Abstracts
This study aimed to evaluate the role of the Amazon River prawn, Macrobrachium amazonicum, as carrier of Candida spp., by analyzing the correlation between Candida spp. from these prawns and their environment (surface water and sediment), through M13-PCR fingerprinting and RAPD-PCR. For this purpose, 27 strains of Candida spp. were evaluated. These strains were recovered from the gastrointestinal tract of adult M. amazonicum (7/27) from Catú Lake, Ceará State, Brazil and from the aquatic environment (surface water and sediment) of this lake (20/27). Molecular comparison between the strains from prawns and the aquatic environment was conducted by M13-PCR fingerprinting and RAPD-PCR, utilizing the primers M13 and OPQ16, respectively. The molecular analysis revealed similarities between the band patterns of eight Candida isolates with the primer M13 and 11 isolates with the primer OPQ16, indicating that the same strains are present in the digestive tract of M. amazonicum and in the aquatic environment where these prawns inhabit. Therefore, these prawns can be used as sentinels for environmental monitoring through the recovery of Candida spp. from the aquatic environment in their gastrointestinal tract
Macrobrachium amazonicum prawn; environmental sentinel; Candida spp.; pollution, monitoring
Este estudo teve como objetivo avaliar o papel do camarão Macrobrachium amazonicum como carreador de Candida spp. do ambiente aquático, por meio da análise da correlação entre Candida spp. isoladas desse camarão e do seu ambiente (água de superfície e sedimento) através das técnicas de M13-PCR fingerprinting e RAPD-PCR. Para tanto, 27 cepas de Candida spp. foram avaliadas. Essas cepas foram recuperadas a partir do trato gastrointestinal de M. amazonicum adultos (7/27), oriundos da lagoa do Catú, Ceará, Brasil, e do meio aquático (águas superficiais e sedimentos) desse lago (20/27). A comparação molecular entre as cepas de camarões e o ambiente aquático foi conduzida por M13-PCR fingerprinting e RAPD-PCR, utilizando os iniciadores M13 e OPQ16, respectivamente. A análise molecular revelou semelhanças entre os padrões de bandas de oito isolados de Candida com o iniciador M13 e 11 isolados com o primer OPQ16, indicando que elas estão presentes no trato digestivo de M. amazonicum e no ambiente aquático, onde esses camarões habitam. Portanto, essa espécie de camarão pode ser usada como sentinela para monitoramento ambiental através da recuperação de Candida spp. do ambiente aquático, a partir do seu trato gastrointestinal
camarão Macrobrachium amazonicum ; sentinela ambiental; Candida spp; poluição; monitoramento
INTRODUCTION:
Several studies have identified Candida spp. as potential biological
indicators of environmental degradation (MEDEIROS et
al., 2008MEDEIROS, A.O. et al. Diversity and antifungal susceptibility of yeasts
from tropical freshwater environments in Southeastern Brazil. Water Research, v.42,
n.14, p.3921-3929, 2008. Disponível em:
<http://www.sciencedirect.com/science/article/pii/S0043135408002522>. Accessed:
Nov. 08, 2013. doi: 10.1016/j.watres.2008.05.026.
http://www.sciencedirect.com/science/art...
; BRILHANTE et al., 2011,
2012BRILHANTE, R.S.N. et al. Yeast microbiota of raptors: a possible tool
for environmental monitoring. Environmental Microbiology Reports, v.4, n.2,
p.189-193, 2012. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1758-2229.2011.00319.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1758-2229.2011.00319.x.
http://onlinelibrary.wiley.com/doi/10.11...
; CASTELO-BRANCO et al., 2013CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a
wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance?
Medical Mycology, v.51, n.5, p.555-560, 2013. Available from:
<http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed:
Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
http://informahealthcare.com/doi/abs/10....
),
particularly in samples obtained from aquatic sources (BUTINAR et al., 2005BUTINAR, L. et al. Yeast diversity in hypersaline habitats. FEMS
Microbiology Letters, v.15, n.2, p.229-234, 2005. Available from:
<http://onlinelibrary.wiley.com/doi/10.1016/j.femsle.2005.01.043/pdf>.
Accessed: Nov. 08, 2013. doi:10.1016/j.femsle.2005.01.043.
http://onlinelibrary.wiley.com/doi/10.10...
