Abstracts
Fruits from temperate and tropical climates which have high levels of antioxidant compounds are the source of numerous studies concerning the correlation with benefits to human health. The objectives of this study were to quantify the anthocyanins and phenolic compounds and also to measure the antioxidant activity (ferric reducing antioxidant power - FRAP) of blackberries from two varieties grown in southern Brazil ('Brazos' and 'Tupy') at three stages of ripening; unripe, semi-ripe, ripe and their products (pulp and fermented products). During fruit ripening it was observed that weight, size, diameter and sugars increase significantly and acidity decreased significantly. The anthocyanin content ranged from 4.19 (semi-ripe 'Tupy' variety) to 205.75mg 100g-1 (ripe 'Brazos' variety). The highest levels of phenolic compounds were observed for the unripe fruit of both varieties, while antioxidant activity showed no significant difference during the ripening stages. The studied pulp showed a high content of phenolic compounds (ten times higher than that found in the ripe fruits). The anthocyanin content and antioxidant activity did not show the same increase due to the degradation of anthocyanins caused by the heat treatment that was used. The alcoholic fermented beverage made from blackberries remained stable (total phenolic compounds and antioxidant activity) during two years of storage, but the in third year a significant reduction in antioxidant activity was observed. These results can be important for establishing the shelf life of this kind of product made with blackberry
Rubus spp.; antioxidant activity; ripening stage; processing
Algumas frutas de clima temperado e tropical, principalmente as do tipo "berries", têm como característica o acúmulo de compostos antioxidantes e são objeto de inúmeros estudos, haja vista a sua correlação com os benefícios para a saúde humana. Os objetivos deste trabalho foram quantificar as antocianinas, os compostos fenólicos e mensurar a capacidade antioxidante (ferric reducing antioxidant power - FRAP) de amoras-pretas de duas cultivares ('Brazos' e 'Tupy'), nos três estágios de maturação, considerados verde, semi-maduro e maduro, e dos seus produtos (polpas e fermentados). No processo de maturação da fruta, observou-se que o peso, tamanho, diâmetro e teor de açúcar aumentaram significativamente enquanto a acidez diminuiu. Os teores de antocianinas aumentaram de 4,19 (FSM cv. 'Tupy') (frutos semi-maduros) para 205,75mg 100g-1 (FM cv. 'Brazos') (frutos maduros). Os maiores teores de compostos fenólicos foram verificados para os frutos verdes (FV) das duas cultivares, enquanto a atividade antioxidante não apresentou diferença significativa nos estádios de maturação. A polpa estudada acumulou um elevado teor de compostos fenólicos (dez vezes superior ao encontrado nas frutas maduras). Os teores de antocianinas e de atividade antioxidante não apresentaram o mesmo perfil em função da degradação das antocianinas pelo processo térmico utilizado. O fermentado alcoólico de amora permaneceu estável (fenóis totais e atividade antioxidante) durante dois anos de estocagem, porém, no terceiro ano, foi observada uma redução significativa da atividade antioxidante. Estes resultados são importantes para o estabelecimento da vida-de-prateleira desses produtos derivados da amora
Rubus spp.; atividade antioxidante; estádio de maturação; processamento
INTRODUCTION:
Due to the fact that it is a very rustic plant and easy to handle, the cultivation of
blackberries (Rubus spp.) is a promising alternative source of income for
small family farms in Brazil (CLERICI &
CARVALHO-FILHO, 2011CLERICI, M.T.P.S.; CARVALHO-SILVA, L.B. Nutritional bioactive compounds
and technological aspects of minor fruits grown in Brazil. Food Research
International, v.44, n.7, p.1658-1670, 2011. Available from:
<http://dx.doi.org/10.1016/j.foodres.2011.04.020>. Accessed: Jan. 11, 2011.
doi: 10.1016/j.foodres.2011.04.020.
http://dx.doi.org/10.1016/j.foodres.2011...
; FERREIRA et al.,
2010FERREIRA, D.S. et al. Compostos bioativos presentes em amora-preta
(Rubus spp.). Revista Brasileira de Fruticultura, v.32, n.3, p.664-674, 2010.
Available from: <http://dx.doi.org/
http://dx.doi.org/10.1590/S0100-294520110005000110>. Accessed: Jan. 11, 2013. doi:
10.1590/S0100-294520110005000110.
http://dx.doi.org/
...
; HIRSCH et al., 2012HIRSCH, G.E. et al. Caracterização físico-química de variedades de
amora-preta da região sul do Brasil., Ciência Rural v.42, n.5, p.942-947, 2012.
Available from: <http://dx.doi.org/10.1590/S0103-847820120050000021>. Accessed:
Jan. 11, 2013. doi: 10.1590/S0103-847820120050000021.
http://dx.doi.org/10.1590/S0103-84782012...
). The
blackberry is a small fruit and it is characterized by being labor-intensive and also
for the possibility of providing a high economic return. Small fruits are recognized,
both in the literature and in everyday life, as beneficial to human health with
properties linked with the prevention of disease and the remission of symptoms.
