Abstracts
To evaluate the effect of follicular fluid on in vitro maturation, pig oocytes were cultured in the presence of hormones where 10% of fetal calf serum (FCS), 10% of follicular fluid from large follicles (l-pFF), 10% of follicular fluid from medium follicles (m-pFF) or no supplement were added. When oocytes where matured in medium containing the hormones the addition of different supplements did not affect (P<0.05) nuclear maturation. However, changing the supplement altered the cytoplasmic maturation, with higher rate (P<0.05) observed in the l-pFF group. To determine the effect of the presence of hormone and/or supplement during maturation, the oocytes were cultured either in presence of TCM-199 alone, with hormones, with 10% l-pFF or with hormones and 10% l-pFF. The highest proportion of oocytes undergoing nuclear and cytoplasmic maturation was obtained when both hormones and follicular fluid were present. Cumulus expansion had a significant (P<0.05) effect on cytoplasmic maturation with the non-expanded groups showing a lower percentage of maturation in all groups. When the adequacy of gonadotropins levels were evaluated by adding higher or lower concentrations into the maturation medium neither beneficial nor detrimental effects were observed in either nuclear or cytoplasmic maturation. These results suggest that changes in the composition of the medium can alter the percentage of oocytes completing maturation. Follicular fluid combined with hormones was found to give better conditions for pig oocyte maturation in vitro.
follicular fluid; oocyte maturation; gonadotrophins; porcine
O presente estudo avaliou o efeito da presença do líquido folicular e hormônios durante a maturação nuclear e citoplasmática em ovócitos suínos. Para avaliar o efeito do líquido folicular, os oócitos foram maturados in vitro em TCM-199 na presença de hormônios, em que o meio foi suplementado com 10% de soro fetal bovino (SFB), 10% de líquido folicular de folículos grandes (l-pFF), 10% de líquido folicular de folículos médios (m-pFF) ou nenhum suplemento. Quando os oócitos foram maturados no meio contendo hormônios, a adição de diferentes suplementos não afetou (P<0,05) a maturação nuclear. Entretanto, mudanças no suplemento provocaram uma alteração na maturação citoplasmática, sendo que as maiores taxas (P<0,05) foram observadas no grupo com l-pFF. Para determinar o efeito da presença de hormônios e/ou suplemento durante a maturação, os oócitos foram cultivados em TCM-199, TCM-199 com hormônios, TCM-199 com 10% l-pFF ou TCM-199 com hormônio e 10% l-pFF. A maior proporção de oócitos que sofreram maturação nuclear e citoplasmática ocorreu no grupo em que ambos, hormônios e líquido folicular, estavam presentes. A expansão do cumulus influenciou (P<0,05) na maturação citoplasmática, sendo que os oócitos sem expansão apresentaram menor taxa de maturação em todos os grupos. Quando diferentes concentrações de gonadotrofinas foram utilizadas durante a maturação, não foi observado nenhum efeito na maturação nuclear ou citoplasmática. Os resultados deste estudo sugerem que as mudanças na composição do meio podem afetar as taxas de maturação, sendo que o líquido folicular, combinado com hormônios, proporciona as melhores condições para maturação de oócitos suínos.
líquido folicular; maturação; oócito; gonadotrofinas; suíno
INFLUENCE OF HORMONES AND FOLLICULAR FLUID ON MATURATION OF PIG OOCYTES
INFLUÊNCIA DE HORMÔNIOS E LÍQUIDO FOLICULAR NA MATURAÇÃO DE OÓCITOS SUÍNOS
Margot Alves Nunes Dode1 1 Médico Veterinário, PhD., Embrapa Gado de Corte, Rodovia BR 262 km 4, CP 154, 79002-970 Campo Grande, MS. E-mail: margot@cenargen.embrapa.br. Autor para correspondência. Charles Graves2 1 Médico Veterinário, PhD., Embrapa Gado de Corte, Rodovia BR 262 km 4, CP 154, 79002-970 Campo Grande, MS. E-mail: margot@cenargen.embrapa.br. Autor para correspondência.
