ABSTRACT:
As a gas signaling molecule, endogenous hydrogen sulfide (H2S) plays a crucial role in the plant stress response. However, the role of H2S in the response to organic pollutants specifically has not been studied. Here, the effects of H2S addition on soybean (Glycine max) seedlings tolerance of 1,4-dichlorobenzene (1,4-DCB) were investigated. Under 1,4-DCB stress, the growth of soybean seedlings roots and stems was inhibited, while L-/D-cysteine desulfhydrase (LCD/DCD) activity was induced and endogenous H2S increased. When applied jointly with sodium hydrosulfide (NaHS), a H2S donor, root growth inhibition was effectively alleviated. Pre-treatment of seedlings with 0.4mmol L-1 NaHS reduced the malondialdehyde (MDA) and reactived oxygen species (ROS) content, mitigating root cell toxicity significantly. Further experiments confirmed that NaHS enhanced soybean seedlings peroxidase (POD) and superoxide dismutase (SOD) enzyme activities. In contrast, these effects were reversed by hypotaurine (HT), a H2S scavenger. Therefore, H2S alleviated 1,4-DCB toxicity in soybean seedlings by regulating antioxidant enzyme activity to reduce cell oxidative damage.
Key words:
hydrogen sulfide (H2S); soybean seedlings; 1,4-dichlorobenzene (1,4-DCB); toxicity; oxidative stress
RESUMO:
Tal como uma molécula de sinal de gás, sulfureto de hidrogenio endógena (H2S) desempenha um papel crucial na resposta ao stress das plantas. Mas não foi relatado o papel de H2S em plantas poluentes orgânicos stress. Este estudo sobre a variação de H2S envolvido em plântulas de soja tolerância 1,4-Diclorobenzeno foi investigada. Os resultados mostraram sob o 1,4-diclorobenzeno stress, que o crescimento da soja (Glycine max) de raiz de mudas e caule foram inibidas, L- / D-cisteína desulfhydrase (LCD / DCD) atividades enzimáticas foram empossados, em seguida, H2S endógeno aumentado. Quando aplicado com hidrossulfureto de sódio (NaSH), um doador de H2S, raiz de plântulas de soja, a inibição do crescimento pode ser melhorada. Tratamentos prévios com 0,4mmol L-1 NaHS, malondialdeído (MDA) e espécies de oxigênio reactivas conteúdo (ROS) foi reduzida, em seguida, a toxicidade celular da raiz foi reduzida significativamente. Outros experimentos confirmaram que NaSH melhorou a peroxidase de plântulas de soja (POD), superóxido dismutase (SOD) atividades enzimáticas. Em contraste, estes efeitos foram revertidos por hypotaurine (HT), um eliminador de H2S. Então H2S pode aliviar toxicidade 1,4-diclorobenzeno em plântulas de soja por meio da regulamentação das atividades de enzimas antioxidantes para manter a integridade da estrutura celular.
Palavras-chave:
sulfeto de hidrogênio (H2S); das plântulas de soja; 1,4-diclorobenzeno; toxicidade; estresse oxidativo
Introduction:
Hydrogen sulfide (H2S) has been recently discovered to act as a gaseous transmitter. It is therefore the third discovered gaseous signaling molecule, following the discoveries of Nitric oxide (NO) and Carbon monoxide (CO). In plants, H2S is mainly produced via the L-/D-cysteine desulfhydrase enzymes (LCD/DCD) and it has been reported to be involved in stress responses to drought, heat, heavy metal and salt during seed germination and seedling growth (ZHANG et al., 2010aZHANG, H. et al. Hydrogen sulfide alleviated chromium toxicity in wheat. Biologia Planta rum, v. 54, p.743-747, 2010a. Available from: <http://link.springer.com/article/10.1007%2Fs10535-010-0133-9>. Accessed: Jan. 08, 2014. doi:10.1007/s10535-010-0133-9.
http://link.springer.com/article/10.1007...
; JIN et al., 2011JIN, Z. et al. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, v.414, p.481-486, 2011. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/21986537>. Accessed: Nov. 21, 2015. doi: 10.1016/j.bbrc.2011.09.090.
http://www.ncbi.nlm.nih.gov/pubmed/21986...