Scientific papers have been widely publicized and have encouraged the creation of a
special group of fruits, still in the process of definition, but with the name of
"superfruits" (HASSIMOTTO et al., 2008HASSIMOTTO, N.M.A. et al. Physico-chemical characterization and
bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Ciência e
Tecnologia de Alimentos, v.28, n.3; p.702-708, 2008. Available from:
<http://dx.doi.org/10.1590/S0101-20612008000300029>. Accessed: Aug. 20, 2012.
doi: 10.1590/S0101-20612008000300029.
http://dx.doi.org/10.1590/S0101-20612008...
; ACOSTA-MONTOYA et al., 2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
; VIZZOTTO et al., 2012VIZZOTTO, M. et al. Teor de compostos fenólicos e atividade antioxidante
em diferentes genótipos de amoreira-preta (Rubus sp.). Revista Brasileira de
Fruticultura, v.34, n.3, p.853-858, 2012. Available from:
<http://dx.doi.org/10.1590/S0100-29452012000300027>. Accessed: Jan. 11, 2013.
doi: 10.1590/S0100-29452012000300027.
http://dx.doi.org/10.1590/S0100-29452012...
).
Among these small red fruits the blackberry shows some of the highest levels of
anthocyanins closely following the black raspberry and superior to the strawberry (WANG & LIN, 2000WANG, S.Y.; LIN, H.S. Antioxidant activity in fruits and leaves of
blackberry, raspberry, and strawberry varies with cultivar and developmental stage.
Journal of Agricultural and, Food Chemistry v.48, p.140-146, 2000. Available from:
<http://dx.doi.org/10.1021/jf9908345>. Accessed: Aug. 20, 2012. doi:
10.1021/jf9908345.
http://dx.doi.org/10.1021/jf9908345...
; ACOSTA-MONTOYA et al., 2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
). These flavonoids are considered to be primarily
responsible for antioxidant activity, which explains the functionality of the fruits in
general, and more specifically, that of the blackberry. The pigments responsible for
their typical color (anthocyanins) are already well known and studied as potential
antioxidants that provide benefits to the human health. Anthocyanins are hydrophilic
pigments that give the various shades of orange, red and blue there are present in
fruits, vegetables, leaves, flowers and roots, due to reversible structural changes of
the flavylium cation, which is sensitive to pH changes intrinsic to plants (MOTA, 2006MOTA, R.V. Caracterização do suco de amora-preta elaborado em extrator
caseiro. Ciência e Tecnologia de Alimentos v.26, n.2, p.303-308, 2006. Available
from: <http://dx.doi.org/10.1590/S0101-20612006000200012>. Accessed: Aug. 20,
2012. doi: 10.1590/S0101-20612006000200012.
http://dx.doi.org/10.1590/S0101-20612006...
). The main anthocyanic compounds in
blackberries, associated with antioxidant activity, are the anthocyanins,
cyanidin-3-glucoside and cyanidin-3-(6"-malonyl) glucoside; as well as ellagitannins,
lambertianin sanguiin C; and H-6 complex (ACOSTA-MONTOYA
et al., 2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
).
Although there are native species of the Rubus genus in Brazil, the
blackberry only started to be studied in the 1970s, and the first varieties introduced
to the country were the 'Brazos', 'Comanche' and 'Cherokee', all from the United States,
the world's largest producer. After a genetic program conducted by the Brazilian
Agricultural Research Company (EMBRAPA), new Brazilian varieties came into the market,
including 'Tupy', launched in the late 1980s, which is now the main variety produced in
the country (ANTUNES, 2002ANTUNES, L.E.C. Amora-preta: nova opção de cultivo no Brasil. Ciência
Rural, v.32, n.1, p.151-158, 2002. Available from:
<http://dx.doi.org/10.1590/S0103-84782002000100026>. Accessed: Aug. 20, 2012.
doi: 10.1590/S0103-84782002000100026.
http://dx.doi.org/10.1590/S0103-84782002...
). There is still only
little technical and scientific information regarding the use of the blackberry as raw
material for adding value to agricultural produce that has begun to emerge in southern
Brazil in a promising manner. So, this study seeks to identify the effects of processing
on the quality of products made from different varieties of blackberries. Considering
the lack of scientific data on blackberries cultivated in Brazil, the objective was to
quantify the total phenolic compounds and total monomeric anthocyanins. It was also
intended to measure the antioxidant activity of the 'Brazos' and 'Tupy' varieties in
three stages of ripeness (unripe, semi-ripe and ripe) and of the pulp from ripe fruits
from the 2009 harvest, and also from alcoholic fermented products from 2007, 2008 and
2009.