; MEDEIROS et al.,
2008MEDEIROS, A.O. et al. Diversity and antifungal susceptibility of yeasts
from tropical freshwater environments in Southeastern Brazil. Water Research, v.42,
n.14, p.3921-3929, 2008. Disponível em:
<http://www.sciencedirect.com/science/article/pii/S0043135408002522>. Accessed:
Nov. 08, 2013. doi: 10.1016/j.watres.2008.05.026.
http://www.sciencedirect.com/science/art...
). In these studies, the isolation of this genus was greater than that of
other microorganisms, including bacteria, demonstrating the potential use of this yeast
for environmental monitoring.
Monitoring aquatic environments requires an adequate water sampling technique, including
the selection of representative collection sites and considering environmental factors,
such as seasonality, temperature, the water column and the presence of affluent or
effluent waters (APHA/AWWA/WEF, 1998AMERICAN PUBLIC HEALTH ASSOCIATION (APHA); AMERICAN WATER WORKS
ASSOCIATION (AWWA); WATER ENVIRONMENTAL FEDERATION (WEF). Standard methods for the
examination of water and wastewater. 20.ed. Washington, 1998. 64p.). These
requirements may represent an obstacle for the adequate monitoring of fresh water
environments, because of the large number of samples needed. Hence, it is important to
seek alternatives to facilitate monitoring of aquatic ecosystems. In this context, the
use of aquatic crustaceans has been reported as a reliable alternative for that purpose,
especially because of their feeding habits (filter feeding) and benthic behavior, as
described by VIRGA et al. (2007)VIRGA, R.H.P. et al. Assessment of heavy metal contamination in blue
crab specimes. Food Science and Technology, v.27, n.4, p.779-785, 2007. Available
from:
<http://www.scielo.br/scielo.php?pid=S0101-20612007000400017&script=sci_arttext>.
Accessed: Nov. 08, 2013. doi: 10.1590/S0101-20612007000400017.
http://www.scielo.br/scielo.php?pid=S010...
and BRILHANTE et al. (2011)BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
.
More recently, BRILHANTE et al. (2011)BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
performed
a research with the freshwater prawn M. amazonicum (Amazon River prawn) in
captivity and from the natural environment for the isolation of yeasts and Candida
was the most isolated genus, showing that it belongs to the gastrointestinal
microbiota of these animals. In addition, these authors suggested that these prawns may
be an important environmental sentinels if they harbor in their gastrointestinal tract
Candida spp. from the aquatic environment. Thus, in light of these
findings and considering the wide distribution of the species M. amazonicum
in South America, the objective of the present study was to evaluate the role of
these prawns as carriers of Candida spp. from the aquatic environment.
MATERIALS AND METHODS:
Microorganisms
In this study, 27 strains of Candida spp. were analyzed, out of which seven were recovered from wild-harvested M . amazonicum, while 20 were recovered from the aquatic environment and were deposited in our culture collection. It is important to emphasize that the analyzed Candida strains, from animal and environmental sources, were simultaneously recovered.
Of the 20 environmental strains, 13 were isolated from surface water (SW) and seven from sediment (S). These strains belong to the culture collection of the Specialized Medical Mycology Center of the Federal University of Ceará, where they are kept on 2% Sabouraud dextrose agar. They were manipulated under level II biosafety procedures.
Candida spp. from the aquatic environment were obtained from Catú Lake,
which is located at the municipality of Aquiraz, Ceará state, Brazil, about 35
kilometers from the state capital. It is delimited by the UTM coordinates 0567000E,
9561273N and 0575000E, 9569000N. It is a rich freshwater body with mangrove areas that
have been degraded by uncontrolled occupation of the surrounding area and pollution with
residues from industrial, commercial and farming activities (BRILHANTE et al., 2011BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
).
Water samples were collected, according to MEDEIROS et
al. (2008)MEDEIROS, A.O. et al. Diversity and antifungal susceptibility of yeasts
from tropical freshwater environments in Southeastern Brazil. Water Research, v.42,
n.14, p.3921-3929, 2008. Disponível em:
<http://www.sciencedirect.com/science/article/pii/S0043135408002522>. Accessed:
Nov. 08, 2013. doi: 10.1016/j.watres.2008.05.026.
http://www.sciencedirect.com/science/art...
