ABSTRACT:
The objective of this research was to evaluate the proline synthesis and physiological response of cassava genotypes which were micro propagated and induced to salinity stress in vitro. Micro cuttings of approximately 1.0cm long with a single bud of genotypes TBRS Tapioqueira, BRS Verdinha and Lagoão which were previously established in vitro were inoculated in a MS medium containing different concentrations of NaCl (0; 25; 50; 75; 100mM) and were analyzed after 90th day for: number of roots, number of leaves and shoot dry mass. The proline content of BRS Tapioqueira and Lagoão was assessed at 30th, 60th and 90th day. There was no analysis of proline of the variety Verdinha because of the contamination of the explants. The experimental design was completely randomized in double factorial scheme (3 genotypes x 5 salt treatments), with seven repetitions for growth variables. For comparing proline content, completely randomized design was used in a plot subdivided in time, with genotype and NaCl factors in plot and time in subplot, with two repetitions. For r time and genotypes Tukey test (P<0,05) was used and for NaCl levels regression test (P<0,05). Salinity affected the growth of all varieties; although, BRS Tapioqueira and BRS Verdinha were less affected by induced salt stress. There was an increase in the accumulation of proline from the salt increment, this synthesis of proline being a biochemical indicator of salt stress in cassava plants cultivated in vitro.
Key words:
Manihot esculenta; abiotic stress; osmoprotectors
RESUMO:
O objetivo deste trabalho foi avaliar a síntese de prolina e respostas fisiológicas de variedades de mandioca micropropagadas e induzidas ao estresse salino in vitro. Microestacas das variedades BRS Tapioqueira, BRS Verdinha e Lagoão previamente estabelecidas in vitro foram inoculadas em meio MS com diferentes concentrações de NaCl (0; 25; 50; 75; 100mM) e aos 90 dias foram analisados: número de raiz, número de folhas e massa seca de parte aérea. O teor de prolina das variedades BRS Tapioqueira e Lagoão foi analisado aos 30, 60 e 90 dias. Não houve análise de prolina da variedade Verdinha por causa da contaminação dos explantes. O delineamento experimental foi inteiramente casualizado em esquema fatorial 3 genótipos x 5 tratamentos salinos, com sete repetições para as variáveis de crescimento. Para o conteúdo de prolina foi considerado inteiramente casualizado subdividido no tempo, com genótipos e NaCl na parcela e o tempo na subparcela e duas repetições. Para os fatores variedade e tempo, foi utilizado o teste de Tukey (P<0,05) e para tratamentos salinos, teste de Regressão (P<0,05). A salinidade afetou o crescimento de todas as variedades, porém BRS Tapioqueira e BRS Verdinha mostraram-se menos afetadas pelo estresse salino induzido. Houve aumento no acúmulo de prolina a partir do incremento de sal, sendo então este, um indicador bioquímico de estresse salino em plantas de mandioca cultivadas in vitro.
Palavras-chave:
Manihot esculenta; estresse abiótico; osmoprotetores
Introduction:
Cassava (Manihot esculenta Crantz), belongs to the class of dicotyledons, of the Euphorbiales order, Euphorbiaceae family, Manihot genus. It is a plant that has most of its production destined for human consumption, through its roots, because it has high starch production under conditions considered unsuitable for other species (FIALHO & VIEIRA, 2011FIALHO, J.de F; VIEIRA, E.A. Mandioca no Cerrado: orientações técnicas. Planaltina, DF: Embrapa Cerrados. 208p, 2011.).
The Northeast region has characteristically saline soils (NOBREGA et al., 2012NOBREGA, J.A. et al. Crescimento do pinhão-manso sob irrigação com água salina e adubação orgânica em condições de campo. Revista Verde, v.7, n.1, p.60-61, 2012. Availablefrom:<Availablefrom:http://www.gvaa.com.br/revista/index.php/RVADS/article/view/1169/1124
>. Accessed: Jun. 15, 2016.
http://www.gvaa.com.br/revista/index.php...