MATERIALS AND METHODS:
The blackberry fruits ('Brazos' and 'Tupy' varieties) harvested in 2009, alcoholic fermented blackberries (from 2007, 2008 and 2009 harvests) and blackberry pulp were kindly provided by Adega Porto Brazos (Ponta Grossa, PR, Brazil). The fruits were harvested unripe (>75% of the skin green), semi-ripe (>75% with red skin), and ripe (100% of the skin dark purple). In the case of the alcoholic fermented blackberries ('Brazos' variety), their production involved juice extraction from ripe fruits by using a horizontal de-pulper (D-008, Mecamau, Espirito Santo do Pinhal, São Paulo, Brazil), sugar addition to 22.5° Brix and inoculation with 106 cells mL-1 of Saccharomyces cerevisiae Fermol Bouquet (AEB Group, São José dos Pinhais, Paraná, Brazil). The alcoholic fermentation started in open tanks, with intercalated addition of sugar (commercial sucrose). After the tumultuous phase, the fermentation was racked into 10m3 tanks (JAPA Components, Garibaldi, Rio Grande do Sul, Brazil) which were closed with a bung for 30 days. After that, the product was filtrated (10μm, Hidro filtros of Brazil, Caxias do Sul, Rio Grande do Sul, Brazil) and stored without preservative in a 10m³ bulk fermenter at 20°C. The analyses were carried out at the beginning of 2010 for all the samples. At that time, the fermented product from 2009 had just concluded fermentation whereas the others had been stored for one and two years. The blackberry pulp (marketed by the producer) consisted of the solid residue obtained from the de-pulping processing. The pulp was pasteurized (two hours at boiling temperature at 97°C), cooled and packaged (low density polyethylene) being stored frozen (-18°C).
Extraction of anthocyanins from fruit and pulp: The fruit and pulp (separately) were macerated in mortar and the soluble fractions were extracted by vacuum filtration with a mixture of acidified methanol (99:1, MeOH: HCl conc.) with Whatman paper no2 in a Büchner funnel. The extract was concentrated using a rotary evaporator (Tecnal TE-211, Piracicaba SP, Brazil) at low temperature (40ºC) up to 10% of the initial volume, filtered through a 0.45µm PVDF membrane (Millipore) and stored in an amber bottle at 4.0±1.0°C (LEES & FRANCIS, 1972LEES, D.H.; FRANCIS, F.G. Standardization of pigment analysis in cranberries. Hortscience, v.7, p.83-84, 1972.).
Extraction of total phenolic compounds from fruit and pulp: The fruit
and pulp (separately) were macerated in mortar and the soluble fractions were extracted
by vacuum filtration using a mixture of 80:20:1, 70° ethanol: distilled water: 3% formic
acid (ZARDO et al., 2009ZARDO, D.M. et al. Intensidade de pigmentação vermelha em maçãs e sua
relação com os teores de compostos fenólicos e capacidade antioxidativa. Ciência e
Tecnologia de Alimentos v.29, n.1, p.148-154, 2009. Available from:
<http://dx.doi.org/10.1590/S0101-20612009000100023>. Acessed: Jan. 11, 2013.
doi: 10.1590/S0101-20612009000100023.
http://dx.doi.org/10.1590/S0101-20612009...
).
Physicochemical analysis: The weight and size (length and width) of the
fruit were determined by measuring an individual number of each sample. The weight was
determined using a digital scale AX200 model (SHIMADZU) and the overall length and width
with a caliper (INSIZE). The dry extract, mineral residue (ash), acidity and dietary
fiber were determined using the methods of IAL (2008). Reducing sugars were determined
according NELSON (1944NELSON, N. A photometric adaptation of the Somogyi method for the
determination of glucose. Journal of Biological Chemistry, v.153, p.375-380,
1944.) and SOMOGYI (1945SOMOGYI, M. A new reagent for the determination of sugars., Journal of
Biological Chemistry v.160, n.1, p.61-68, 1945.) and glucose was determined by the enzymatic method
using a glucose oxidase kit (Gold Analisa, Belo Horizonte MG, Brazil) with D-glucose as
standard (DAHLQVIST, 1961DAHLQVIST, A. Determination of maltase and isomaltase activities with a
glucose-oxidase reagent. Biochemical Journal, v.80, p.547-551, 1961.). Fructose was
indirectly estimated by performing a subtraction of the levels of the reducing sugars
from glucose. The total phenolic compounds of phenolic extracts and
fermentation were quantified by the Folin-Ciocalteu reagent using catechin as standard
(SINGLETON & ROSSI, 1965SINGLETON, V.; ROSSI, J.A. Colorimetry of total phenolics with
phosphomolybdic- phosphotungstic acid reagents. American Journal of Enology and
Viticulture, v.16, n.3, p.144-158, 1965.) and results were
expressed in mg100g-1. Quantification of total monomer anthocyanins,
anthocyanin extracts and fermentations was carried out using the differential pH method
(GIUSTI & WORSLTAD, 2001GIUSTI, M.M.; WORSLTAD, R.E. Characterization and measurement of
anthocyanins by UV-visible spectroscopy. Curr. In: WROLSTAD, R.E. Protocols in Food
Analytical Chemistry. New York: Wiley, 2001.) and results were
expressed in mg of cyanidin 3-glucoside 100g-1 fruit. For the analysis of
antioxidant activity, the phenolic extracts and fermentations were measured by FRAP
methodology as described by BENZIE & STRAIN
(1996BENZIE, I.F.F.; STRAIN, J.J. The ferric reducing ability of plasma
(FRAP) as a measure of "antioxidant power": the FRAP assay. Analytical Biochemistry,
v.239, n.1, p.70-76, 1996. Available from:
<http://dx.doi.org/10.1006/abio.1996.0292>. Accessed: Aug. 20, 2012. doi:
10.1006/abio.1996.0292.