; LAI et al., 2014LAI, D. et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, v.225, p.117-129, 2014. Available from: <http://www.sciencedirect.com/science/article/pii/S016894521400140X>. Accessed: Jun. 12, 2015. doi: 10.1016/j.plantsci.2014.06.006.
http://www.sciencedirect.com/science/art...
; LI et al., 2014LI, Z.G. et al. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia, v.69, p.1001-1009, 2014. Available from: <http://link.springer.com/article/10.2478/s11756-014-0396-2>. Accessed: Jun. 12, 2015. doi:10.2478/s11756-014-0396-2.
http://link.springer.com/article/10.2478...
). Applications of H2S could also significantly improve root morphology, chlorophyll content and photosynthetic activity under Pb stress (ALI et al., 2014ALI, B. et al. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastr-uctural changes in oilseed rape. Ecotoxicology and Environmental Safety, v.102, p.25-33, 2014. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/24580818>. Accessed: Feb. 01, 2015. doi:10.1016/j.ecoenv.2014.01.013.
http://www.ncbi.nlm.nih.gov/pubmed/24580...
).
Chlorobenzene dissolved in an organic solvent is an important raw material and intermediate in the pharmaceutical, leather, dye and other industries, where it is widely used. With the discharge of industrial waste, chlorobenzene, which is non-biodegradable and can accumulate in the environment, enters in ecosystems in different ways, as polluted air, soil and water, potentially causing lasting harm. For example, the presence of 1,2,4-trichlorobenzene reduced maximum root length, plant height, tillers per hill, shoot and root dry weight in rice plants, as a result of changes in the activity of antioxidant enzymes, reactive oxygen species (ROS), malondialdehyde (MDA) and membrane lipid peroxidation (DING et al., 2014DING, X.W. et al. Effects of 1,2,4-trichlorobenzene on growth and physiological characteristics of rice at maximum tillering stage. Acta Agronomica Sinica, v.40, p.487-496, 2014. ). Chlorobenzene also inhibited maize cell division and seedling growth, and the oxidative stress response increased proportional to the compound's degree of chlorination (MIGUEL et al., 2012MIGUEL, A.S. et al. Biological responses of maize (Zea mays) plants exposed to chlorobenzenes case study of monochloro-,1,4-dichloro and 1,2,4-trichloro-benzenes. Ecotoxicology, v.21, p.315-324, 2012. Available from: <http://link.springer.com/article/10.1007%2Fs10646-011-0792-0>. Accessed: Jan. 12, 2015. doi: 10.1007/s10646-011-0792-0.
http://link.springer.com/article/10.1007...
).
H2S has been identified to play an important role in diverse physiological processes in plants. However, the relationship between H2S and plant chlorobenzene stress tolerance remains poorly characterized, and whether or not oxidation reactions are involved in the process is not clear. The aim of this study was to provide more evidences for potential mechanisms of H2S-related mitigation of developmental inhibition caused by the organic pollutant, 1, 4-dichlorobenzene (1,4-DCB). Changes in root and stem elongation, MDA and ROS contents, and antioxidant enzyme levels were investigated. The results revealed that the presence of H2S alleviated 1, 4-DCB-induced stress in soybean seedlings by restoring oxidation balance.
materials and methods:
plant materials, growth conditions and processing methods
Seeds of the soybean (Glycine max) variety Zhong huang 57 were surface-sterilized with a 10min long soak in 5% NaOCl. After being rinsed with deionized water, the seeds were then soaked in an appropriate amount of deionized water for 24h to initiate germination. Uniform seedlings were selected and planted in seedling trays containing treated soil. The soil was purchased from Kaifeng Seed Company and contained at least 28% total organic matter and 2% total nitrogen, phosphorus and potassium. Varying concentrations of sodium hydrosulfide (NaHS, H2S donor) or hypotaurine (HT, H2S scavenger) solutions were added to the dry soil mix, increasing the total water content to 30%, and 1,4-DCB dissolved in acetone was then added to make up the different treatments, with acetone for the controls. Soybean seedlings were maintained at 25°C±1°C with a 14h photoperiod (the light intensity was 200µmol m-2 s-1). One week old seedlings were harvested for analysis.