), causing damages to the productions, such as cassava culture. Among the mechanisms of tolerance to salt stress, plants stand out for their osmoregulation, which includes an increase in the concentration of solutes in the cells. These play a key role in osmotic balance, protection of enzymes in the presence of high concentrations of electrolytes in the cytoplasm (GREENWAY & MUNNS, 1980GREENWAY, H.; MUNS, R. Mechanism of salt tolerance in nonhalophytes. Annual Review of Plant Physiology, v.31, p.149-190, 1980. Available from: :<Available from: :http://dx.doi.org/10.1146/annurev.pp.31.060180.001053
>. Accessed: Dec. 08, 2015. doi: 10.1146/annurev.pp.31.060180.001053.
http://dx.doi.org/10.1146/annurev.pp.31....
).
Among the organic solutes that accumulate in the cytoplasm in response to stress, proline (PONTE et al., 2011PONTE, L.F.A. et al. Variabilidade de indicadores fisiológicos de resistência à salinidade entre genótipos de cajueiro-anão e gigante. Pesquisa Agropecuária Brasileira, v.46, n.1, p.1-8, 2011. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2011000100001
>. Accessed: June 15, 2016. doi: 10.1590/S0100-204X2011000100001.
http://dx.doi.org/10.1590/S0100-204X2011...
; KANAWAPEE et al., 2012KANAWAPEE, N. et al. Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil, v.358, p.235-249, 2012. Available from: <Available from: http://dx.doi.org/10.1007/s11104-012-1179-6
>. Accessed: May, 08, 2016. doi: 10.1007/s11104-012-1179-6.
http://dx.doi.org/10.1007/s11104-012-117...
) stands out because it is a solute with high sensitivity of response to environmental changes (ASHRAF et al., 2011ASHRAF, M. et al. Drought tolerance: roles of organic osmolytes, growth regulators, and mineral nutrients. Advances in Agronomy, v.111, p.249-296, 2011. Available from: <Available from: http://dx.doi.org/10.1016/B978-0-12-387689-8.00002-3
>. Accessed: Nov. 16, 2016. doi: 10.1016/B978-0-12-387689-8.00002-3.
http://dx.doi.org/10.1016/B978-0-12-3876...
) and may be considered an osmolyte which serves as biochemical and physiological indicator of the effects of saline stress in plants cultivated under these adverse conditions (MONTEIRO et al., 2014MONTEIRO, J.G. et al. Crescimento e conteúdo de prolina em plântulas de guandu submetidas a estresse osmótico e à putrescina exógena. Pesquisa Agropecuária Brasileira, v.49, n.1, p.18-25, 2014. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2014000100003
>. Accessed: Oct. 18, 2016. doi: 10.1590/S0100-204X2014000100003.
http://dx.doi.org/10.1590/S0100-204X2014...
).
Under natural conditions, there may be difficulty in evaluating the tolerance of cultivated species to some types of stress. The in vitro technique emerged as an alternative for the development of plants resistant to this type of limitation (LIMA et al., 1998LIMA, G.P. et al. Peroxidase activity and carboydrate content alteractions in cassava cultivated in vitro under saline stress. Scientia Agricola, v.55, n.3, p.413-417, 1998. Available from: <Available from: http://dx.doi.org/10.1590/S0103-90161998000300009
>. Accessed: Dec. 22, 2016. doi: 10.1590/S0103-90161998000300009.
http://dx.doi.org/10.1590/S0103-90161998...
). It is a method of vegetative propagation of the plant, carried out in vitro under aseptic conditions (LEMA-RUMIŃSKA & KULUS, 2014LEMA-RUMIŃSKA, J.; KULUS, D. Micropropagation of cacti - a Review. Haseltonia, v.19, p.46-63, 2014. Available from: <Available from: http://dx.doi.org/10.2985/026.019.0107
>. Accessed: Sept. 16, 2016. doi: 10.2985/026.019.0107.
http://dx.doi.org/10.2985/026.019.0107...
).