http://dx.doi.org/10.1006/abio.1996.0292...
) with modifications. The results were expressed as µM of total
equivalent capacity (TEAC) per g of sample.
Statistical analysis: The results of the experimental procedures were analyzed using analysis of variance (ANOVA), with 95% reliability and if there were differences these were distinguished by Tukey's differential test at 5% probability using the statistical software STATISTICA 7.0 for Windows (Statsoft, Inc., São Caetano do Sul, São Paulo, Brazil).
RESULTS AND DISCUSSION:
During the ripening the physical and chemical characteristics for both blackberries
varieties were evaluated. Characteristics in relation to weight, size and diameter
showed a significant increase with the evolution of ripening (Table 1). The parameters related to dry matter and mineral content
showed a significant decrease in relation to unripe fruit compared to semi-ripe and ripe
fruit. The levels of fibers decreased significantly during the maturation period. This
occurs due to increased synthesis of endogenous enzymes during ripening which makes the
fruit with soft texture. The sugar contents (Table
1) confirm the ripening stages (ACOSTA-MONTOYA
et al., 2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
). The levels of glucose and fructose increased significantly
during the period. The fruits of both 'Brazos' and 'Tupy' varieties showed a decrease in
the levels of acidity during the ripening period, as expected, making the fruits tasty
and ready to be consumed or processed. Similar results were described for tropical
highland blackberry (Rubus adenotrichus Schltdl.) by ACOSTA-MONTOYA et al. (2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
). The physical results and sugar and
acidity contents changed significantly during the blackberry ripening stage (Table 1).
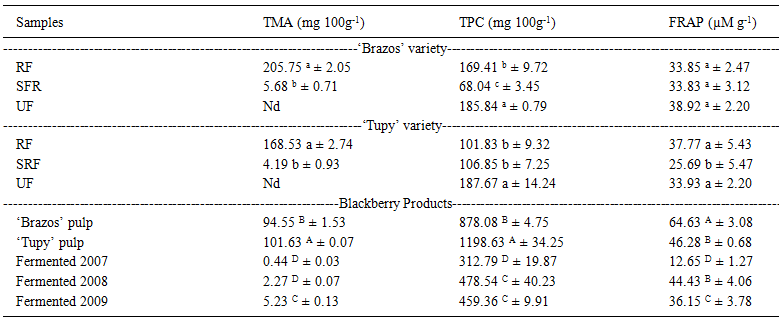
The highest levels of total monomeric anthocyanins (TMA) were found in the ripe fruit
(RF) (205.75mg 100g-1 'Brazos' and 168.53mg 100g-1 'Tupy' (Table 2). When assessing the levels of anthocyanins
for 'Brazos' and 'Tupy', HASSIMOTTO et al. (2008HASSIMOTTO, N.M.A. et al. Physico-chemical characterization and
bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Ciência e
Tecnologia de Alimentos, v.28, n.3; p.702-708, 2008. Available from:
<http://dx.doi.org/10.1590/S0101-20612008000300029>. Accessed: Aug. 20, 2012.
doi: 10.1590/S0101-20612008000300029.
http://dx.doi.org/10.1590/S0101-20612008...
)
determined values below those found in this study (133.0mg 100g-1 and 116.0mg
100g-1) and FERREIRA et al. (2010FERREIRA, D.S. et al. Compostos bioativos presentes em amora-preta
(Rubus spp.). Revista Brasileira de Fruticultura, v.32, n.3, p.664-674, 2010.
Available from: <http://dx.doi.org/
http://dx.doi.org/10.1590/S0100-294520110005000110>. Accessed: Jan. 11, 2013. doi:
10.1590/S0100-294520110005000110.
http://dx.doi.org/
...
)
found 104.1mg 100g-1 for the 'Tupy' variety. The values found in the present
study were within those presented by KOCA &
KARADENIZ (2009KOCA, I.; KARADENIZ, B. Antioxidant properties of blackberry and
blueberry fruits grown in the Black Sea Region of Turkey. Scientia Horticulturae,
v.121, n.4, p.447-450, 2009. Available from:
<http://dx.doi.org/10.1016/j.scienta.2009.03.015>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.scienta.2009.03.015.
http://dx.doi.org/10.1016/j.scienta.2009...