Endogenous H2S content determination and L-/D-cysteine desulfhydrase activity assay
The methylene blue method was used to determine the H2S content in soybean seedling roots, following ZHANG et al. (2008ZHANG, H. et al. Hydrogen Sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. Journal of Integrative Plant Biology, v.50, p.1518-1529, 2008. Available from: <http://onlinelibrary.wiley.com/doi/10.1111/j.1744-7909.2008.00769.x/>. Accessed: Jun. 12, 2015. doi: 10.1111/j.1744-7909.2008.00769.x.
http://onlinelibrary.wiley.com/doi/10.11...
), while root cysteine desulfhydrase activity was determined using the method of JIN et al. (2011JIN, Z. et al. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, v.414, p.481-486, 2011. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/21986537>. Accessed: Nov. 21, 2015. doi: 10.1016/j.bbrc.2011.09.090.
http://www.ncbi.nlm.nih.gov/pubmed/21986...
). The activity of L-cysteine desulfhydrase was established using H2S (including L-DTT) release. D-cysteine desulfhydrase enzyme activity was also measured by replacing L-cysteine with D-cysteine and using pH 8.0 Tris-HCl buffer; otherwise, the steps remained the same. Control and treatment data were expressed as means plus standard errors of three independent experiments.
MDA content determination
The MDA concentration was calculated using the formula of ZHANG et al. (2011ZHANG, H. et al. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology, v.60, p.251-257, 2011. Available from: <http://www.sciencedirect.com/science/article/pii/S092552141100007X>. Accessed: Jan. 08, 2014. doi: 10.1016/j.postharvbio.2011.01.006.
http://www.sciencedirect.com/science/art...
).
Determination of hydrogen H2O2 content and the rate of O2.- generation
H2O2 content and the rate of O2.- generation were determined using assay kits produced by the first branch of the Nanjing Jiancheng Biological Engineering Institute.
Histochemical staining and tissue sections
Evans Blue dye was used to detect the viability of root tip cells in the soybean seedlings following BAKER & MOCK's method (1994BAKER, C.J.; MOCK, N.M. An improved method for monitoring cell death incell suspension and leaf disc assays using Evans blue. Plant Cell Tissue and Organ Culture, v.39, p.7-12, 1994. Available from: <http://rd.springer.com/article/10.1007/BF00037585>. Accessed: Jan. 08, 2014. doi: 10.1007/BF00037585.
http://rd.springer.com/article/10.1007/B...
). A 2-3cm root tip was stained for 10 minutes in a 0.25% Evans Blue solution at room temperature. After a wash with double distilled water, the root tip was photographed.
Antioxidant enzyme activity assay
POD activity was measured using the guaiacol method, where a 0.01 change per minute at A470 was calculated as one enzyme activity unit (U). CAT activity was determined according to LANG & ZHU's method (2014LANG, J.; ZHU, Y. Comparison of two methods for determination of catalase activity in rice. Journal of the Chinese Cereals and Oils Association, v.29, p.89-99, 2014.). SOD activity was determined using the nitro blue tetrazolium chloride (NBT) photochemical reduction method.
Results AND Discussion:
1, 4-DCB inhibited soybean seedlings growth and promoted the synthesis of H2S
In order to assess toxicity symptoms in soybean seedlings under chlorobenzene stress, different concentrations (0, 4, 8, 16, 24mM L-1) of 1,4-DCB were applied in this study. It was determined that 1,4-DCB significantly inhibited the elongation of soybean seedling roots and stems in a dose-dependent manner, with greater inhibition with increasing 1,4-DCB concentrations. Maximum response occurred at doses of 24mmol L-1, where the length of seedling roots and stems were 33.3% and 34.7%, respectively, of controls (Figure 1A, B). In order to investigate whether H2S was associated with this process, endogenous H2S concentrations were measured in soybean seedlings. H2S content increased with 1, 4-DCB concentration; when the 1, 4-DCB concentration was between 8 and 24mmol kg-1, the endogenous H2S content in treated seedlings differed significantly from controls (Figure 1C). Therefore, a concentration of 8mmol kg-1 1, 4-DCB was selected to induce a stress response for further experiments. H2S was mainly produced from L-/D-cysteine in plants (JIN, et al., 2015JIN, Z.P. et al. Physiological implications of hydrogen sulfide in plants: pleasant exploration behind its unpleasant odour. Oxidative Medicine and Cellular Longevity, v.2015, p.1-6, 2015. Available from: <http://dx.doi.org/10.1155/2015/397502>. Accessed: Nov. 10, 2015. doi: 10.1111/j.1742-4658.2005.04567.x.
http://dx.doi.org/10.1155/2015/397502...