The influence of in vitro salt stress on the growth and development of plants has been subject of studies in other crops such as potatoes (MARTINEZ et al., 1996MARTINEZ, C.A. et al. In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance. Plant Science, v.116, p.117-184, 1996. Available from: <Available from: http://dx.doi.org.ez20.periodicos.capes.gov.br/10.1016/0168-9452(96)04374-9
>. Accessed: May, 20, 2016. doi: 10.1016/0168-9452(96)04374-9.
http://dx.doi.org.ez20.periodicos.capes....
), rice (BENITEZ et al., 2010BENITEZ, L.C. et al. Salinity tolerance evaluated in genotypes of in vitro cultivated rice. Revista Ceres, v.57, n.3, p.330-337, 2010. Available from: <Available from: http://dx.doi.org/10.1590/S0034-737X2010000300007
>. Accessed: Nov. 20, 2016. doi: 10.1590/S0034-737X2010000300007.
http://dx.doi.org/10.1590/S0034-737X2010...
) and sugarcane (GANDONOU et al., 2015GANDONOU, C.H. et al. Effects of NaCl on growth and ion and proline accumulation in sugarcane (Saccharum sp.) callus culture. Belgian Journal of Botany, v.138, n.2, p.173-180, 2015. Available from: <Available from: http://www.jstor.org/stable/20794582
>. Accessed: Apr.15, 2016.
http://www.jstor.org/stable/20794582...
). The objective of this research was to evaluate the proline synthesis and physiological response of cassava genotypes which were induced to in vitro salt stress.
Materials and methods:
As plant material, microcuttings of approximately 1.0cm long with a single bud were used, extracted from pre-established plants multiplied in vitro of the BRS Verdinha (tolerant to dry and with higher production of starch), BRS Tapioqueira (high yield) and Lagoão (easy-to-adapt variety) genotypes. The initial apical meristems were acquired from Instituto Biofábrica Cacau (IBC).
In a laminar flow cabinet, the micro-cuttings were inoculated in glass jars (9.5 x 5.5cm) containing 30mL of MS medium (MURASHIGE & SKOOG, 1962MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, v.15, n.3, p.437-497, 1962. Available from: <Available from: http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
>. Accessed: Jul. 20, 2016. doi: 10.1111/j.1399-3054.1962.tb08052.x.
http://dx.doi.org/10.1111/j.1399-3054.19...
), 30g L-1 of sucrose and different concentrations of NaCl (0; 25; 50; 75; 100mM), with 4g L-1 of Phytagel® and pH adjusted to 5.7±0.1. After inoculation, the containers were sealed with polyethylene plastic film and the cultures were transferred to a growth room at 25 °C±2 °C, average relative humidity around 70%, photo period of 12 hours and luminous intensity of 60µmol m-2 s-1. Two experiments were mounted under the same conditions: one to analyze growth, another to analyze proline.
At 90th day, the number of roots, number of leaves and shoot dry mass of each experimental unit were evaluated. Proline content was assessed only for BRS Tapioqueira and BRS Lagoão cultivars due to high bacterial contamination of BRS Verdinha on the proliferation phase. Free proline content was determined according to BATES et al. (1973BATES, L.S. et al. Rapid determination of free proline for water-stress studies. Plant and Soil, v.39, p.205-207, 1973. Available from: <Available from: https://link.springer.com/article/10.1007/BF00018060
>. Accessed: Jan., 10 2015. doi: 10.1007/BF00018060.
https://link.springer.com/article/10.100...
) modified by replacing the centrifuge filtration of the extracts (all extracts were taken to the centrifuge at 3,220 xg for 20 minutes at 2 °C). Leaf samples (1.00 g) were homogenized in aqueous sulfosalicylic acid (3% w/v;12 mL). The filtered homogenate (2 mL) was reacted with equal volume each of acid ninhydrin and acetic acid at 100°C for 1 h and the reaction was terminated in an ice bath. The reaction mixture was extracted with 4 mL toluene and mixed vigorously with a stirrer for 10-15 s. The chromophore containing toluene was aspirated from the aqueous phase and warmed to room temperature. The absorbance was recorded at 520 nm using toluene as a blank. Proline concentration (µg g-1 FW) was determined from a standard curve prepared with L-proline. Extraction of proline was made at the 30th, 60th and 90th day after inoculation of the explants into the medium with salt treatments.