) for ten different blackberries varieties grown in Turkey
(12.0 to 326.0mg 100g-1). Other results similar to the levels of anthocyanins
have also been reported by other authors (WANG &
LIN, 2000WANG, S.Y.; LIN, H.S. Antioxidant activity in fruits and leaves of
blackberry, raspberry, and strawberry varies with cultivar and developmental stage.
Journal of Agricultural and, Food Chemistry v.48, p.140-146, 2000. Available from:
<http://dx.doi.org/10.1021/jf9908345>. Accessed: Aug. 20, 2012. doi:
10.1021/jf9908345.
http://dx.doi.org/10.1021/jf9908345...
; SIRIWOHARN et al., 2004SIRIWOHARN, T. et al. Influence of cultivar, maturity, and sampling on
blackberry (Rubus L. hybrids) anthocyanins, polyphenolics, and antioxidant
properties. Journal of Agricultural and, Food Chemistry v.52, n.26, p.8021-8030,
2004. Available from: <http://dx.doi.org/10.1021/jf048619y>. Accessed: Aug. 20,
2012. doi: 10.1021/jf048619y.
http://dx.doi.org/10.1021/jf048619y...
).
Considering the maximum sensory acceptability of ripe fruits due to their high contents
of sugars, low acidity, soft texture and color due high anthocyanins content and the
importance of these compounds for human health principally as antioxidants, the results
of the present study confirm that this is the preferred stage for fruit consumption as
well as for its use as raw material for processing. The results show that functional
molecules (anthocyanins and total phenolic compounds) as well as the antioxidant
capacity are not decreased as blackberry fruits become ripe.
The levels of total phenolic compounds (TPC) decreased with the advancement of ripening
and were determined in a higher concentration for unripe fruit of both varieties. In the
'Tupy' variety, semi-ripe fruits did not differ significantly from ripe fruits, while in
the 'Brazos' variety it was observed that between these two stages the highest level was
for ripe fruit. As reported by ACOSTA-MONTOYA et al.
(2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
), the main phenolic compounds determined of blackberries were
ellagitannins (lambertianin C and sanguiin H-6) and anthocyanins (cyanindin-3-glucoside
and cyanidin-3-(6'-malonyl) glucoside), and in the semi-ripe fruit ellagitannins
accounted for more than 92% of phenolic compounds and 61 % in ripe fruit. Ellagitannins,
also referred to as hydrolysable tannins or ellagic acid derivatives (LEE et al., 2012LEE, J. et al. Rubus fruit phenolic research: The good, the bad, and the
confusing. Food Chemistry, v.130, n.4, p.785-796, 2012. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2011.08.022>. Accessed: Apr. 30, 2013.
doi: 10.1016/j.foodchem.2011.08.022.
http://dx.doi.org/10.1016/j.foodchem.201...
) which are identified as active
principles in traditional Chinese medicines (QUIDEAU,
2009QUIDEAU, S. Chemistry and biology of ellagitannins: an underestimated
class of bioactive plant polyphenols. Singapore: World Scientific Publishing, 2009.
374p.) and are major phenolic compounds. These compounds are certainly
associated with the antioxidant activity (SIRIWOHARN et
al., 2004SIRIWOHARN, T. et al. Influence of cultivar, maturity, and sampling on
blackberry (Rubus L. hybrids) anthocyanins, polyphenolics, and antioxidant
properties. Journal of Agricultural and, Food Chemistry v.52, n.26, p.8021-8030,
2004. Available from: <http://dx.doi.org/10.1021/jf048619y>. Accessed: Aug. 20,
2012. doi: 10.1021/jf048619y.
http://dx.doi.org/10.1021/jf048619y...
; ACOSTA-MONTOYA et al., 2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
).
Although they decrease during ripening of the blackberries, anthocyanins increase with
the degree of maturity, as was observed for the 'Brazos' and 'Tupy'
varieties.
The antioxidant activity determined by FRAP for the 'Brazos' variety did not present
significant difference between the different stages of maturation. For the 'Tupy'
variety the unripe and ripe fruits were those with the highest values. The antioxidant
activities of the ripe fruits of both varieties were similar to those presented by KOCA & KARADENIZ (2009KOCA, I.; KARADENIZ, B. Antioxidant properties of blackberry and
blueberry fruits grown in the Black Sea Region of Turkey. Scientia Horticulturae,
v.121, n.4, p.447-450, 2009. Available from:
<http://dx.doi.org/10.1016/j.scienta.2009.03.015>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.scienta.2009.03.015.
http://dx.doi.org/10.1016/j.scienta.2009...
) for ten different
varieties of blackberries at a ripe stage of maturity (35.05 to 70.41µmol
g-1). As found in the present paper, VIZZOTTO et al. (2012VIZZOTTO, M. et al. Teor de compostos fenólicos e atividade antioxidante
em diferentes genótipos de amoreira-preta (Rubus sp.). Revista Brasileira de
Fruticultura, v.34, n.3, p.853-858, 2012. Available from:
<http://dx.doi.org/10.1590/S0100-29452012000300027>. Accessed: Jan. 11, 2013.
doi: 10.1590/S0100-29452012000300027.
http://dx.doi.org/10.1590/S0100-29452012...
