Abstracts
The effects of topical and intraluminal Carolina Rinse Solution (CRS) in p44/42 (ERK 1/2) and p38 MAPK activation profile, in rabbit jejunum after ischemia and reperfusion (I/R), were investigated in this study. Fifteen New Zealand rabbits were allocated in three groups: Sham-operated (A), Ischemia and reperfusion (B) and CRS (C). Groups B and C were subjected to one hour of ischemia and two hours of reperfusion. In group C, ten minutes prior to reperfusion, the bowel lumen was filled with CRS, and the segment was immersed in CRS until reperfusion onset. Ischemia and reperfusion stimulated the phosphorylation of the p44/42 MAPK and p38 MAPK pathways in some layers of jejunum. Progressive activation of p44/42 MAPK was chiefly localized in the crypts of Lieberkühn, circular and longitudinal muscle layers, whereas p38 MAPK was prominently activated in myenteric plexus and both muscle layers. The results of this research indicate that the chosen model of topical and intraluminal CRS does not interfere in p44/42 and p38 MAPK activation profile in rabbit jejunum subjected to I/R.
ERK 1/2; intestine; ischemia; p38; reperfusion
Os efeitos do uso tópico e intraluminal da Solução de Carolina Rinse (CRS), no perfil de ativação das MAP quinases p44/42 (ERK 1/2) e p38, no jejuno de coelhos após isquemia e reperfusão (I/R), foram investigados neste estudo. Quinze coelhos da raça Nova Zelândia foram alocados em três grupos: Instrumentado (A), Isquemia e Reperfusão (B) e CRS (C). Os grupos B e C foram submetidos a uma hora de isquemia e duas horas de reperfusão. No grupo C, dez minutos antes da reperfusão, o lúmen do segmento isolado foi preenchido com CRS e o segmento foi imerso em CRS até o início da reperfusão. A isquemia e reperfusão resultou em estímulo da fosforilação das MAP quinases p44/42 e p38 em algumas camadas do jejuno. A ativação progressiva de p44/42 ocorreu principalmente nas criptas de Lieberkühn e camadas musculares longitudinal e circular, enquanto p38 foi ativada principalmente no plexo mioentérico e em ambas as camadas musculares. Os resultados deste trabalho indicam que o modelo escolhido de uso tópico e intraluminal de CRS não interfere no perfil de ativação das MAP quinases p44/42 e p38 no jejuno de coelhos submetidos à I/R.
ERK 1/2; intestino; isquemia; p38; reperfusão
ARTICLES
CLINIC AND SURGERY
Topical and intraluminal Carolina Rinse Solution in p44/42 and p38 MAP Kinase activation profile in rabbit jejunum after ischemia and reperfusion
Uso tópico e intraluminal da Solução de Carolina Rinse no perfil de ativação das MAP quinases p44/42 e p38 no jejuno de coelhos após isquemia e reperfusão
Luciana Ramos Gaston BrandstetterI,1 1 Autor para correspondência. ; Eugênio Gonçalves de AraújoI; Juan Carlos Duque MorenoI; Maria Clorinda Soares FioravantiI; Veridiana Maria Brianezi Dignani de MouraI; Patrícia de Almeida MachadoI
IUniversidade Federal de Goiás (UFG), Escola de Veterinária e Zootecnia, Rod. Goiania, Nova Veneza, km 0, Campus Samambaia, CP 131, 74001-970, Goiânia, GO, Brasil. E-mail: lubrands@yahoo.com.br
ABSTRACT
The effects of topical and intraluminal Carolina Rinse Solution (CRS) in p44/42 (ERK 1/2) and p38 MAPK activation profile, in rabbit jejunum after ischemia and reperfusion (I/R), were investigated in this study. Fifteen New Zealand rabbits were allocated in three groups: Sham-operated (A), Ischemia and reperfusion (B) and CRS (C). Groups B and C were subjected to one hour of ischemia and two hours of reperfusion. In group C, ten minutes prior to reperfusion, the bowel lumen was filled with CRS, and the segment was immersed in CRS until reperfusion onset. Ischemia and reperfusion stimulated the phosphorylation of the p44/42 MAPK and p38 MAPK pathways in some layers of jejunum. Progressive activation of p44/42 MAPK was chiefly localized in the crypts of Lieberkühn, circular and longitudinal muscle layers, whereas p38 MAPK was prominently activated in myenteric plexus and both muscle layers. The results of this research indicate that the chosen model of topical and intraluminal CRS does not interfere in p44/42 and p38 MAPK activation profile in rabbit jejunum subjected to I/R.