For growth variables, was considered the experimental design was completely randomized in double factorial scheme (3 genotypes x 5 salt treatments), with seven repetitions, being the experimental unit composed of four plants each. For proline content, completely randomized design was used in a plot subdivided in time, with the factors genotypes and NaCl in the plot and time in the subplot, respectively, using all saline treatments with seven replications by four replicate samples. All samples were made in triplicates. For the NaCl levels regression test was used and for time and genotypes Tukey test with 5% of significance was used. For all analyses the statistical software SISVAR was used (Version 5.6, Build 86).
Results and Discussion:
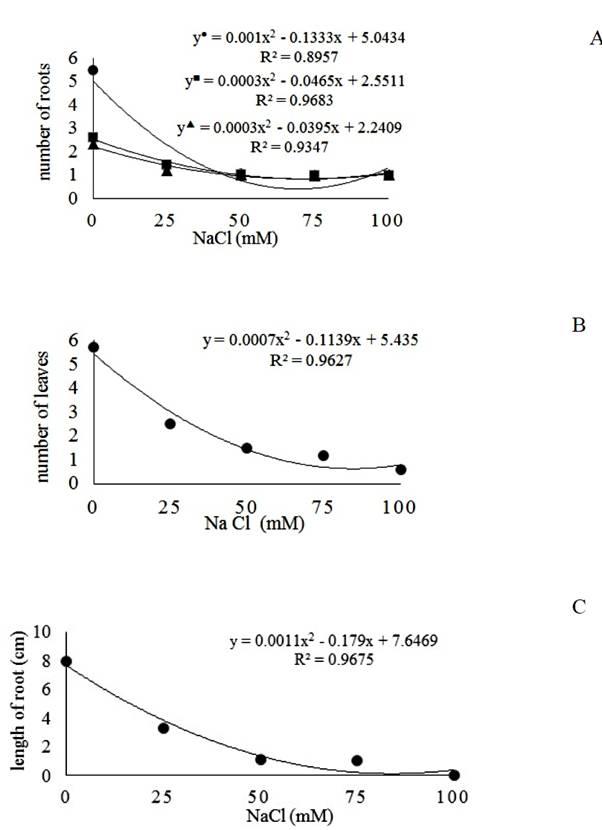
The presence of NaCl significantly affected the in vitro growth pattern of explants of cassava. There was significant effect of the interaction between the saline treatments and genotypes for the number of roots. The effect of the factors genotype and saline treatment alone was significant for the number of leaves. For the length of the root, there was significant effect of the saline treatment only.
There was a quadratic response with significant reduction in the production of roots in the three genotypes with the increase of NaCl in the medium (Figure 1A). The lowest productions were observed at 66.65 mM (Lagoão); 77.5mM (BRS Tapioqueira) and 83.33 mM (BRS Verdinha), with estimated averages of 0.60, 0.75 and 1.03 roots per plant respectively. In the absence of NaCl, the highest production occurred in cultivar BRS Tapioqueira and in the more severe treatment (100mM), there were no differences between genotypes (Table 1).
Effect of in vitro salt stress in ●Lagoão; ■ BRS Tapioqueira; ▲BRS Verdinha, (A) number of roots, (B) number of leaves* and (C) length of roots* (cm) of the cassava genotypes cultivated under different concentrations of NaCl (0; 25; 50; 75 and 100mM). *Only salt concentrations were significant.
Reduction in the production of roots can be explained by the inhibition of cell division and cell expansion in the growing tissue caused by salinity (FORNER - GINER et al., 2011FORNER-GINER, M.A. et al. Nutritional responses of citrus rootstocks to salinity: performance of new hybrids Forner-Alcaide 5 and Forner-Alcaide 13. Journal of Plant Nutrition, v.34, p.1437-1452, 2011. Available from: <Available from: http://dx.doi.org/10.1080/01904167.2011.585202
>. Accessed: Mar. 10, 2016. doi: 10.1080/01904167.2011.585202.
http://dx.doi.org/10.1080/01904167.2011....