. Then, four collection sites were included, as follows:
recreational area and prawn collection site (point 1, 3°55'59.79" S and 38°21'50.10" W),
agricultural wastewater (point 2, 3°55'47.25" S and 38°22'14.16" W), industrial
wastewater (point 3, 3°56'03.70" S and 38°22'25.15" W) and residential area (point 4,
3°56'56.72" S and 38°22'31.57" W). Two water samples (SW and S) were monthly collected
from each collection site, during one year (from March 2011 to February 2012). Overall,
a total of 96 water samples were obtained for this study.
Adults of M
.
amazonicum were monthly harvested from Catú Lake (point 1) in the same
period as the water samples. Afterwards, the digestive tracts of 10 individuals were
removed, placed in sterile slants containing sterile saline and treated as one single
sample (BRILHANTE et al., 2011BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
). Overall, 12
collections were performed. This study was previously approved by the Chico Mendes
Institute for Conservation of Biodiversity/Biodiversity Authorization and Information
System - SISBIO, under the number 28175-1.
Microbiological processing
Samples were processed in a biosafety level II cabinet and were seeded on 2% Sabouraud
agar plus chloramphenicol (0.5g L-1), in Petri dishes for primary isolation.
Water samples were processed according to MEDEIROS et
al. (2008)MEDEIROS, A.O. et al. Diversity and antifungal susceptibility of yeasts
from tropical freshwater environments in Southeastern Brazil. Water Research, v.42,
n.14, p.3921-3929, 2008. Disponível em:
<http://www.sciencedirect.com/science/article/pii/S0043135408002522>. Accessed:
Nov. 08, 2013. doi: 10.1016/j.watres.2008.05.026.
http://www.sciencedirect.com/science/art...
with some modifications. Briefly, a 100-µL aliquot of the SW
samples was spread on the medium, after homogenization, while the S samples were
processed, after centrifuging for 20 minutes at 3,000rpm. Then, the supernatant was
removed and the substrate was suspended again in 2mL of a sterile 0.9% solution of NaCl.
Afterwards, the suspension was agitated in a vortex mixer for 3 minutes and left to rest
for 30 minutes at 25°C. Next, 100-µL aliquots of the supernatant of each sample were
seeded on the culture medium. The digestive tracts of adult prawns were processed as
described by BRILHANTE et al. (2011)BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
and seeded
onto the culture medium. The inoculated Petri dishes containing the culture media were
incubated at 25oC, for 10 days, and were daily observed (BRILHANTE et al., 2011BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
).
The yeast colonies were identified through specific morphological and biochemical tests,
including growth on chromogenic medium (CHROMagar Candida, BD, USA), micromorphology on
cornmeal-Tween 80 agar, carbohydrate and nitrogen assimilation and urease production
(BRILHANTE et al., 2011BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
), and the results were
interpreted according to (DE HOOG et al., 2000DE HOOG, G.S. et al. Atlas of clinical fungi. 2.ed. The Nederlands:
Centraalbureau voor Schimmelcultures, Baarn, 2000. 1126 p.).
Strains that presented dubious identity were also identified through VITEK II automated
system (BioMérieux, USA). Additionally, the susceptibility of these microorganisms to
amphotericin B, fluconazole and itraconazole was evaluated through broth microdilution
method. Minimum inhibitory concentrations (MIC) of >1, ≥64, ≥1µg mL-1 were
considered resistant to amphotericin B, fluconazole and itraconazole, respectively
(CLSI, 2008CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a
wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance?
Medical Mycology, v.51, n.5, p.555-560, 2013. Available from:
<http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed:
Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
http://informahealthcare.com/doi/abs/10....
).
M13-PCR fingerprinting and OPQ-16 RAPD
The DNA from the strains was extracted after 48 hours of growth on potato dextrose agar,
according to the methodology described by CASTELO-BRANCO
et al. (2013)CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a
wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance?
Medical Mycology, v.51, n.5, p.555-560, 2013. Available from:
<http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed:
Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
http://informahealthcare.com/doi/abs/10....
.
For molecular comparison between the Candida isolates from the aquatic
environment (SW and S) and from prawns, the PCR-fingerprinting technique was used,
according to the method described by CASTELO-BRANCO et
al. (2013)CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a
wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance?