Key words: ERK 1/2, intestine, ischemia, p38, reperfusion.
RESUMO
Os efeitos do uso tópico e intraluminal da Solução de Carolina Rinse (CRS), no perfil de ativação das MAP quinases p44/42 (ERK 1/2) e p38, no jejuno de coelhos após isquemia e reperfusão (I/R), foram investigados neste estudo. Quinze coelhos da raça Nova Zelândia foram alocados em três grupos: Instrumentado (A), Isquemia e Reperfusão (B) e CRS (C). Os grupos B e C foram submetidos a uma hora de isquemia e duas horas de reperfusão. No grupo C, dez minutos antes da reperfusão, o lúmen do segmento isolado foi preenchido com CRS e o segmento foi imerso em CRS até o início da reperfusão. A isquemia e reperfusão resultou em estímulo da fosforilação das MAP quinases p44/42 e p38 em algumas camadas do jejuno. A ativação progressiva de p44/42 ocorreu principalmente nas criptas de Lieberkühn e camadas musculares longitudinal e circular, enquanto p38 foi ativada principalmente no plexo mioentérico e em ambas as camadas musculares. Os resultados deste trabalho indicam que o modelo escolhido de uso tópico e intraluminal de CRS não interfere no perfil de ativação das MAP quinases p44/42 e p38 no jejuno de coelhos submetidos à I/R.
Palavras-chave: ERK 1/2, intestino, isquemia, p38, reperfusão.
INTRODUCTION
In mammalian intestine, ischemia results from local (mechanical vascular obstruction) or systemic (hypovolemia, hypotension, sepsis) factors (BARIE, 1999). However, the reperfusion damage frequently exceeds the original ischemic insult (STALLION et al., 2002). There are evidences of MAP (Mitogen Activated Protein) kinases (MAPK) activation during ischemia and reperfusion and its contribution to structural and functional changes in affected organs (ABE et al., 2000). MAPK p38 and JNK are usually related to apoptosis (CROSS et al., 2000), while activated ERK 1/2 (p44/42) have a protection function, inhibiting apoptosis induced by ischemia and reperfusion (YUE et al., 2000).
Carolina Rinse Solution (CRS), a compound of electrolytes similar to plasma, allopurinol and glutathione as antioxidants, desferrioxamine as an iron chelator, the calcium channel blocker nicardipine, adenosine for improved microcirculation, and fructose and glucose as adenosine triphosphate substrates, was designed by North Carolina University, USA, with the purpose of minimizing reperfusion injury after liver transplant in rats (CURRIN et al., 1990).
The present study aimed to investigate p38 and p44/42 MAPK activation profile in a rabbit model of jejunal ischemia and reperfusion, and also to verify the influence of CRS in both enzymes activation.
MATERIAL AND METHODS
Animals and surgical procedure
Fifteen young adult male New Zealand rabbits (Oryctolagus cuniculus), weighing 2-3.5kg, were randomly allocated to three groups of five: Sham-operated (A), Ischemia and reperfusion (B), Carolina Rinse solution (C). Anesthesia was induced with an intravenous injection of 15 to 25mg kg-1 sodium thiopental (Thipentax®, Cristália, São Paulo, Brazil). Maintenance of anesthesia was obtained by continuous infusion of 40 to 60mg kg-1 hour-1 of 1.25% Thiopenthal. A midline incision was made starting caudal from the xiphoid process and extending approximately seven centimeters caudally. A 10cm segment of distal jejunum was exteriorized and the most distal part of its mesenteric artery and vein were identified. The vessels were isolated and sterile 0.2mm fishing line nylon monofilament was used to bring both vein and artery together into a 2cm section of polyvinyl urethral tube #4 in order to establish ischemia of the segment. Extramural circulation was blocked by tying #2 chromic catgut (Ethicon, Inc, New Brunswick, USA) sutures around the circumference of the small bowel at both ends of the segment. After one hour of ischemia, sutures were removed and the isolated segment remained in reperfusion for two hours. During ischemia and reperfusion the segment was maintained inside the abdominal cavity in order to keep it warm and moist. In group A, jejunum was exteriorized, and samples were harvested without occlusion of mesenteric vessels. In groups B and C, ischemia of the isolated segment was established for one hour and reperfusion for two hours. In group C, ten minutes prior to reperfusion, the lumen of the isolated segment was filled, but not distended with 1ml kg-1 body weight of CRS (YOUNG et al., 2002). The segment was also immersed in 30ml of CRS in a sterile small plastic bag until reperfusion was established. CRS solution was warmed up to 37°C prior to lumen injection and immersion. Animals were euthanized with a lethal dose of thiopental.