SUMMARY
To evaluate the effect of follicular fluid on in vitro maturation, pig oocytes were cultured in the presence of hormones where 10% of fetal calf serum (FCS), 10% of follicular fluid from large follicles (l-pFF), 10% of follicular fluid from medium follicles (m-pFF) or no supplement were added. When oocytes where matured in medium containing the hormones the addition of different supplements did not affect (P<0.05) nuclear maturation. However, changing the supplement altered the cytoplasmic maturation, with higher rate (P<0.05) observed in the l-pFF group. To determine the effect of the presence of hormone and/or supplement during maturation, the oocytes were cultured either in presence of TCM-199 alone, with hormones, with 10% l-pFF or with hormones and 10% l-pFF. The highest proportion of oocytes undergoing nuclear and cytoplasmic maturation was obtained when both hormones and follicular fluid were present. Cumulus expansion had a significant (P<0.05) effect on cytoplasmic maturation with the non-expanded groups showing a lower percentage of maturation in all groups. When the adequacy of gonadotropins levels were evaluated by adding higher or lower concentrations into the maturation medium neither beneficial nor detrimental effects were observed in either nuclear or cytoplasmic maturation. These results suggest that changes in the composition of the medium can alter the percentage of oocytes completing maturation. Follicular fluid combined with hormones was found to give better conditions for pig oocyte maturation in vitro.
Key words: follicular fluid, oocyte maturation, gonadotrophins, porcine
RESUMO
O presente estudo avaliou o efeito da presença do líquido folicular e hormônios durante a maturação nuclear e citoplasmática em ovócitos suínos. Para avaliar o efeito do líquido folicular, os oócitos foram maturados in vitro em TCM-199 na presença de hormônios, em que o meio foi suplementado com 10% de soro fetal bovino (SFB), 10% de líquido folicular de folículos grandes (l-pFF), 10% de líquido folicular de folículos médios (m-pFF) ou nenhum suplemento. Quando os oócitos foram maturados no meio contendo hormônios, a adição de diferentes suplementos não afetou (P<0,05) a maturação nuclear. Entretanto, mudanças no suplemento provocaram uma alteração na maturação citoplasmática, sendo que as maiores taxas (P<0,05) foram observadas no grupo com l-pFF. Para determinar o efeito da presença de hormônios e/ou suplemento durante a maturação, os oócitos foram cultivados em TCM-199, TCM-199 com hormônios, TCM-199 com 10% l-pFF ou TCM-199 com hormônio e 10% l-pFF. A maior proporção de oócitos que sofreram maturação nuclear e citoplasmática ocorreu no grupo em que ambos, hormônios e líquido folicular, estavam presentes. A expansão do cumulus influenciou (P<0,05) na maturação citoplasmática, sendo que os oócitos sem expansão apresentaram menor taxa de maturação em todos os grupos. Quando diferentes concentrações de gonadotrofinas foram utilizadas durante a maturação, não foi observado nenhum efeito na maturação nuclear ou citoplasmática. Os resultados deste estudo sugerem que as mudanças na composição do meio podem afetar as taxas de maturação, sendo que o líquido folicular, combinado com hormônios, proporciona as melhores condições para maturação de oócitos suínos.
Palavras-chave: líquido folicular, maturação, oócito, gonadotrofinas, suíno.
INTRODUCTION
There is some suggestion that addition of blood serum as a supplement to the culture medium provides a superior environment for oocyte maturation. Bovine and porcine oocytes matured and fertilized in vitro were shown to be capable of developing to the blastocyst stage in serum-free medium (PINYOPUMMINYTR & BAVISTER, 1991; ABEYDEERA et al., 1998). Nevertheless, superior fertilizability of bovine (LEIBFRIED-RUTLEDGE et al., 1986; ZUELK & BRACKETT, 1990) oocytes was obtained in medium containing serum. On the other hand, the use of follicular fluid to replace serum in order to increase the efficiency of maturation has been proposed, since the follicular fluid is composed of follicular secretions, which in vivo supports oocyte maturation. Results of some studies have shown a positive effect of follicular fluid on nuclear and cytoplasmic maturation (NAITO et al., 1989; RATH et al., 1995), while some investigators reported no beneficial influence of the follicular fluid in male pronucleus (MPN) formation (YOSHIDA et al., 1992).