). Here, DCD activity increased with the concentration of 1, 4-DCB and the activity of LCD was significantly elevated at a 1,4-DCB concentration of 8mmol kg-1; however, as 1,4-DCB levels rose above 8mmol kg-1, the total activity of LCD did not differ from the control (Figure 1D). Thus, these results indicated a possible inter-relationship between LCD/DCD-related H2S homeostasis and chlorobenzene response in soybean seedlings. The H2S pathway was similar to the generation of H2S in stomatal closure (HOU et al., 2011HOU, Z.H. et al. H2S may function downstream of H2O2 in jasmonic acid-induced stomatal closure in Vicia faba. Chinese Bulletin of Botany, v.46, p.396-406, 2011. ).
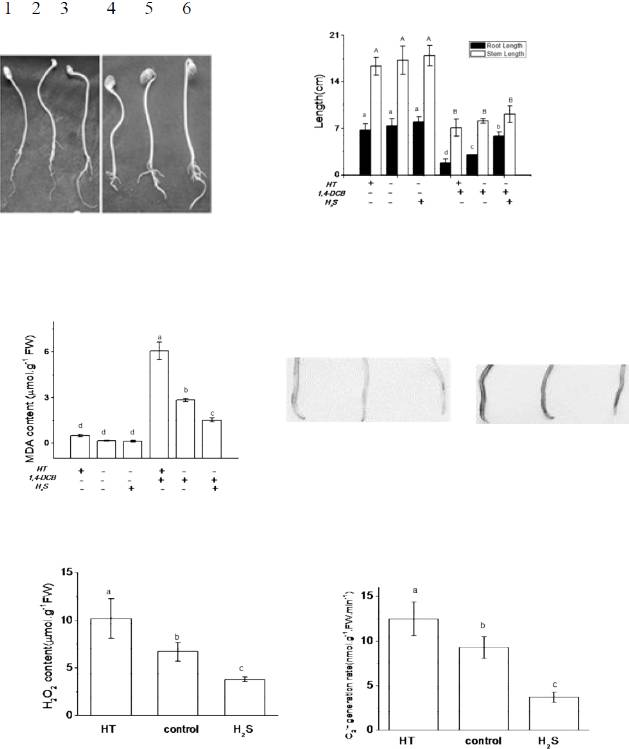
Effects of 1,4-DCB and NaHS treatments on soybean seedlings. The root and stem lengths (A), endogenous H2S content (B), and activities of DCD (C) and LCD (D) were measured in soybean seedlings under the 1,4-DCB stress. NaHS treatments of different concentrations on root length and stem length (E) and intracellular MDA (F) in soybean seedlings under 1,4-DCB stress. Note that values are means ± the SE of three independent experiments, and that different letters indicate significant differences (P<0.05), in all figures.
NaHS alleviated 1, 4-DCB-induced toxicity in soybean seedlings
NaHS has been reported to be a H2S donor in previous research (LAI et al., 2014LAI, D. et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, v.225, p.117-129, 2014. Available from: <http://www.sciencedirect.com/science/article/pii/S016894521400140X>. Accessed: Jun. 12, 2015. doi: 10.1016/j.plantsci.2014.06.006.
http://www.sciencedirect.com/science/art...
). Therefore, NaHS was applied in this study to examine the effects of H2S on soybean seedlings under 1, 4-DCB-induced stress. Experiments were carried out on soybean seedlings planted in soil with different concentrations of NaHS (0, 0.2, 0.4, 0.6, 0.8, 1.0mmol L-1) and 8mmol kg-1 1, 4-DCB. Root length inhibition observed under 1, 4-DCB stress was mitigated progressively as the NaHS concentration increased; this effect might be even further enhanced above 1mmol L-1 of NaHS. However, there was no detoxification effect of NaHS on stem growth (Figure 1E). This finding agreed with previous results showing that pretreatment with NaHS could improve heat tolerance (LI et al., 2015LI, Z.G. et al. Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. In Vitro Cellular and Developmental Biology-Plant, v.51, p.428-437, 2015. Available from: <http://link.springer.com/article/10.1007/s11627-015-9705-x>. Accessed: Jan. 25, 2016. doi:10.1007/s11627-015-9705-x.
http://link.springer.com/article/10.1007...