Samples harvesting and processing
Full-thickness 3mm biopsy specimens were harvested in five different time points during surgery: T0: Before vascular compromise; T5i: 5 minutes after ischemia onset; T5r: 5 minutes after reperfusion onset; T60r: 60 minutes after reperfusion onset and T120r: 120 minutes after reperfusion onset. In group A, samples were harvested at similar times, but vessels were not occluded. After sample harvesting, the intestinal defect was sutured using # 3-0 nylon monofilament (Moura e Draschi Ltda, Goiania, Brazil).
Immunohistochemistry
Biopsy specimens were immediately fixed in buffered 10% formalin for 24 hours and kept in 70% ethyl alcohol solution until dehydrated and embedded in paraffin. Five micrometers thick sections mounted onto glass slides were deparaffinized, hydrated and endogenous peroxidase activity was blocked using 10% hydrogen peroxide (H2O2) diluted in phosphate-buffered saline solution (PBS) for 15 minutes. Epitopes were retrieved using citrate buffer (pH 6,0) in a pressure cooker for six minutes and 3% bovine serum albumin (BSA) was added to block unspecific bindings. The tissues were then incubated overnight in a humid chamber at 4°C with mouse monoclonal phospho-ERK 1/2 (1:4000) and phospho-p38 (1:2000) antibodies. Primary antibodies clones used were p-ERK (E-4): sc-7383 and p-p38 (D-8): sc-7973 (Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA). Secondary antibody incubation was achieved by using Labeled streptavidin biotin (LSABTM + System-HRP, Dako, Carpinteria, California, USA). In order to immunolocalize the antigens, chromogen 3.3 diaminobenzidine (Liquid DAB Substrate Chromogen System®, Dako, Carpinteria, California, USA) was used for three minutes. Sections were dehydrated and slides were mounted with glass coverslips.
Data acquisition and statistical analysis
Digital images of the sections were obtained with a 20x objective (LeicaTM DM 4000 B, Germany) and analyzed for optical density of phospho-p44/42 and phospho-p38 MAPK labeling using semi-automatic software Image Processing and Analysis in Java (Image J, National Institutes of Health, Bethesda, Maryland, USA). Different jejunal areas were evaluated separately. Comparison between groups and times was analyzed by Kruskal Wallis test, and Student Newman-Keuls multiple comparison test using BioEstat software (Instituto de Desenvolvimento Sustentável Mamirauá, Tefé, Brazil). P<0.05 was considered significant. Mean values were used as indicators for central tendency.
RESULTS AND DISCUSSION
Progressive activation of p44/42 MAPK in response to ischemia and reperfusion was chiefly localized to the crypts of Lieberkühn, circular and longitudinal muscle layers, whereas p38 MAPK was prominently activated in myenteric plexus and both muscle layers. Interestingly, in all groups, all layers that did respond to I/R insult with increasing in p44/42 and p38 MAPK activation showed low baseline phosphorylation levels as compared to those that did not respond to the insult (Figures 1F and 2F).
In the lamina propria and crypts of Lieberkühn, activation of p38 MAPK showed no progressive activation in any group. However, in these layers, basal activation level was considerably higher than in other layers, in all time points in all groups (Figures 1A and 1B). A possible reason for this is that p38 MAPK expression is related to the differentiation of intestinal crypts and villi (HOUDE et al., 2001). Also, growth factors like vascular endothelial growth factor (VEGF) could trigger p38 MAPK activation in certain cell types (ROUSSEAU et al., 1997).
In the myenteric plexus, progressive activation of p38 MAPK was observed in all groups (Figure 1E). Previous studies have reported high expression of phospho-p38 MAPK in neurons of rabbit duodenum (GONZALO et al., 2010). Also, it has been showed that surgical manipulation can lead to oxidative stress (ANUP et al., 1999).
