318, 1995.
- ZAMIR, D., JONES, R.A., KEDAR, N. Anther culture of male-sterile tomato (Lycopersicon esculentum Mill.) mutants. Plant Science Letters, Limerick, v. 17, p. 353361, 1980.
- ZAMIR, D., TANKSLEY, S.D., JONES, R.A. Genetic analysis of the origin of plants regenerated from anther tissues of Lycopersicon esculentum Mill. Plant Science Letters, Limerick, v. 21, p. 223227, 1981.
- ZIV, M., HADARY, D., KEDAR, N. Dihaploid plants regenerated from tomato anthers in vitro Plant Tissue Culture. Proceedings 5th International Congress of Plant Tissue & Cell Culture p. 549550, 1982.
Publication Dates
-
Publication in this collection
08 Dec 2006 -
Date of issue
Dec 1999
History
-
Accepted
27 Jan 1999 -
Received
21 Oct 1998