), but differs from results showing that high concentrations of NaHS produce toxic effects (LAI et al., 2014). This variation in results might be due to differing physiological concentrations of H2S experienced as a result of variation in the plant material, developmental stage, tissue and organs, and growth environment studied. In addition, this study reported relationship between NaHS concentration and the release characteristics of different H2S donors. Pretreatment with increasing doses of NaHS caused a considerable decrease in the level of 1,4-DCB-induced MDA (Figure 1F). This effect occurred above NaHS concentrations of 0.4mmol L-1, hence this concentration of NaHS was used in the follow-up experiments.
H2S scavengers aggravated toxic effects of 1,4-DCB in soybean seedlings
In order to clarify the role of endogenous H2S homeostasis in regulating the stress response of soybean seedlings to 1,4-DCB, applications of HT were made in experiments. When HT was applied (at 0.4mmol L-1), soybean seedlings had an opposite phenotype compared to the NaHS treatment. The growth of roots and stems was arrested, and in the presence of both chlorobenzene and HT, this growth inhibition was further aggravated, with root and stem lengths only 26.5% and 37.4%, respectively, of controls (Figure 2A, B); roots also became thicker.
Effects of HT and NaHS treatments on the root length and stem length of soybean seedlings (A & B), intracellular MDA content (C), H2O2 content, and the rate of O2. - generation (E) in soybean seedlings under 1,4-DCB stress. The apical part of treated roots was stained (D).
H2S scavengers aggravated the toxic effect of 1,4-DCB on cells
H2S reduced the impact of 1,4-DCB stress on plants, mainly by lowering the ROS content, which helped to maintain the integrity of the cell membrane (ZHANG et al., 2015ZHANG, L. et al. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. Pekine-nsis. Oxidative Medicine and Cellular Longevity , v.2015, p.1-11, 2015. Available from: <http://www.hindawi.com/journals/omcl/2015/804603>. Accessed: Jun. 12, 2015. doi: 10.1155/2015/804603.
http://www.hindawi.com/journals/omcl/201...
). Under 1,4-DCB stress, pretreatment with HT significantly increased the MDA content (Figure 2C), hydrogen peroxide (H2O2) content and the rate of superoxide radical (O2.-) generation (Figure 2E). This implied that a large number of lipid peroxides were produced, which could eventually damage the cell membrane. In contrast, these effects were reversed by NaHS, indicating that such damage had been alleviated. Similar phenomena have been previously observed; for example, H2S maintained low concentrations of MDA and H2O2, thereby alleviating aluminum stress (ZHANG et al., 2010b). This effect was confirmed here by using tissue staining. when there was no stress treatment, the root staining was relatively lighter. under 1,4-DCB stress, pretreatment with HT enhanced the chlorobenzene-related staining pattern, as indicated by a stronger deposition of blue-colored precipitates in root tips (Figure 2D). This result agreed with the effects seen on soybean seedling phenotypes. Hence, the presence of a H2S scavenger, HT, aggravated the toxic effects of 1,4-DCB on cells.
H2S restored redox equilibrium
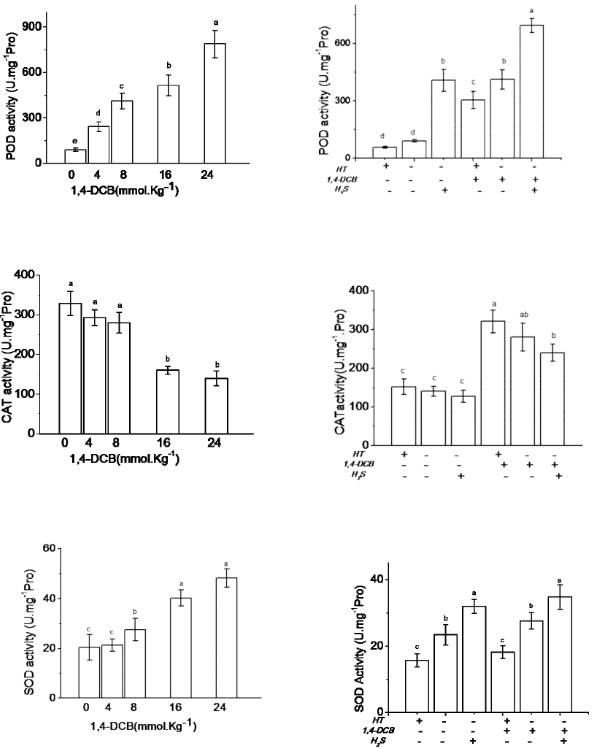