After reperfusion was established, the activation of p38 MAPK in both circular and longitudinal muscles increased progressively, what is in accordance to other studies that showed that intestinal ischemia alone cannot activate significantly the p38 MAPK pathway, but ischemia and reperfusion can (FU et al., 2003). Labeling was significantly higher in groups B and C, as compared to group A after one hour of reperfusion, although there was no difference between groups B and C. There was no time course difference in p38 MAPK activation in group A, and levels remained low within two hours of reperfusion (Figures 1C and 1D).
Activation of p38 MAPK is inhibited by allopurinol and deferoxamine (WANG et al., 2003). However, the effects of allopurinol are dose dependent, and also the rout and duration of drug administration can potentiate the beneficial effects (TERZI et al., 2001). In our study, allopurinol was used intraluminal and as a rinse solution. The duration of drug activities can be questioned and might explain the results.
The basal level of p 44/42 MAPK activation in the crypts of Lieberkhun was moderate for all groups at the T0 sample. This could be related to the proliferative cells that reside in this layer (VAN DER FLIER & CLEVERS, 2009) and also to the response to growth factors stimuli, which is essential to cell differentiation and proliferation (MANSOUR et al., 1994).
In the lamina propria and myenteric plexus, no difference in activation of p44/42 MAPK was observed between groups. However, in these layers, the basal activation levels in all groups, and all times, were considerably higher than in other layers, particularly in myenteric plexus (Figures 2A and 2E), what had also been described recently in rabbits (GONZALO et al., 2011). Ischemia initially produced mild increase in mean p44/42 MAPK activation levels in the myenteric plexus, reaching a peak after five minutes of ischemia, and plateaued throughout the procedure. Such results were similar to those observed in the myenteric plexus of equine jejunum subjected to ischemia and reperfusion (BRANDSTETTER, 2006). The activation of p44/42 MAPK seems to have neuroprotective effects, besides mediating several extrinsic signals of neural cells survival (HETMAN & GOZDZ, 2004) and that could partly concur for the high constitutive level of activated p44/42 MAPK in these cells.
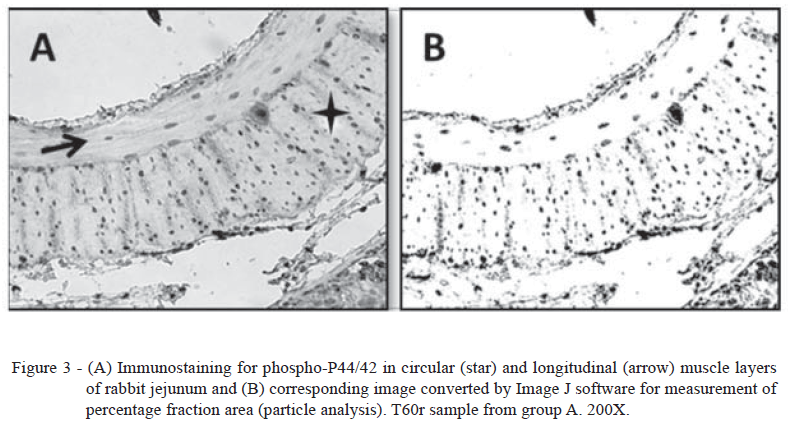
In both circular and longitudinal muscles, from the T0 time point, p44/42 MAPK activation increased significantly and reached a peak after one hour of reperfusion (T60r time point) in groups B and C, but there was no difference between these groups. After one hour of reperfusion, the activation levels decreased slowly. These results are similar to the response to ischemic stress related in previous studies (EL-ASSAL & BESNER, 2005, BRANDSTETTER, 2006). In group A, kinase activation remained low throughout the experimental procedure (Figures 2C and 2D) as previously described (GONZALO et al., 2011). Representative images of immunostaining for phospho-p44/42 MAPK are shown in figure 3.
The presence of extracellular adenosine is essential for maximal p44/42 MAPK activation. However, supplemental adenosine does not lead to an additional increase in ERK 1/2 activation during repletion (MAMMEN, 2009). The results of this research indicate that the chosen model of topical and intraluminal CRS does not interfere in p44/42 and p38 MAPK activation profile in rabbit jejunum subjected to ischemia and reperfusion. Further studies unfold beneficial effects of Carolina Rinse Solution in reperfusion injury related to a modulation of p44/42 and p38 MAPK phosphorylation. Although some CRS compounds have been proven to interfere in some MAPK activities, the experimental model and application mode of CRS in this study did not elicit any changes in p44/42 or p38 MAPK activation profiles.