), due to it being the first organ affected by stress (BHATNAGAR-MATHUR et al., 2008BHATNAGAR-MATHUR P. et al. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Reports, v.27, p.411-424, 2008. Available from: <Available from: http://dx.doi.org/10.1007 / s00299-007-0474-9
>. Accessed: Jul. 26, 2017. doi: 10.1007 / s00299-007-0474-9.
http://dx.doi.org/10.1007 / s00299-007-0...
). This result was also obtained by MARTINEZ et al. (1996MARTINEZ, C.A. et al. In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance. Plant Science, v.116, p.117-184, 1996. Available from: <Available from: http://dx.doi.org.ez20.periodicos.capes.gov.br/10.1016/0168-9452(96)04374-9
>. Accessed: May, 20, 2016. doi: 10.1016/0168-9452(96)04374-9.
http://dx.doi.org.ez20.periodicos.capes....
) with potato species and rice genotypes.
The number of leaves had a quadratic behavior, being the lowest average at 81.35 mM of NaCl, reaching 0.80 leaves per plant (Figure 1B). This result evidences the effect of salt toxicity in plants, corroborating with KHOUSHBAKHT et al. (2010KHOUSHBAKHT, D. et al. Effect of salinity on growth parameters of 9 citrus rootstocks. Iran Journal of Agricultural Science, v.40, p.71-81, 2010. Available from: <Available from: http://en.journals.sid.ir/ViewPaper.aspx?ID=179101
>. Accessed: May 10, 2016.
http://en.journals.sid.ir/ViewPaper.aspx...
), who evidenced the decline in the number of leaves as a result of the inhibition of growth by salinity, but also by toxicity, which causes losses in production, falling of leaves or damaged leaves. The genotype with the highest number of leaves was BRS Verdinha, with an average of 3.65 (Table 1).
In what concerns the relative performance regarding the length of the roots, the lowest value was achieved in the 81.36mM saline concentration (Figure 1C), showing that the excess of salts in the root zone promotes a reduction in the length and number of roots, causing adverse effects on the growth and development of the plant (RHOADES et al., 2000RHOADES, J.D. et al. Uso de águas salinas para a produção agrícola. In: GHEYI, H.R.; et al. (Eds.) Estudos FAO de irrigação e drenagem. Campina Grande, UFPB. 2000. p.40-48.). Studies by BENITEZ et al. (2010BENITEZ, L.C. et al. Salinity tolerance evaluated in genotypes of in vitro cultivated rice. Revista Ceres, v.57, n.3, p.330-337, 2010. Available from: <Available from: http://dx.doi.org/10.1590/S0034-737X2010000300007
>. Accessed: Nov. 20, 2016. doi: 10.1590/S0034-737X2010000300007.
http://dx.doi.org/10.1590/S0034-737X2010...
) in rice genotypes, showed that BRS Pelota maintained its normal production of the root system, even with addition of salt to the medium in the 4 and 8g L-1 concentrations. The potato varieties Spunta and Cardinal showed tolerance to 40 and 80mM of NaCl; however, variety Bartina had 40% of reduction in the length of its roots in the salt concentration of 40mM (KHENIFI et al., 2011KHENIFI, M.L. et al. Effects of salt stress on micropropagation of potato (Solanum tuberosum L.). African Journal of Biotechnology, v.10, n.40, p.7840-7845, 2011. Available from: <Available from: http://dx.doi.org/10.5897/AJB10.982
>. Accessed: Sep. 12, 2016. doi: 10.5897/AJB10.982.
http://dx.doi.org/10.5897/AJB10.982...
). These results evidenced that the presence of salts influences the root elongation (SHARMA et al., 2013SHARMA, L.K. et al. Evaluation of rough lemon (Citrus jambhiri Lush.) as rootstock for salinity tolerance at seedling stage under in vitro conditions. African Journal of Biotechnology, v.12, n.44, p.6267-6275, 2013. Available from: <Available from: http://dx.doi.org/10.5897/AJB2013.12994
>. Accessed: Mar. 30, 2016. doi: 10.5897/AJB2013.12994.
http://dx.doi.org/10.5897/AJB2013.12994...