Medical Mycology, v.51, n.5, p.555-560, 2013. Available from:
<http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed:
Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
http://informahealthcare.com/doi/abs/10....
, using the single primer M13 (59-GAGGGTGGCG GTTCT-39) and the PCR
mix (25 µL), containing 10mM of Tris/HCI (pH 8.3), 50mM of KCl, 1.5mM of MgCl2, 0.2mM of
dNTPs, 0.15mM of the primer, 2.5U of Taq polymerase (MBI Fermentas) and 25ng of yeast
DNA. The RAPD reactions were performed with the primer OPQ16 (5' AAGAGCCCGT3'),
according to the method described by CASTELO-BRANCO et
al. (2013)CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a
wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance?
Medical Mycology, v.51, n.5, p.555-560, 2013. Available from:
<http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed:
Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
http://informahealthcare.com/doi/abs/10....
. The RAPD reaction was carried out with a total volume of 10µL,
containing 50ng of genomic DNA, 1X buffer, 1mM of MgCl2, 2pmol of primer,
0.5mM each of deoxynucleoside triphosphate and 1 U μL-1 of Hot Start Taq
polymerase.
Dice similarity coefficient was measured and a dendrogram was obtained through the use of the Unweighted Pair Group Method with Arithmetic Average (UPGMA), through the software BioNumerics (version 6.6), resulting in the analysis of clusters and measure of relatedness among isolates.
RESULTS:
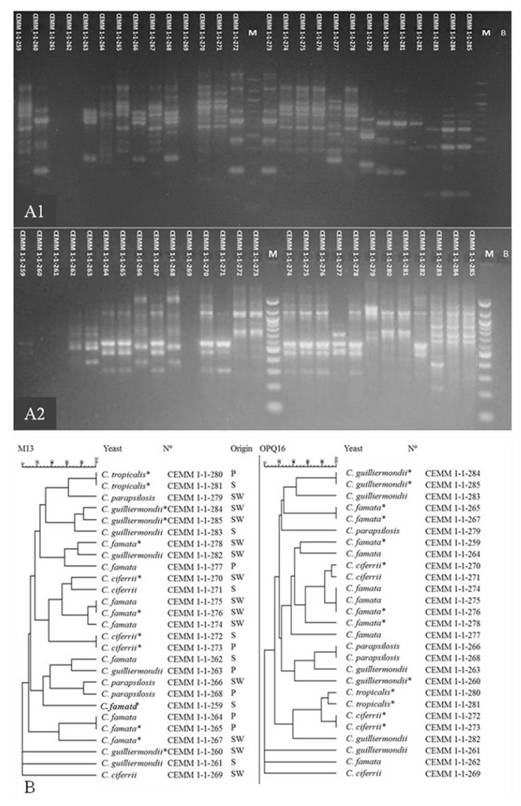
Data referring to the identity, the origin and the antifungal susceptibility profile of the recovered Candida strains are listed in table 1. Five to eight DNA bands were generated through the M13-PCR fingerprinting, while three to ten DNA bands were generated through RAPD-PCR with the primer OPQ16. The molecular analysis employing both techniques revealed strong similarities between the DNA band patterns of the isolates belonging to the same Candida species. For the primer M13, eight isolates of Candida spp. with 100% band similarity were obtained, while with the primer OPQ16, 11 isolates were obtained with 100% band similarity (Figure 1).
: Dendrograms resulting from the analysis of 27 isolates of Candida spp. obtained from the gastrointestinal tract of Macrobrachium amazonicum (n=7) and the natural environment (n=20) through M-13-fingerprinting and RAPD-PCR with primer OPQ16. P: prawn; SW: surface water; S: sediment. *indicates antifungal resistance. Dendrograms generated by the BioNumerics program (Applied Math, Inc.).
The M13-PCR fingerprinting identified 100% similarity between two C. tropicalis strains from prawn (n=1) and sediment (point 3, n=1); four C. famata strains from prawns (n=2) and surface water (points 1 and 3; n=2) and two C. ciferrii strains from prawns (n=1) and sediment (point 1, n=1). In turn, the RAPD-PCR with the primer OPQ16 allowed identifying 100% similarity between two C. guilliermondii strains from surface water (points 1 and 4); five C. famata strains, two from prawn (n=1) and surface water (point 1, n=1) and three from surface water (points 1 and 2); two strains of C. parapsilosis from prawn (n=1) and surface water (point 4, n=1) and two C. ciferrii strains from prawn (n=1) and sediment (point 1, n=1) (Figure 1).