Another factor that affects oocyte maturation in vitro is the presence of hormones, especially gonadotropins (LIU et al., 1997). Although the mechanism of action of LH and FSH during maturation is not very well understood, the addition of these hormones during maturation is a current procedure. The concentration of the gonadotropin added to the maturation medium varies from 0.5mg/m to 10mg/m
to 10mg/m (MATTIOLI et al.1988; ZHENG & SIRARD, 1992; ROSE & BAVISTER, 1992); however, the circulating levels after the LH surge in the pig are not higher than 20ng/m
(MATTIOLI et al.1988; ZHENG & SIRARD, 1992; ROSE & BAVISTER, 1992); however, the circulating levels after the LH surge in the pig are not higher than 20ng/m of FSH and 5.9ng/m
of FSH and 5.9ng/m of LH (VAN DE WIEL et al., 1981). In addition, the concentration of these hormones in the follicular fluid has been shown in humans to be approximately 30% of the serum levels. Thus, the concentrations usually added to the maturation medium are much higher than those present in either the serum or the follicular fluid.
of LH (VAN DE WIEL et al., 1981). In addition, the concentration of these hormones in the follicular fluid has been shown in humans to be approximately 30% of the serum levels. Thus, the concentrations usually added to the maturation medium are much higher than those present in either the serum or the follicular fluid.
Therefore, the objectives of this study were to examine the effect of different supplements added to the medium on nuclear and cytoplasmic maturation and to determine the adequacy of the gonadotropin levels added during maturation in vitro of pig oocytes.
MATERIAL AND METHODS
The cumulus oocyte complexes (COC) were recovered by aspiration of 2mm to 5mm diameter follicles. After washing the COCs were transfered to culture medium and cultured for 42 hours at 39ºC in an atmosphere 5% CO2 in air. The basic medium for maturation was TCM-199 supplemented with pyruvic acid (44mg/m ), 100IU/m
), 100IU/m of penicillin, 50mg/m
of penicillin, 50mg/m of streptomycin, 25mM Hepes , 5mg/m
of streptomycin, 25mM Hepes , 5mg/m of LH , 2.5mg/m
of LH , 2.5mg/m of FSH, 0.5mg/m
of FSH, 0.5mg/m estradiol-17-b and 10% of fetal calf serum (FCS). Changes in the basic medium were made according to each aspect to be examined in oocyte maturation.
estradiol-17-b and 10% of fetal calf serum (FCS). Changes in the basic medium were made according to each aspect to be examined in oocyte maturation.
Porcine follicular fluid was withdrawn with a syringe from follicles of two sizes, medium-size (m-pFF) from 2mm to 5mm in diameter and large-size follicles (l-pFF) from 6mm to 8mm in diameter. After collection the follicular fluid was filtered and stored at -20ºC in 500m aliquots. After culture the oocytes were fixed for 48 hours in acetic acid-alcohol (1:3) and then stained with 1% lacmoid in 45% glacial acetic acid. The maturational stage of each oocyte was determined by using phase contrast microscopy (400-2000x) with only the oocyte in metaphase II being considered nuclear matured. Oocytes having one female pronucleus (FPN) and at least one male pronucleus (MPN), after fertilization, were considered as cytoplasmic matured.
aliquots. After culture the oocytes were fixed for 48 hours in acetic acid-alcohol (1:3) and then stained with 1% lacmoid in 45% glacial acetic acid. The maturational stage of each oocyte was determined by using phase contrast microscopy (400-2000x) with only the oocyte in metaphase II being considered nuclear matured. Oocytes having one female pronucleus (FPN) and at least one male pronucleus (MPN), after fertilization, were considered as cytoplasmic matured.
For in vitro fertilization, boar fresh semen samples were diluted in a 1:2 dilution with a commercial extender (Modema), and incubated for 24 hours at 16ºC. Before use the spermatozoa were washed twice with BSA-saline (10mg/100m ), and then incubated at 39ºC for 20-30 minutes, during which time the oocytes were processed.
), and then incubated at 39ºC for 20-30 minutes, during which time the oocytes were processed.
After the maturation, oocytes from each treatment were washed and transfered to fertilization medium (YOSHIDA et al., 1990), which was composed of modified TCM-199 with 10% of FCS and 0.04g of caffeine/100m , with pH of 7.4. The spermatozoa were diluted to make a final concentration of 5x105 cells/m
, with pH of 7.4. The spermatozoa were diluted to make a final concentration of 5x105 cells/m and were incubated with the oocytes for 8 hours. After this time, the oocytes were transfered to a 50m
and were incubated with the oocytes for 8 hours. After this time, the oocytes were transfered to a 50m drop of BMOC-2 medium and cultured for an additional 12 hours. At the end of the incubation the oocytes were fixed and stained.
drop of BMOC-2 medium and cultured for an additional 12 hours. At the end of the incubation the oocytes were fixed and stained.