To reduce free radical damage to the cell membrane, plants may activate an antioxidant defense system. In this study, soybean seedlings were subjected to 1,4-DCB stress and the activities of different antioxidant enzymes were measured. POD was the most sensitive to the treatment: even at low 1,4-DCB concentrations (4mmol kg-1), POD activity increased rapidly. With increasing 1,4-DCB stress, POD activity gradually increased. At the highest concentration of 1,4-DCB, POD activity was more than 8 times greater than in the control. As a key regulator of H2O2, CAT exhibited the opposite trend; as the 1,4-DCB concentration increased, CAT activity decreased. At high 1,4-DCB concentrations, this decline was significant. The activity of SOD, a scavenging superoxide anion, was more stable (Figure 3A). In order to examine the relationship between H2S and antioxidase levels, the H2S inhibitor HT and donor NaHS were applied experimentally. Activities of three kinds of antioxidases were then observed and, as expected, HT acted to further reduce POD and SOD activity. In contrast, NaHS increased the activity of POD and SOD (Figure 3B). The activity levels of CAT, POD and SOD were thus adjusted in soybean seedlings so as to limit the amount of active oxygen species, in order to mitigate the damage from 1,4-DCB. H2S also helped alleviate 1,4-DCB-induced injury by increasing the activity of antioxidant enzymes, maintaining a balance of active oxygen. This finding was consistent with previous research in peas that showed H2S increased soluble protein content, as well as the activity of APX, POD and SOD, but decreased CAT activity in root tissues (LI et al., 2010LI, D.B. et al. Effects of exogenous hydrogen sulfide(H2S) on the root tip and root border cells of PiSum sativum. Chinese Bulletin of Botany , v.45, p.354-362, 2010. ). it was also similar to results, which showed that higher CAT and SOD activity relieved heat and salt stress (LI et al., 2014; LAI et al., 2014LAI, D. et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, v.225, p.117-129, 2014. Available from: <http://www.sciencedirect.com/science/article/pii/S016894521400140X>. Accessed: Jun. 12, 2015. doi: 10.1016/j.plantsci.2014.06.006.
http://www.sciencedirect.com/science/art...
). Although the role of antioxidant enzymes in the stress response versus the reaction to H2S was not the same, antioxidant enzymes acted in the cases to maintain stability in levels of oxygen free radicals, keeping their concentration within a low enough range to reduce cellular damage. Results of this study were also consistent with the finding that, through increasing the activity of antioxidant enzymes, plants resisted the effects of aluminum, boron, and tissue hypoxia stresses (WANG et al., 2010WANG, B.L. et al. Boron toxicity is alleviated by hydrogen sulfide in cucum ber (Cucumb sativus L.) seedlings. Planta, v.231, p.1301-1309, 2010. Available from: <http://link.springer.com/article/10.1007%2Fs00425-010-1134-9>. Accessed: Jun. 12, 2015. doi: 10.1007/s00425-010-1134-9.
http://link.springer.com/article/10.1007...
., CHEN et al., 2013CHEN, J. et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant and Soil, v.362, p.301-308, 2013. Available from: <http://link.springer.com/article/10.1007%2Fs11104-012-1275-7>. Accessed: Feb. 01, 2014. doi: 10.1007/s11104-012-1275-7.
http://link.springer.com/article/10.1007...
; CHENG et al., 2013CHENG, W. et al. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiology and Biochemistry, v.70, p.278-286, 2013. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/23800663>. Accessed: Apr. 11, 2014. doi: 10.1016/j.plaphy.2013.05.042.
http://www.ncbi.nlm.nih.gov/pubmed/23800...
). However, owing to the ubiquity of H2S and its versatile properties, the enhancement of antioxidant capacity to decrease ROS accumulation is just the first step in the H2S-mediated stress response, and the full range of potential molecular mechanisms remain unknown, especially how H2S may interact with other signaling molecules, and so, further research is needed.
Effects of 1, 4-DCB (A), HT and NaHS (B) treatments on the activities of antioxidant enzymes in soybean seedlings.