).
Considering the shoot dry mass, there was decrease in all genotypes in response of the salt increase (Table 1). This result was expected due to the reduced of others variables as number of leaves and number of roots in the presence of high levels of salt. The Lagoão and BRS Verdinha genotypes presented lower shoot dry mass (0.01 and 0.04g) than BRS Tapioqueira (0.08g).
There was a significant (P<0.05) effect of the triple interaction (data not shown) between the factors time, genotype and saline treatment on the production of proline. In general, there was an increase in the production of proline with the increase in NaCl (Table 2). This result corroborated with GANDONOU et al. (2015GANDONOU, C.H. et al. Effects of NaCl on growth and ion and proline accumulation in sugarcane (Saccharum sp.) callus culture. Belgian Journal of Botany, v.138, n.2, p.173-180, 2015. Available from: <Available from: http://www.jstor.org/stable/20794582
>. Accessed: Apr.15, 2016.
http://www.jstor.org/stable/20794582...
) who, when studying varieties of sugarcane under conditions of saline stress in vitro, observed that both the sensitive variety CP65-357 and resistant variety NCo310 behave similarly, accumulating greater amounts of proline in the more severe saline treatments (102 mM). In gandu, under stress, the osmoprotector was accumulated in both roots and shoot, establishing a relationship between disturbance and biochemical response (MONTEIRO et al., 2014MONTEIRO, J.G. et al. Crescimento e conteúdo de prolina em plântulas de guandu submetidas a estresse osmótico e à putrescina exógena. Pesquisa Agropecuária Brasileira, v.49, n.1, p.18-25, 2014. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2014000100003
>. Accessed: Oct. 18, 2016. doi: 10.1590/S0100-204X2014000100003.
http://dx.doi.org/10.1590/S0100-204X2014...
).
The interaction between genotypes, saline concentrations and exposure time of the explant to the saline medium were statistically significant. In the control treatment, the genotypes Lagoão and BRS Tapioqueira had the highest average proline accumulation at 30 days (18.66 and 10.43 μmol.g-1) (Table 2). In a short period of exposure to salinity (30 days), the genotype Lagoão presented greater accumulation of proline. However, with the more severe and longer treatment (100mM at 90 days), the BRS Tapioqueira genotype showed a significantly higher proline increase than Lagoão (21.59 μmol g -1). Simple linear regression models were adjusted for the studied genotypes. At 30, 60 and 90 days, the accumulation of proline by the genotypes Lagoão and BRS Tapioqueira increased as there was increase of NaCl in the culture medium (Figure 2).
Effect of in vitro salt stress on the proline accumulation in cassava genotypes cultivated under different concentrations of NaCl (0; 25; 50; 75 and 100mM). (A) Lagoão; (B) BRS Tapioqueira. *Only salt concentrations were significant.
Proline synthesis in cassava plants is a consequence by a salt stress that induced changes in osmotic pressure with increased water absorption. This osmoprotector is produced as a first response mechanism of plants under different types of stress and also is related to the improvement of the salinity tolerance (ASHRAF & FOOLAD, 2007ASHRAF, M.; FOOLAD, M. R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, v. 59, p.206-216, 2007. Available from: <Available from: http://dx.doi.org/10.1016/j.envexpbot.2005.12.006
>. Accessed: Nov. 16, 2016. doi: 10.1016/j.envexpbot.2005.12.006.
http://dx.doi.org/10.1016/j.envexpbot.20...
; HASANUZZAMAN et al., 2014HASANUZZAMAN, M et al. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Research International, v. 2014, p. 757219, 2014. Avaiable: < Avaiable: http://dx.doi.org/10.1155/2014/757219
>. Acessed: Oct. 13, 2018. doi: 0.1155/2014/757219.
http://dx.doi.org/10.1155/2014/757219...