DISCUSSION:
This study demonstrated the similarity among Candida spp. isolated from wild-harvested prawns and the aquatic environment where the animals inhabit, including surface water and sediment. The molecular analysis through M13-PCR fingerprinting and RAPD-PCR with OPQ-16 allowed evaluating this correlation, since these techniques generated varied band patterns among different Candida species and similar ones within the same species, thus presenting desirable and reliable results. In the present study, the primer OPQ16 was used to complement the results obtained through the M13-PCR fingerprinting and it generated a greater variety of DNA bands and identified a greater number of strains with 100% of similarity.
The recovered Candida species were simultaneously isolated from prawns and the aquatic environment and some of these isolates presented 100% similarity, even when recovered from different collection points. Thus, it was demonstrated that M . amazonicum contains in its gastrointestinal tract a representative cross-section of Candida spp. that colonize the water and the substrate where they live.
In addition, three sets of azole resistant strains were observed among the isolates from
prawns and aquatic environment that presented 100% similarity. These findings are in
accordance with those of BRILHANTE et al. (2011)BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus
on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology
Ecology, v.76, n.2, p.268-277, 2011. Available from:
<http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>.
Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
http://onlinelibrary.wiley.com/doi/10.11...
,
who observed that 28.6% of the Candida spp. recovered from the intestinal
tract of wild-harvested M
.
amazonicum isolated from Catú Lake were resistant to azole antifungals.
Considering that the main mechanism of azole resistance among Candida spp.
is the overexpression of efflux pumps (FENG et
al., 2010FENG, L. et al. Relationship between antifungal resistance of
fluconazole resistant Candida albicans and mutations in ERG11 gene. Chinese Medical
Journal, v.123, n.5, p.544-548, 2010. Available from:
<http://www.cmj.org/ch/reader/view_abstract_ext.aspx?file_no=20103842232480&flag=1>.
Accessed: Dec. 20, 2013. doi:
10.3760/cma.j.issn.0366-6999.2010.05.007.
http://www.cmj.org/ch/reader/view_abstra...
), which is possibly related to the exposure of
these microorganisms to chemical compounds, as an unspecific mechanism of cellular
detoxification (JUNGWIRTH & KUCHLER,
2006JUNGWIRTH, H.; KUCHLER, K. Yeast ABC transporters - a tale of sex,
stress, drugs and aging. FEBS Letters, v.580, n.4, p.1131-1138, 2006. Available from:
<http://www.sciencedirect.com/science/article/pii/S0014579305015334>. Accessed:
Dec. 20, 2013. doi:10.1016/j.febslet.2005.12.050.
http://www.sciencedirect.com/science/art...
), we strongly believe that Candida spp. could be used as
indicators of environmental pollution, through the phenotypical assessment of their
in vitro susceptibility profile.
Crustaceans accumulate pollutants in their tissues, such as hydrocarbons, pesticides and
heavy metals (YILMAZ & YILMAZ, 2007YILMAZ, A.B.; YILMAZ, L. Influences of sex and seasons on levels of
heavy metals in tissues of green tiger shrimp (Penaeus semisulcatus de Hann, 1844).
Food Chemistry, v.101, n.4, p.1664-1669, 2007. Available from:
<http://www.sciencedirect.com/science/article/pii/S0308814606003438>. Accessed:
Nov. 08, 2013. doi: 10.1016/j.foodchem.2006.04.025.
http://www.sciencedirect.com/science/art...
),
which might increase the azole resistance rate among yeasts from the microbiota, due to
the overexpression of efflux pumps (KEENAN et al.,
2007KEENAN, P.O. et al. Clear and present danger? The use of a yeast
biosensor to monitor changes in the toxicity of industrial effluents subjected to
oxidative colour removal treatments. Journal of Environmental Monitoring, v.9, n.12,
p.1394-1401, 2007. Available from:
<http://pubs.rsc.org/en/Content/ArticleLanding/2007/EM/b710406e#!divAbstract>.