CONCLUSION
Although MAPK are known to be related to structural and functional changes in tissues subjected to ischemia and reperfusion, the modulation of p44/42 and p38 phosphorylation is not influenced by the brief topical and intraluminal CRS in small intestine of rabbits, subjected to experimental ischemia and reperfusion. The contact with CRS for a short period of time may have influenced the results. However, immersion for longer periods could increase surgical stress, leading to additional lesions, especially when considering non experimental situations. Further studies are necessary, in order to verify the need for long-term contact of CRS with small intestine and its applicability, and also the influence of CRS in other molecular signaling pathways.
ETHICS COMMITTEE AND BIOSECURITY
This study has been approved by the Ethics Committee (CoEP) of the Federal University of Goias (protocol number 011/1).
ACKNOWLEDGEMENTS
We thank Dr. Ekaterina Akimova Botovchenco Rivera for animal facility privileges and Halex Istar Industria Farmacêutica for providing the animals.
REFERENCES
ABE, J. et al. Role of mitogen-activated protein kinases in ischemia and reperfusion injury: the good and the bad. Circulation Research, v.86, n.6, p.607-609, 2000. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10746992>. Accessed: ago, 29, 2011. doi: 10.1161/01.RES.86.6.607.
ANUP, R. et al. Surgical stress and the small intestine: role of oxygen free radicals. Surgery, v.125, n.5, p.560-569, 1999. Available from: <http://www.ncbi.nlm.nih.gov/entrez- /query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10330946>. Accessed: 05 nov. 2010. doi: S0039606099001294 [pii].
BARIE, P.S. Schemes against ischemia; solutions for reperfusion (injury)? Critical Care Medicine, v.27, n.4, p.684-685, 1999. Available from: <http://www.ncbi.nlm.nih.gov/entrez-/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10321649>. Accessed: 12 abr. 2011. doi: 10.1097/00003246-199904000-00009.
BRANDSTETTER, L.R.G. Perfil de ativação das MAP quinases ERK 1/2 no intestino delgado de equinos submetidos à isquemia e reperfusão. 2006. 55f. Dissertação (Mestrado em Ciência Animal) - Escola de Veterinária e Zootecnia, Universidade Federal de Goiás, Goiânia, GO.
CROSS, T.G. et al. Serine/threonine protein kinases and apoptosis. Experimental Cell Research, v.256, n.1, p.34-41, 2000. Available from: <http://www.ncbi.nlm.nih.gov/entrez-/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10739649>. Accessed: 16 out. 2010. doi: 10.1006/excr.2000.4836.
CURRIN, R.T. et al. Evidence that Carolina rinse solution protects sinusoidal endothelial cells against reperfusion injury after cold ischemic storage of rat liver. Transplantation, v.50, n.6, p.1076-1078, 1990. Available from: <http://www.ncbi.nlm.nih.gov/entrez-/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1701572>. Accessed: 10 out. 2010.
EL-ASSAL, O.N.; BESNER, G.E. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology, v.129, n.2, p.609-625, 2005. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query-.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16083716>. Accessed: 18 jul. 2011. doi: S0016-5085(05)01079-6[pii]10.1016/j.gastro.2005.05.054.
FU, X.B. et al. Activation of phosphorylating-p38 mitogen-activated protein kinase and its relationship with localization of intestinal stem cells in rats after ischemia-reperfusion injury. World Journal of Gastroenterology, v.9, n.9, p.2036-2039, 2003. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12970901>. Accessed: 18 jun. 2011.
GONZALO, S. et al. Inhibition of p38 MAPK improves intestinal disturbances and oxidative stress induced in a rabbit endotoxemia model. Neurogastroenterology & Motility, v.22, n.5, p.564-572, e123, 2010. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.-fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20003078>. Accessed: 24 ago. 2012. doi: NMO1439[pii]10.1111/j.1365-2982.2009.01439.x.