). Furthermore, other proline features are related to antioxidant by reducing the effects of reactive oxygen species (ROS) (MOLINARI et al., 2007MOLINARI, H. B. C. et al. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.) osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiologia Plantarum, v. 130, p. 218-229, 2007. Avaiable from: < Avaiable from: https://doi.org/10.1111/j.1399-3054.2007.00909
>. Accessed: Oct. 13, 2018. doi: 10.1111/j.1399-3054.2007.00909.
https://doi.org/10.1111/j.1399-3054.2007...
), plasma membrane and integrity of macromolecules protector (SILVEIRA et al., 2003SILVEIRA, J. A. G. et al. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. Journal of Plant Physiology, v. 160, p. 115-123, 2003. Available: <Available: https://doi.org/10.1078/0176-1617-00890
>. Acessed: Oct. 13, 2018. doi: 10.1078/0176-1617-00890.
https://doi.org/10.1078/0176-1617-00890...
) besides of being a source of carbon and nitrogen (GUPTA & HUANG, 2014GUPTA, B.; HUANG, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, v. 1, p. 1-18, 2014. Available from: Available from: https://doi.org/10.1155/2014/701596
. Accessed: Oct. 13, 2018. doi: 10.1155/2014/701596.
https://doi.org/10.1155/2014/701596...
), implying an osmotic equilibrium and providing continuous development (GREENWAY & MUNNS, 1980GREENWAY, H.; MUNS, R. Mechanism of salt tolerance in nonhalophytes. Annual Review of Plant Physiology, v.31, p.149-190, 1980. Available from: :<Available from: :http://dx.doi.org/10.1146/annurev.pp.31.060180.001053
>. Accessed: Dec. 08, 2015. doi: 10.1146/annurev.pp.31.060180.001053.
http://dx.doi.org/10.1146/annurev.pp.31....
).
Variation in the proline accumulation observed between the genotypes and in the different treatments probably is due to the low proline synthesis or greater degradation of the proline under high salinity stress (KIBRIA et al., 2017KIBRIA et al. Antioxidant Defense Mechanisms of Salinity Tolerance in Rice Genotypes. Rice Science, v. 24, n. 3, p. 155-162, 2017. Available: <Available: https://doi.org/10.1016/j.rsci.2017.05.001
>. Acessed: Oct. 13, 2018. doi: 10.1016/j.rsci.2017.05.001.
https://doi.org/10.1016/j.rsci.2017.05.0...
), corroborating with KIBRIA et al., (2017) LEMA-RUMIŃSKA, J.; KULUS, D. Micropropagation of cacti - a Review. Haseltonia, v.19, p.46-63, 2014. Available from: <Available from: http://dx.doi.org/10.2985/026.019.0107
>. Accessed: Sept. 16, 2016. doi: 10.2985/026.019.0107.
http://dx.doi.org/10.2985/026.019.0107...
in their studies using sensitive and salinity tolerant rice genotypes. A positive correlation was reported between proline accumulation and stress tolerance in rice (KANAWAPE et al, 2012KANAWAPEE, N. et al. Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil, v.358, p.235-249, 2012. Available from: <Available from: http://dx.doi.org/10.1007/s11104-012-1179-6
>. Accessed: May, 08, 2016. doi: 10.1007/s11104-012-1179-6.
http://dx.doi.org/10.1007/s11104-012-117...
), alfalfa (FARISSI et al., 2013FARISSI, M. et al. Changes in water deficit saturation and photosynthetic pigments of Alfafa populations under salinity and assessment of proline role in salt tolerance. Agricultural Science Research Journals, v.3, n.1, p.29-35, 2013. Available from: <Available from: https://www.researchgate.net/publication/258978898
>. Accessed: Feb. 01, 2016.
https://www.researchgate.net/publication...
) and sugarcane (OLIVEIRA et al., 2018OLIVEIRA, L.A.R et al. Effects of in vitro Drought Stress on Growth, Proline Accumulation and Antioxidant Defense in Sugarcane. Journal of Agricultural Science; v. 10, n. 5, 2018. Available from: <Available from: https://doi.org/10.5539/jas.v10n5p135
>. Accessed: Oct. 13, 2018. doi: 10.5539/jas.v10n5p135.
https://doi.org/10.5539/jas.v10n5p135...