) also reported a lack of correlation between total
phenolic compounds and antioxidant activity in blackberries, although in many papers
considering different vegetables a positive correlation exists. GORDON et al. (2012GORDON, A. et al. Chemical characterization and evaluation of
antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food
Chemistry, v.133, n.2, p.256-263, 2012. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2011.11.150>. Accessed: Jan. 11, 2013.
doi: 10.1016/j.foodchem.2011.11.150.
http://dx.doi.org/10.1016/j.foodchem.201...
) reported decreasing antioxidant activity in
açaí fruits (Euterpe oleraceae Mart) during ripening,
as did PINELI et al. (2011PINELI, L.L.O. et al. Antioxidants and other chemical and physical
characteristics of two strawberry cultivars at different ripeness stages. Journal of
Food Composition and Analysis, v.24, p.11-16, 2011. Available from:
<http://dx.doi.org/10.1016/j.jfca.2010.05.004>. Accessed: Jan. 11, 2013. doi:
10.1016/j.jfca.2010.05.004.
http://dx.doi.org/10.1016/j.jfca.2010.05...
) when considering
different ripening stages of strawberries. ACOSTA-MONTOYA
et al. (2010ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of
tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible
maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from:
<http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012.
doi: 10.1016/j.foodchem.2009.09.032.
http://dx.doi.org/10.1016/j.foodchem.200...
) reported high values of antioxidant activity of unripe
blackberry fruits and related this fact to non-anthocyanin phenolic compounds such as
ellagitannins (Table 2). The results of the
present research did not show decrease in the antioxidant activity during fruit ripening
and that is the most important characteristic for the consumer.
The highest levels of anthocyanins were found among the blackberry pulp ('Brazos' and 'Tupy' varieties) and the 'Tupy' variety had the highest content (101.63mg 100mL-1). The levels of total phenolic compounds and antioxidant activity were high for the pulp. The 'Tupy' variety presented the highest content of phenolic compounds (1198.63mg 100mL-1) and the 'Brazos' variety produced the highest antioxidant activity (64.63mg 100mL-1), up to eleven times and twice as much as the concentration in fruit, respectively (Table 2). The high values of phenolic compounds can be due to the concentration by thermal process. The anthocyanins did not increase with this operation and this could be due to their instability at high temperatures (SHAHIDI & NACZK, 1995SHAHIDI, F.; NACZK, M. Food phenolics: sources, chemistry, effects and applications. Lancaster: Technomic, 1995. 331p.).
It was observed that in the fermented products of the berries there was a decrease in
the levels of anthocyanins during the storage period; the fermented berries with the
greatest storage period had the lowest anthocyanin content across all the products
(0.44mg 100mL-1) (Table 2). PÉREZ-MAGARIÑO & JOSÉ (2004PÉREZ-MAGARIÑO, S.; JOSÉ, M.L.G. Evolution of flavanols, anthocyanins,
and their derivatives during the aging of red wines elaborated from grapes harvested
at different stages of ripening. Journal of Agricultural and, Food Chemistry v.52,
p.1181-1189, 2004. Available from: <http://dx.doi.org/10.1021/jf035099i>.
Accessed: Aug. 20, 2012. doi: 10.1021/jf035099i.
http://dx.doi.org/10.1021/jf035099i...
) evaluated the
evolution of flavonoids and anthocyanins during the ageing of wines and found a decrease
in free anthocyanins and flavonoids and an increase in anthocyanin derivatives that were
responsible for maintaining the intensity of color in the aged wines. These reactions
not only modify wine color but also bring about changes in such other attributes as
astringency and bitter flavors. Fermented blackberries have a superior phenolic content
when compared to that found in other fruit, possibly due to phenolic extraction by
ethanol and carbon dioxide release in the initial stages of alcoholic fermentation.
However, the free anthocyanin content reduces during processing and storage losing the
function quality. This anthocyanin decrease is due to degradation reactions and
sedimentation during long periods of storage (MARKAKIS,
1982MARKAKIS, P. Stability of anthocyanins in foods. In: MARKAKIS,
P.Anthocyanins as food colors. New York: Academic, 1982. p.163-180.). In the present study, antioxidant activity was similar to that found in
fruits during the first two years of storage, but in the third year the decrease was
greater than 50% (Table 2).
CONCLUSION:
Blackberry pulp had the high levels of phenolic compounds and highest antioxidant activity, what should be related with its production method involving juice extraction in a depulper that concentrates fiber and other solids. The fermented beverages indicated the instability of anthocyanins with long storage period (loss of 92% in three years) and that antioxidant activity was stable for two years but there was a significant loss during the third year of storage (>50%).