GONZALO, S. et al. Extracellular signal-regulated kinase (ERK) is involved in LPS-induced disturbances in intestinal motility. Neurogastroenterology & Motility, v.23, n.2, p.e80-90, 2011. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db-=PubMed&dopt=Citation&list_uids=21087357>. Accessed: 24 ago. 2012. doi: 10.1111/j.1365-2982.2010.01632.x.
HETMAN, M.; GOZDZ, A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. European Journal of Biochemistry, v.271, n.11, p.2050-2055, 2004. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=Pub-Med&dopt=Citation&list_uids=15153093>. Accessed: 24 ago. 2012. doi: 10.1111/j.1432-1033.2004.04133.x.
HOUDE, M. et al. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. Journal of Biological Chemistry, v.276, n.24, p.21885-21894, 2001. Available from: <http://www-.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11283019>. Accessed: 24 ago. 2012. doi: 10.1074/jbc.M100236200 M100236200[pii].
MAMMEN, J.M.V. Ischemia/reperfusion injury in the intestine: important roles for PKC, MAPK, and adenosine. 2009. 121f. Thesis (PhD in Molecular and Cellular Physiology) - Department of Molecular and Cellular Physiology of the College Medicine, University of Cincinnati, Cincinnati.
MANSOUR, S.J. et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science, v.265, n.5174, p.966-970, 1994. Available from: <http://www.ncbi-.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8052857>. Accessed: 24 ago. 2012.
ROUSSEAU, S. et al. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene, v.15, n.18, p.2169-2177, 1997. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi-?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9393975>. Accessed: 24 ago. 2012. doi: 10.1038/sj.onc.1201380.
STALLION, A. et al. IL-10 is not protective in intestinal ischemia reperfusion injury. Journal of Surgical Research, v.105, n.2, p.145-152, 2002. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12121701>. Accessed: 24 ago. 2012. doi: S0022480402963985 [pii].
TERZI, C. et al. Prevention of deleterious effects of reperfusion injury using one-week high-dose allopurinol. Digestive Diseases and Sciences, v.46, n.2, p.430-437, 2001. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=-Citation&list_uids=11281195>. Accessed: 14 set. 2011.
VAN DER FLIER, L.G.;CLEVERS, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology, v.71, p.241-260, 2009. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=-Citation&list_uids=18808327>. Accessed: 13 nov. 2011. doi: 10.1146/annurev.physiol-.010908.163145.
WANG, Q. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. Journal of Biological Chemistry, v.278, n.48, p.47731-47743, 2003. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=-Retrieve&db=PubMed&dopt=Citation&list_uids=14504278>. Accessed: 14 set. 2011. doi: 10.1074/jbc.M308466200M308466200[pii].
YOUNG, B.L. et al. Treatment of ischaemic jejunum with topical and intraluminal Carolina Rinse. Equine Veterinary Journal, v.34, n.5, p.469-474, 2002. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12358049>. Accessed: 30 abr. 2009.
YUE, T.L. et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circulation Research, v.86, n.6, p.692-699, 2000. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve-&db=PubMed&dopt=Citation&list_uids=10747006>. Accessed: 24 ago. 2012. doi: 10.1161/01.RES.86.6.692.
Received 12.17.12
Approved 07.04.13
Returned by the author 11.01.13
CR-2012-1303.R1
- ABE, J. et al. Role of mitogen-activated protein kinases in ischemia and reperfusion injury: the good and the bad. Circulation Research, v.86, n.6, p.607-609, 2000. Available from: <http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10746992>. Accessed: ago, 29, 2011. doi: 10.1161/01.RES.86.6.607.
- ANUP, R. et al. Surgical stress and the small intestine: role of oxygen free radicals. Surgery, v.125, n.5, p.560-569, 1999. Available from: <http://www.ncbi.nlm.nih.gov/entrez- /query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10330946>. Accessed: 05 nov. 2010. doi: S0039606099001294 [pii].
- BARIE, P.S. Schemes against ischemia; solutions for reperfusion (injury)? Critical Care Medicine, v.27, n.4, p.684-685, 1999. Available from: <http://www.ncbi.nlm.nih.gov/entrez-/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10321649>. Accessed: 12 abr. 2011. doi: 10.1097/00003246-199904000-00009.
Publication Dates
-
Publication in this collection
22 Nov 2013 -
Date of issue
Jan 2014
History
-
Accepted
04 July 2013 -
Received
17 Dec 2012