Experiment 1 evaluated the effect of follicular fluid during in vitro maturation. COCs were cultured in TCM-199 in the presence of hormones where 10% of FCS, 10% l-pFF, 10% m-pFF or no supplement were added. After maturation oocytes were fertilized in vitro, fixed and stained.
In experiment 2 the effect of the presence of hormone and/or supplement during maturation was determined. The oocytes were cultured either in presence of TCM-199 alone, TCM-199 with hormones (LH, FSH and estradiol), TCM-199 with 10% l-pFF (v/v) or TCM-199 with hormones and 10% l-pFF.
Experiment 3 was designed to determine whether cumulus expansion is an important factor for cytoplasmic maturation independent of the maturation conditions. The COCs were divided into three treatments: no supplement, 10% FCS and 10% l-pFF. For IVF, the oocytes from each treatment were placed in separated dishes according with their cumulus appearance.
In the experiment 4 the adequacy of the levels of the LH and FSH used in our system was investigated. Oocytes were matured in TCM-199 supplemented with 10% l-pFF and estradiol in the absence of gonadotropin or in the presence of 0.25mg/m and 0.5mg/m
and 0.5mg/m , 2.5mg/m
, 2.5mg/m and 5.0mg/m
and 5.0mg/m or 25mg/m
or 25mg/m and 50mg/m
and 50mg/m of FSH and LH, respectively. After maturation and fertilization, the eggs were fixed and stained. Levels of LH were measured in the follicular fluid by a RIA method described previously (MATTERI et al., 1987).
of FSH and LH, respectively. After maturation and fertilization, the eggs were fixed and stained. Levels of LH were measured in the follicular fluid by a RIA method described previously (MATTERI et al., 1987).
All the data were analyzed by analysis of variance (SAS Institute Inc., Cary, NC 27512-8000, USA), for a randomized complete block design. When a significant (P<0.05) effect was detected, Least Significant Difference (LSD) test was performed to determine differences among treatment means
RESULTS AND DISCUSSION
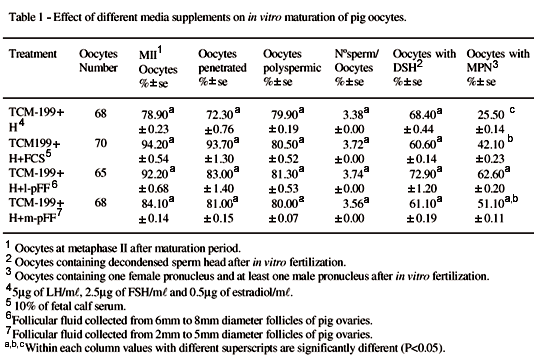
When COCs were incubated in medium containing hormones the addition of different supplement did not affect (P<0.05) nuclear maturation (Table 1). Follicular fluid and serum have many common elements such as hormones, proteins and growth factors, and both have been shown as to be beneficial for maturation. Although the results of this study suggest that a protein supplement in the medium is not required for nuclear maturation, cytoplasmic maturation can be increased by adding different source of protein. The development to the pronuclei stage was significantly higher in l-pFF (P<0.05) than that observed in other treatment groups except for the m-pFF which did not differ from l-pFF. The various components of follicular fluid have been shown to change in different phases of follicular development (AINSWORTH et al., 1980). Therefore, the levels of maturation obtained with the l-pFF group suggest that maybe there is an effective substance/s that is present in l-pFF which is lacking or is present in lower concentration in m-pFF and serum. In fact, ROMERO-ARREDONDO & SEIDEL (1996) showed that follicular fluid obtained 20 hours after LH surge gave better results in the in vitro maturation than the 0 hour follicular fluid.
As shown in table 2, addition of supplement alone to the medium failed to cause significant stimulus in nuclear and cytoplasmic maturation, showing that follicular fluid in the absence of hormones is unable to support maturation. On the other hand, the addition of hormones to the medium, in the absence of pFF, did not improved the percentage of oocytes with MPN formation, suggesting that gonadotropins alone are also insufficient to provide suitable environment for maturation. A marked increase in nuclear and cytoplasmic maturation of pig oocytes was only obtained when both hormones and supplement were present in the medium. This is consistent with others reports which show that secretion of follicle cells and gonadotropins are needed to promote the better conditions for maturation of pig oocytes (RATH et al., 1995; LIU et al., 1997).