Accessed: Nov. 08, 2013. doi: 10.1039/B710406E.
http://pubs.rsc.org/en/Content/ArticleLa...
; MÜLLER et al., 2007MÜLLER, F.M.C. et al. Cross-resistance to medical and agricultural azole
drugs in yeasts from the oropharynx of human immunodeficiency virus patients and from
environmental bavarian vine grapes. Antimicrobial Agents and Chemotherapy, v.51, n.8,
p.3014-3016, 2007. Available from: <http://aac.asm.org/content/51/8/3014.long>.
Accessed: Nov. 08 2013. doi:10.1128/AAC.00459-07.
http://aac.asm.org/content/51/8/3014.lon...
), as a
consequence of the chronic exposure to these chemical compounds. In this context, the
use of this prawn as a sentinel for the isolation of Candida spp. seems
potentially advantageous.
CONCLUSION:
In conclusion, based on the obtained results, the use of M . amazonicum as a sentinel for the isolation of Candida spp. from aquatic environments is an interesting alternative for evaluating the environmental quality, considering that these animals harbor yeasts from the environment in their gastrointestinal tract. Additionally, due to their capacity to accumulate chemical pollutants in their tissues, they simulate the environmental conditions to which these yeasts are exposed, potentially contributing for monitoring the presence of resistant Candida spp. in the environment.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq; Brazil; Processes 302574/2009-3, 481614/2011-7, 504189/2012-3) and the Coordination Office for the Improvement of Higher Education Personnel (CAPES/PNPD 2103/2009, AE1-0052-000650100/11, Casadinho/PROCAD 552215/2011-2).
- AMERICAN PUBLIC HEALTH ASSOCIATION (APHA); AMERICAN WATER WORKS ASSOCIATION (AWWA); WATER ENVIRONMENTAL FEDERATION (WEF). Standard methods for the examination of water and wastewater. 20.ed. Washington, 1998. 64p.
- BRILHANTE, R.S.N. et al. Yeast microbiota of raptors: a possible tool for environmental monitoring. Environmental Microbiology Reports, v.4, n.2, p.189-193, 2012. Available from: <http://onlinelibrary.wiley.com/doi/10.1111/j.1758-2229.2011.00319.x/abstract>. Accessed: Nov. 08, 2013. doi: 10.1111/j.1758-2229.2011.00319.x.
» https://doi.org/10.1111/j.1758-2229.2011.00319.x» http://onlinelibrary.wiley.com/doi/10.1111/j.1758-2229.2011.00319.x/abstract - BRILHANTE, R.S.N. et al. Yeasts from Macrobrachium amazonicum: a focus on antifungal susceptibility and virulence factors of Candida spp. FEMS Microbiology Ecology, v.76, n.2, p.268-277, 2011. Available from: <http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract>. Accessed: Nov. 08, 2013. doi: 10.1111/j.1574-6941.2011.01050.x.
» https://doi.org/10.1111/j.1574-6941.2011.01050.x» http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01050.x/abstract - BUTINAR, L. et al. Yeast diversity in hypersaline habitats. FEMS Microbiology Letters, v.15, n.2, p.229-234, 2005. Available from: <http://onlinelibrary.wiley.com/doi/10.1016/j.femsle.2005.01.043/pdf>. Accessed: Nov. 08, 2013. doi:10.1016/j.femsle.2005.01.043.
» https://doi.org/10.1016/j.femsle.2005.01.043» http://onlinelibrary.wiley.com/doi/10.1016/j.femsle.2005.01.043/pdf - CASTELO-BRANCO, D.S.C.M. et al. Azole-resistant Candida albicans from a wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance? Medical Mycology, v.51, n.5, p.555-560, 2013. Available from: <http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878>. Accessed: Nov. 08, 2013. doi: 10.3109/13693786.2012.752878.
» https://doi.org/10.3109/13693786.2012.752878» http://informahealthcare.com/doi/abs/10.3109/13693786.2012.752878 - CLINICAL AND LABORATORY STANDARDS INSTITUTE. Reference method for broth dilution antifungal susceptibility testing of yeast: approved guideline M27-A3. Wayne, PA: CLSI, 2008. V.28, p.1-25.