CONCLUSION:
This study revealed that the presence of endogenous H2S, associated with the total activity of LCD/DCD, effectively improved soybean seedlings' tolerance of 1,4-DCB. Study results further illustrated the role of endogenous H2S homeostasis in the stress response, showing that antioxidant activities were changed to block the elevation of ROS.
ACKNOWLEDGMENTS
This research was supported by Research Fund for Young Backbone Teacher of Higher Education of Henan (2014GGJS-184) and the Key Scientific Research Project of Higher Education of Henan ( 16B210005).
References:
- ALI, B. et al. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastr-uctural changes in oilseed rape. Ecotoxicology and Environmental Safety, v.102, p.25-33, 2014. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/24580818>. Accessed: Feb. 01, 2015. doi:10.1016/j.ecoenv.2014.01.013.
» https://doi.org/10.1016/j.ecoenv.2014.01.013» http://www.ncbi.nlm.nih.gov/pubmed/24580818 - BAKER, C.J.; MOCK, N.M. An improved method for monitoring cell death incell suspension and leaf disc assays using Evans blue. Plant Cell Tissue and Organ Culture, v.39, p.7-12, 1994. Available from: <http://rd.springer.com/article/10.1007/BF00037585>. Accessed: Jan. 08, 2014. doi: 10.1007/BF00037585.
» https://doi.org/10.1007/BF00037585.» http://rd.springer.com/article/10.1007/BF00037585 - CHEN, J. et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant and Soil, v.362, p.301-308, 2013. Available from: <http://link.springer.com/article/10.1007%2Fs11104-012-1275-7>. Accessed: Feb. 01, 2014. doi: 10.1007/s11104-012-1275-7.
» https://doi.org/10.1007/s11104-012-1275-7.» http://link.springer.com/article/10.1007%2Fs11104-012-1275-7 - CHENG, W. et al. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiology and Biochemistry, v.70, p.278-286, 2013. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/23800663>. Accessed: Apr. 11, 2014. doi: 10.1016/j.plaphy.2013.05.042.
» https://doi.org/10.1016/j.plaphy.2013.05.042.» http://www.ncbi.nlm.nih.gov/pubmed/23800663 - DING, X.W. et al. Effects of 1,2,4-trichlorobenzene on growth and physiological characteristics of rice at maximum tillering stage. Acta Agronomica Sinica, v.40, p.487-496, 2014.
- HOU, Z.H. et al. H2S may function downstream of H2O2 in jasmonic acid-induced stomatal closure in Vicia faba. Chinese Bulletin of Botany, v.46, p.396-406, 2011.
- JIN, Z. et al. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, v.414, p.481-486, 2011. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/21986537>. Accessed: Nov. 21, 2015. doi: 10.1016/j.bbrc.2011.09.090.
» https://doi.org/10.1016/j.bbrc.2011.09.090.» http://www.ncbi.nlm.nih.gov/pubmed/21986537 - JIN, Z.P. et al. Physiological implications of hydrogen sulfide in plants: pleasant exploration behind its unpleasant odour. Oxidative Medicine and Cellular Longevity, v.2015, p.1-6, 2015. Available from: <http://dx.doi.org/10.1155/2015/397502>. Accessed: Nov. 10, 2015. doi: 10.1111/j.1742-4658.2005.04567.x.
» https://doi.org/10.1111/j.1742-4658.2005.04567.x.» http://dx.doi.org/10.1155/2015/397502 - LANG, J.; ZHU, Y. Comparison of two methods for determination of catalase activity in rice. Journal of the Chinese Cereals and Oils Association, v.29, p.89-99, 2014.
- LAI, D. et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, v.225, p.117-129, 2014. Available from: <http://www.sciencedirect.com/science/article/pii/S016894521400140X>. Accessed: Jun. 12, 2015. doi: 10.1016/j.plantsci.2014.06.006.
» https://doi.org/10.1016/j.plantsci.2014.06.006.» http://www.sciencedirect.com/science/article/pii/S016894521400140X - LI, D.B. et al. Effects of exogenous hydrogen sulfide(H2S) on the root tip and root border cells of PiSum sativum. Chinese Bulletin of Botany , v.45, p.354-362, 2010.
- LI, Z.G. et al. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia, v.69, p.1001-1009, 2014. Available from: <http://link.springer.com/article/10.2478/s11756-014-0396-2>. Accessed: Jun. 12, 2015. doi:10.2478/s11756-014-0396-2.