When the COCs were matured in different supplements and the results grouped according to cumulus expansion (Table 3), no COC underwent expansion in the absence of either the FCS or l-pFF. The expansion of cumulus cells has been reported to be induced in vitro by FSH (HILLENSJO & CHANNING, 1980) with the presence of serum required to retain the secreted hyaluronic acid within the cumulus cell complex (EPPIG, 1980). Therefore, the absence of some components in the medium explains the low percentage or the absence of expansion when no supplement was used.
Cumulus expansion was not an important aspect in obtaining nuclear maturation since similar (P>0.05) percentages of oocytes underwent nuclear maturation in COCs possessing either an expanded or non-expanded cumulus (Table 3). Therefore, the lower maturation rate observed in the non supplemented group could be more associated with the poor environment than with the cumulus expansion. However, the ability to form a MPN was markedly influenced (P<0.05) not only by the addition of a supplement to the maturation medium but also to the ability of the cumulus to undergo expansion during maturation (Table 3). Thus, expansion seems to be an important factor in the pig oocyte maturation according to the present study, because expansion of the cumulus was consistently associated with increase in cytoplasmic maturation, independent of the supplement present. This is supported by NAKAYANA et al. (1996), who reported that in the pig the synthesis of hyaluronic acid is dependent of the oocyte, therefore the expansion of the cumulus does indicate the presence of a healthy COC that can achieve cytoplasmic maturation.
The percentage of oocytes penetrated by sperm was lower (P<0.05) in the non supplemented group than in the FCS or l-pFF groups (Table 3). In addition, that group also showed no expansion of the cumulus, low decondensed sperm head and low MPN formation. Studies in the pig oocyte showed that only oocytes functionally connected to the cumulus cells throughout maturation could be successfully penetrated by sperm. In fact, GALEATI et al. (1991) established the effect of somatic cells on cortical granule distribution, and confirmed that premature loss of intercellular coupling can cause a premature migration of cortical granules. Therefore, the conditions of the non supplemented medium could cause a premature loss of contact between the oocyte and the cumulus cells, resulting in premature exocytosis of the cortical granules and subsequent change in the egg penetrability.
The follicular fluid used in this experiment contained almost undetectable levels of LH (0.33ng/m ). The addition of 2.5mg/m
). The addition of 2.5mg/m of FSH and 5.0mg/m
of FSH and 5.0mg/m of LH used in our system stimulated a significant increase in the nuclear and cytoplasmic (P<0.05) maturation (Tables 1 and 2). When the adequacy of those levels were evaluated, by adding higher or lower concentrations of the gonadotropins into the maturation medium, neither beneficial nor detrimental effects were observed in nuclear or cytoplasmic maturation (Table 4). The site of action and mechanism of FSH and LH in the COC is unknown although their beneficial effects are well defined. One possibility is that gonadotropins stimulate some substance on the cumulus and/or granulosa cells which would act in the oocyte. This possibility is supported by the results of studies on localization of LH receptors in rat ovaries (BUROVSKY et al., 1993), which showed the presence of LH receptors in granulosa cells surrounding the oocytes of healthy preantral follicles. In fact, MATTIOLI & BARBONI (1998) suggested that the somatic cells read the LH signal and then they elaborate a message which is send to the oocyte inducing maturation. The concentrations of LH and FSH used in this experiment seem to be unnecessary, since much lower concentrations can be used in the maturation medium without losing the effectivess in improving maturation.
of LH used in our system stimulated a significant increase in the nuclear and cytoplasmic (P<0.05) maturation (Tables 1 and 2). When the adequacy of those levels were evaluated, by adding higher or lower concentrations of the gonadotropins into the maturation medium, neither beneficial nor detrimental effects were observed in nuclear or cytoplasmic maturation (Table 4). The site of action and mechanism of FSH and LH in the COC is unknown although their beneficial effects are well defined. One possibility is that gonadotropins stimulate some substance on the cumulus and/or granulosa cells which would act in the oocyte. This possibility is supported by the results of studies on localization of LH receptors in rat ovaries (BUROVSKY et al., 1993), which showed the presence of LH receptors in granulosa cells surrounding the oocytes of healthy preantral follicles. In fact, MATTIOLI & BARBONI (1998) suggested that the somatic cells read the LH signal and then they elaborate a message which is send to the oocyte inducing maturation. The concentrations of LH and FSH used in this experiment seem to be unnecessary, since much lower concentrations can be used in the maturation medium without losing the effectivess in improving maturation.