- DE HOOG, G.S. et al. Atlas of clinical fungi. 2.ed. The Nederlands: Centraalbureau voor Schimmelcultures, Baarn, 2000. 1126 p.
- FENG, L. et al. Relationship between antifungal resistance of fluconazole resistant Candida albicans and mutations in ERG11 gene. Chinese Medical Journal, v.123, n.5, p.544-548, 2010. Available from: <http://www.cmj.org/ch/reader/view_abstract_ext.aspx?file_no=20103842232480&flag=1>. Accessed: Dec. 20, 2013. doi: 10.3760/cma.j.issn.0366-6999.2010.05.007.
» https://doi.org/10.3760/cma.j.issn.0366-6999.2010.05.007» http://www.cmj.org/ch/reader/view_abstract_ext.aspx?file_no=20103842232480&flag=1 - JUNGWIRTH, H.; KUCHLER, K. Yeast ABC transporters - a tale of sex, stress, drugs and aging. FEBS Letters, v.580, n.4, p.1131-1138, 2006. Available from: <http://www.sciencedirect.com/science/article/pii/S0014579305015334>. Accessed: Dec. 20, 2013. doi:10.1016/j.febslet.2005.12.050.
» https://doi.org/10.1016/j.febslet.2005.12.050» http://www.sciencedirect.com/science/article/pii/S0014579305015334 - KEENAN, P.O. et al. Clear and present danger? The use of a yeast biosensor to monitor changes in the toxicity of industrial effluents subjected to oxidative colour removal treatments. Journal of Environmental Monitoring, v.9, n.12, p.1394-1401, 2007. Available from: <http://pubs.rsc.org/en/Content/ArticleLanding/2007/EM/b710406e#!divAbstract>. Accessed: Nov. 08, 2013. doi: 10.1039/B710406E.
» https://doi.org/10.1039/B710406E» http://pubs.rsc.org/en/Content/ArticleLanding/2007/EM/b710406e#!divAbstract - MEDEIROS, A.O. et al. Diversity and antifungal susceptibility of yeasts from tropical freshwater environments in Southeastern Brazil. Water Research, v.42, n.14, p.3921-3929, 2008. Disponível em: <http://www.sciencedirect.com/science/article/pii/S0043135408002522>. Accessed: Nov. 08, 2013. doi: 10.1016/j.watres.2008.05.026.
» https://doi.org/10.1016/j.watres.2008.05.026» http://www.sciencedirect.com/science/article/pii/S0043135408002522 - MÜLLER, F.M.C. et al. Cross-resistance to medical and agricultural azole drugs in yeasts from the oropharynx of human immunodeficiency virus patients and from environmental bavarian vine grapes. Antimicrobial Agents and Chemotherapy, v.51, n.8, p.3014-3016, 2007. Available from: <http://aac.asm.org/content/51/8/3014.long>. Accessed: Nov. 08 2013. doi:10.1128/AAC.00459-07.
» https://doi.org/10.1128/AAC.00459-07» http://aac.asm.org/content/51/8/3014.long - VIRGA, R.H.P. et al. Assessment of heavy metal contamination in blue crab specimes. Food Science and Technology, v.27, n.4, p.779-785, 2007. Available from: <http://www.scielo.br/scielo.php?pid=S0101-20612007000400017&script=sci_arttext>. Accessed: Nov. 08, 2013. doi: 10.1590/S0101-20612007000400017.
» https://doi.org/10.1590/S0101-20612007000400017» http://www.scielo.br/scielo.php?pid=S0101-20612007000400017&script=sci_arttext - YILMAZ, A.B.; YILMAZ, L. Influences of sex and seasons on levels of heavy metals in tissues of green tiger shrimp (Penaeus semisulcatus de Hann, 1844). Food Chemistry, v.101, n.4, p.1664-1669, 2007. Available from: <http://www.sciencedirect.com/science/article/pii/S0308814606003438>. Accessed: Nov. 08, 2013. doi: 10.1016/j.foodchem.2006.04.025.
» https://doi.org/10.1016/j.foodchem.2006.04.025» http://www.sciencedirect.com/science/article/pii/S0308814606003438
Publication Dates
-
Publication in this collection
Nov 2014
History
-
Received
09 Jan 2014 -
Accepted
14 Apr 2014