ACKNOWLEDGEMENTS
The authors are deeply grateful to Universidade Estadual de Ponta Grossa (UEPG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the infrastructure and the scholarships offered in the implementation period of this research and also to Adega Porto Brazos for the supply of fruits and products.
- ACOSTA-MONTOYA, Ó. et al. Phenolic contend and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schtdl.) during three edible maturity stages. Food Chemistry, v.119, p.1497-1501, 2010. Available from: <http://dx.doi.org/10.1016/j.foodchem.2009.09.032>. Accessed: Aug. 20, 2012. doi: 10.1016/j.foodchem.2009.09.032.
» https://doi.org/10.1016/j.foodchem.2009.09.032» http://dx.doi.org/10.1016/j.foodchem.2009.09.032 - ANTUNES, L.E.C. Amora-preta: nova opção de cultivo no Brasil. Ciência Rural, v.32, n.1, p.151-158, 2002. Available from: <http://dx.doi.org/10.1590/S0103-84782002000100026>. Accessed: Aug. 20, 2012. doi: 10.1590/S0103-84782002000100026.
» https://doi.org/10.1590/S0103-84782002000100026» http://dx.doi.org/10.1590/S0103-84782002000100026 - BENZIE, I.F.F.; STRAIN, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Analytical Biochemistry, v.239, n.1, p.70-76, 1996. Available from: <http://dx.doi.org/10.1006/abio.1996.0292>. Accessed: Aug. 20, 2012. doi: 10.1006/abio.1996.0292.
» https://doi.org/10.1006/abio.1996.0292» http://dx.doi.org/10.1006/abio.1996.0292 - CLERICI, M.T.P.S.; CARVALHO-SILVA, L.B. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Research International, v.44, n.7, p.1658-1670, 2011. Available from: <http://dx.doi.org/10.1016/j.foodres.2011.04.020>. Accessed: Jan. 11, 2011. doi: 10.1016/j.foodres.2011.04.020.
» https://doi.org/10.1016/j.foodres.2011.04.020» http://dx.doi.org/10.1016/j.foodres.2011.04.020 - DAHLQVIST, A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochemical Journal, v.80, p.547-551, 1961.
- FERREIRA, D.S. et al. Compostos bioativos presentes em amora-preta (Rubus spp.). Revista Brasileira de Fruticultura, v.32, n.3, p.664-674, 2010. Available from: <http://dx.doi.org/ http://dx.doi.org/10.1590/S0100-294520110005000110>. Accessed: Jan. 11, 2013. doi: 10.1590/S0100-294520110005000110.
» https://doi.org/10.1590/S0100-294520110005000110» http://dx.doi.org/ http://dx.doi.org/10.1590/S0100-294520110005000110 - GIUSTI, M.M.; WORSLTAD, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. In: WROLSTAD, R.E. Protocols in Food Analytical Chemistry. New York: Wiley, 2001.
- GORDON, A. et al. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chemistry, v.133, n.2, p.256-263, 2012. Available from: <http://dx.doi.org/10.1016/j.foodchem.2011.11.150>. Accessed: Jan. 11, 2013. doi: 10.1016/j.foodchem.2011.11.150.
» https://doi.org/10.1016/j.foodchem.2011.11.150» http://dx.doi.org/10.1016/j.foodchem.2011.11.150 - HASSIMOTTO, N.M.A. et al. Physico-chemical characterization and bioactive compounds of blackberry fruits (Rubus sp.) grown in Brazil. Ciência e Tecnologia de Alimentos, v.28, n.3; p.702-708, 2008. Available from: <http://dx.doi.org/10.1590/S0101-20612008000300029>. Accessed: Aug. 20, 2012. doi: 10.1590/S0101-20612008000300029.
» https://doi.org/10.1590/S0101-20612008000300029» http://dx.doi.org/10.1590/S0101-20612008000300029 - HIRSCH, G.E. et al. Caracterização físico-química de variedades de amora-preta da região sul do Brasil., Ciência Rural v.42, n.5, p.942-947, 2012. Available from: <http://dx.doi.org/10.1590/S0103-847820120050000021>. Accessed: Jan. 11, 2013. doi: 10.1590/S0103-847820120050000021.
» https://doi.org/10.1590/S0103-847820120050000021» http://dx.doi.org/10.1590/S0103-847820120050000021 - IAL. Métodos físicos e químicos para análise de alimentos. Normas Analíticas. São Paulo: Instituto Adolfo Lutz, 2008. Total de extrato seco, minerias, acidez e fibra dietética.
- LEE, J. et al. Rubus fruit phenolic research: The good, the bad, and the confusing. Food Chemistry, v.130, n.4, p.785-796, 2012. Available from: <http://dx.doi.org/10.1016/j.foodchem.2011.08.022>. Accessed: Apr. 30, 2013. doi: 10.1016/j.foodchem.2011.08.022.