» https://doi.org/10.2478/s11756-014-0396-2» http://link.springer.com/article/10.2478/s11756-014-0396-2 - LI, Z.G. et al. Involvement of sulfhydryl compounds and antioxidant enzymes in H2S-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension-cultured cells. In Vitro Cellular and Developmental Biology-Plant, v.51, p.428-437, 2015. Available from: <http://link.springer.com/article/10.1007/s11627-015-9705-x>. Accessed: Jan. 25, 2016. doi:10.1007/s11627-015-9705-x.
» https://doi.org/10.1007/s11627-015-9705-x» http://link.springer.com/article/10.1007/s11627-015-9705-x - MIGUEL, A.S. et al. Biological responses of maize (Zea mays) plants exposed to chlorobenzenes case study of monochloro-,1,4-dichloro and 1,2,4-trichloro-benzenes. Ecotoxicology, v.21, p.315-324, 2012. Available from: <http://link.springer.com/article/10.1007%2Fs10646-011-0792-0>. Accessed: Jan. 12, 2015. doi: 10.1007/s10646-011-0792-0.
» https://doi.org/10.1007/s10646-011-0792-0.» http://link.springer.com/article/10.1007%2Fs10646-011-0792-0 - WANG, B.L. et al. Boron toxicity is alleviated by hydrogen sulfide in cucum ber (Cucumb sativus L.) seedlings. Planta, v.231, p.1301-1309, 2010. Available from: <http://link.springer.com/article/10.1007%2Fs00425-010-1134-9>. Accessed: Jun. 12, 2015. doi: 10.1007/s00425-010-1134-9.
» https://doi.org/10.1007/s00425-010-1134-9.» http://link.springer.com/article/10.1007%2Fs00425-010-1134-9 - ZHANG, H. et al. Hydrogen Sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. Journal of Integrative Plant Biology, v.50, p.1518-1529, 2008. Available from: <http://onlinelibrary.wiley.com/doi/10.1111/j.1744-7909.2008.00769.x/>. Accessed: Jun. 12, 2015. doi: 10.1111/j.1744-7909.2008.00769.x.
» https://doi.org/10.1111/j.1744-7909.2008.00769.x.» http://onlinelibrary.wiley.com/doi/10.1111/j.1744-7909.2008.00769.x/ - ZHANG, H. et al. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology, v.60, p.251-257, 2011. Available from: <http://www.sciencedirect.com/science/article/pii/S092552141100007X>. Accessed: Jan. 08, 2014. doi: 10.1016/j.postharvbio.2011.01.006.
» https://doi.org/10.1016/j.postharvbio.2011.01.006.» http://www.sciencedirect.com/science/article/pii/S092552141100007X - ZHANG, H. et al. Hydrogen sulfide alleviated chromium toxicity in wheat. Biologia Planta rum, v. 54, p.743-747, 2010a. Available from: <http://link.springer.com/article/10.1007%2Fs10535-010-0133-9>. Accessed: Jan. 08, 2014. doi:10.1007/s10535-010-0133-9.
» https://doi.org/10.1007/s10535-010-0133-9» http://link.springer.com/article/10.1007%2Fs10535-010-0133-9 - ZHANG, H. et al. Hydrogen sulfide alleviates aluminum toxicity in germinatingwheat seedlings. Journal of Integrative Plant Biology , v.52, p.556-567, 2010b. Available from: <http://www.ncbi.nlm.nih.gov/pubmed/20590986>. Accessed: Jan. 08, 2014. doi: 10.1111/j.1744-7909.2010.00946.x.
» https://doi.org/10.1111/j.1744-7909.2010.00946.x.» http://www.ncbi.nlm.nih.gov/pubmed/20590986 - ZHANG, L. et al. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. Pekine-nsis. Oxidative Medicine and Cellular Longevity , v.2015, p.1-11, 2015. Available from: <http://www.hindawi.com/journals/omcl/2015/804603>. Accessed: Jun. 12, 2015. doi: 10.1155/2015/804603.
» https://doi.org/10.1155/2015/804603.» http://www.hindawi.com/journals/omcl/2015/804603
-
1
CR-2015-1503.R1
Publication Dates
-
Publication in this collection
Oct 2016
History
-
Received
05 Nov 2015 -
Accepted
28 Mar 2016 -
Accepted
12 June 2016