CONCLUSION
The results of this study indicated that neither supplement nor hormone alone are capable of supporting pig oocyte in vitro maturation; cytoplasmic maturation rates can be increased by supplementing with follicular fluid, especially that obtained from large follicles; lower concentrations of gonadotropins can be used during maturation with no detrimental effect in maturation.
2 PhD., Professor Department Animal Science, University of Illinois.
Recebido para publicação em 16.11.99. Aprovado em 10.05.00
- ABEYDEERA, L.A., WANG, W., PRATHER, R.S., et al.. Maturation in vitro of pig oocytes in protein-free culture media: fertilization and subsequent embryo development in vitro Biology of Reproduction, Champaign, v.58, n.5, p.1316-1320, 1998.
- AINSWORTH, L., TSANG, B.K., DOWNEY, B.R. et al Interrelationship between follicular fluid steroid levels, gonadotrophic stimuli and oocyte maturation during preovulatory development of porcine follicles. Biology of Reproduction, Champaign, v.23, n.3, p.621-627, 1980.
- BUROVSKY, A., CHEN,T.T., WIMALASENA, J.Et al Cellular localization of luteinizing hormone receptor imunoreactivity in the ovaries of immature, gonadotropin-primed and normal cycling rats. Biology of Reproduction, Champaign, v.48, p.1367-1382, 1993.
- EPPIG, J.J. Role of serum in FSH-stimulated cumulus expansion by mouse oocyte-cumulus cell complex in vitro Biology of Reproduction, Champaign, v.22, n.3, p. 629-633,1980.
- GALEATI, G., MODINA, S., LAURIA, A., et al.. Follicle somatic cells influence pig oocyte penetrability and cortical granule distribution. Molecular Reproduction and Development, New York, v.29, n.1, p.40-46, 1991.
- HILLENSJO, T., CHANNING, C.P. Gonadotropin stimulation of steroidogenesis and cellular dispersion in the cultured porcine cumuli oophori. Gamete Research, New York, v.3, p.233-240, 1980.
- LEIBFRIED-RUTLEDGE, M.L., CRITSER, E.S., FIRST, N.L. Effects of fetal calf serum and bovine serum albumin onin vitromaturation and fertilization of bovine and hamster cumulus-oocyte complexes. Biology of Reproduction, Champaign, v.35, n.4, p.35:850-857, 1986.
- LIU, L., YANFENG, D., MOOR, R.M. Role of secreted proteins and gonadotrophins in promoting full maturation of porcine oocytes in vitro Molecular Reproduction Development, New York, v.47, n.2, p.191-199, 1997.
- MATTERI, R.L., ROSER, J.F., BALDWIN, D.M., et al Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domestic Animal Endocrinology, New York, v.4, p.157-165, 1987.
- MATTIOLI, M., BARBONI, B. Induction of oocyte maturation. In: LAURIA, A., GANDOLFI, F., ENNE, G., et al (eds.). Gametes: development and function Roma : Serono Symposia, 1998. p.141-153.
- MATTIOLI, M., GALEATI, G., SEREN, E. Effect of follicle somatic cells during pig oocyte maturation on egg penetrability and male pronucleus formation. Gamete Research, New York, v.10, p.177-183, 1988.
- NAKAYAMA, T., INOUE, M., SATO, E. Effect of oocytectomy on glycosaminoglycan composition during cumulus expansion of porcine cumulus-oocyte complexes culture in vitro. Biology of Reproduction, Champaign, v.55, n.6, p.1299-1304, 1996.
- NAITO, K., FUKUDA, Y., ISHIBASHI, I. Developmental ability of porcine ova matured in porcine follicular fluid in vitro and fertilized in vitro Theriogenology, Los Altos, v.31, n.5, p.1049-1057, 1989.
- PINYOPUMMINYTR, T., BAVISTER, B.D. In vitro matured/in vitro fertilized bovine oocytes can develop into morula/blastocyst in chemically defined, protein-free culture media. Biology of Reproduction, Champaign, v.45, p.736-742, 1991.
- RATH, D., NIEMANN, H., TAO, T. In vitro maturation of porcine oocytes in follicular fluid with subsequent effects on fertilization and embryo yield in vitro Theriogenology, Los Altos, v.44, n.4, p.529-538, 1995.
Publication Dates
-
Publication in this collection
10 June 2005 -
Date of issue
Feb 2001
History
-
Accepted
10 May 2000 -
Received
16 Nov 1999