» https://doi.org/10.1016/j.foodchem.2011.08.022» http://dx.doi.org/10.1016/j.foodchem.2011.08.022 - LEES, D.H.; FRANCIS, F.G. Standardization of pigment analysis in cranberries. Hortscience, v.7, p.83-84, 1972.
- KOCA, I.; KARADENIZ, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Scientia Horticulturae, v.121, n.4, p.447-450, 2009. Available from: <http://dx.doi.org/10.1016/j.scienta.2009.03.015>. Accessed: Aug. 20, 2012. doi: 10.1016/j.scienta.2009.03.015.
» https://doi.org/10.1016/j.scienta.2009.03.015» http://dx.doi.org/10.1016/j.scienta.2009.03.015 - MARKAKIS, P. Stability of anthocyanins in foods. In: MARKAKIS, P.Anthocyanins as food colors. New York: Academic, 1982. p.163-180.
- MOTA, R.V. Caracterização do suco de amora-preta elaborado em extrator caseiro. Ciência e Tecnologia de Alimentos v.26, n.2, p.303-308, 2006. Available from: <http://dx.doi.org/10.1590/S0101-20612006000200012>. Accessed: Aug. 20, 2012. doi: 10.1590/S0101-20612006000200012.
» https://doi.org/10.1590/S0101-20612006000200012» http://dx.doi.org/10.1590/S0101-20612006000200012 - NELSON, N. A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, v.153, p.375-380, 1944.
- PÉREZ-MAGARIÑO, S.; JOSÉ, M.L.G. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. Journal of Agricultural and, Food Chemistry v.52, p.1181-1189, 2004. Available from: <http://dx.doi.org/10.1021/jf035099i>. Accessed: Aug. 20, 2012. doi: 10.1021/jf035099i.
» https://doi.org/10.1021/jf035099i» http://dx.doi.org/10.1021/jf035099i - PINELI, L.L.O. et al. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. Journal of Food Composition and Analysis, v.24, p.11-16, 2011. Available from: <http://dx.doi.org/10.1016/j.jfca.2010.05.004>. Accessed: Jan. 11, 2013. doi: 10.1016/j.jfca.2010.05.004.
» https://doi.org/10.1016/j.jfca.2010.05.004» http://dx.doi.org/10.1016/j.jfca.2010.05.004 - QUIDEAU, S. Chemistry and biology of ellagitannins: an underestimated class of bioactive plant polyphenols. Singapore: World Scientific Publishing, 2009. 374p.
- SHAHIDI, F.; NACZK, M. Food phenolics: sources, chemistry, effects and applications. Lancaster: Technomic, 1995. 331p.
- SINGLETON, V.; ROSSI, J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. American Journal of Enology and Viticulture, v.16, n.3, p.144-158, 1965.
- SIRIWOHARN, T. et al. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. hybrids) anthocyanins, polyphenolics, and antioxidant properties. Journal of Agricultural and, Food Chemistry v.52, n.26, p.8021-8030, 2004. Available from: <http://dx.doi.org/10.1021/jf048619y>. Accessed: Aug. 20, 2012. doi: 10.1021/jf048619y.
» https://doi.org/10.1021/jf048619y» http://dx.doi.org/10.1021/jf048619y - SOMOGYI, M. A new reagent for the determination of sugars., Journal of Biological Chemistry v.160, n.1, p.61-68, 1945.
- VIZZOTTO, M. et al. Teor de compostos fenólicos e atividade antioxidante em diferentes genótipos de amoreira-preta (Rubus sp.). Revista Brasileira de Fruticultura, v.34, n.3, p.853-858, 2012. Available from: <http://dx.doi.org/10.1590/S0100-29452012000300027>. Accessed: Jan. 11, 2013. doi: 10.1590/S0100-29452012000300027.
» https://doi.org/10.1590/S0100-29452012000300027» http://dx.doi.org/10.1590/S0100-29452012000300027 - WANG, S.Y.; LIN, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. Journal of Agricultural and, Food Chemistry v.48, p.140-146, 2000. Available from: <http://dx.doi.org/10.1021/jf9908345>. Accessed: Aug. 20, 2012. doi: 10.1021/jf9908345.
» https://doi.org/10.1021/jf9908345» http://dx.doi.org/10.1021/jf9908345 - ZARDO, D.M. et al. Intensidade de pigmentação vermelha em maçãs e sua relação com os teores de compostos fenólicos e capacidade antioxidativa. Ciência e Tecnologia de Alimentos v.29, n.1, p.148-154, 2009. Available from: <http://dx.doi.org/10.1590/S0101-20612009000100023>. Acessed: Jan. 11, 2013. doi: 10.1590/S0101-20612009000100023.
» https://doi.org/10.1590/S0101-20612009000100023» http://dx.doi.org/10.1590/S0101-20612009000100023
Publication Dates
-
Publication in this collection
06 Mar 2015 -
Date of issue
Apr 2015
History
-
Received
21 Aug 2012 -
Accepted
07 Apr 2014