) and cashew nuts (PONTE et al., 2011PONTE, L.F.A. et al. Variabilidade de indicadores fisiológicos de resistência à salinidade entre genótipos de cajueiro-anão e gigante. Pesquisa Agropecuária Brasileira, v.46, n.1, p.1-8, 2011. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2011000100001
>. Accessed: June 15, 2016. doi: 10.1590/S0100-204X2011000100001.
http://dx.doi.org/10.1590/S0100-204X2011...
).
Conclusion:
The salinity affects the in vitro growth of genotypes Lagoão, BRS Tapioqueira and BRS Verdinha. The proline synthesis is intensified with the presence of NaCl in BRS Tapioqueira and Lagoão genotypes. Proline can be used as a biochemical indicator of response to in vitro salt stress for cassava cultures.
ACKNOWLEDGMENTS
To Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Universidade Federal de Sergipe (UFS) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing financial resources and scholarship, and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil - Finance code 001.
REFERENCES
- ASHRAF, M. et al. Drought tolerance: roles of organic osmolytes, growth regulators, and mineral nutrients. Advances in Agronomy, v.111, p.249-296, 2011. Available from: <Available from: http://dx.doi.org/10.1016/B978-0-12-387689-8.00002-3 >. Accessed: Nov. 16, 2016. doi: 10.1016/B978-0-12-387689-8.00002-3.
» https://doi.org/10.1016/B978-0-12-387689-8.00002-3.» http://dx.doi.org/10.1016/B978-0-12-387689-8.00002-3 - ASHRAF, M.; FOOLAD, M. R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, v. 59, p.206-216, 2007. Available from: <Available from: http://dx.doi.org/10.1016/j.envexpbot.2005.12.006 >. Accessed: Nov. 16, 2016. doi: 10.1016/j.envexpbot.2005.12.006.
» https://doi.org/10.1016/j.envexpbot.2005.12.006.» http://dx.doi.org/10.1016/j.envexpbot.2005.12.006 - BATES, L.S. et al. Rapid determination of free proline for water-stress studies. Plant and Soil, v.39, p.205-207, 1973. Available from: <Available from: https://link.springer.com/article/10.1007/BF00018060 >. Accessed: Jan., 10 2015. doi: 10.1007/BF00018060.
» https://doi.org/10.1007/BF00018060.» https://link.springer.com/article/10.1007/BF00018060 - BENITEZ, L.C. et al. Salinity tolerance evaluated in genotypes of in vitro cultivated rice. Revista Ceres, v.57, n.3, p.330-337, 2010. Available from: <Available from: http://dx.doi.org/10.1590/S0034-737X2010000300007 >. Accessed: Nov. 20, 2016. doi: 10.1590/S0034-737X2010000300007.
» https://doi.org/10.1590/S0034-737X2010000300007.» http://dx.doi.org/10.1590/S0034-737X2010000300007 - BHATNAGAR-MATHUR P. et al. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Reports, v.27, p.411-424, 2008. Available from: <Available from: http://dx.doi.org/10.1007 / s00299-007-0474-9 >. Accessed: Jul. 26, 2017. doi: 10.1007 / s00299-007-0474-9.
» https://doi.org/10.1007 / s00299-007-0474-9.» http://dx.doi.org/10.1007 / s00299-007-0474-9 - FARISSI, M. et al. Changes in water deficit saturation and photosynthetic pigments of Alfafa populations under salinity and assessment of proline role in salt tolerance. Agricultural Science Research Journals, v.3, n.1, p.29-35, 2013. Available from: <Available from: https://www.researchgate.net/publication/258978898 >. Accessed: Feb. 01, 2016.
» https://www.researchgate.net/publication/258978898 - FIALHO, J.de F; VIEIRA, E.A. Mandioca no Cerrado: orientações técnicas. Planaltina, DF: Embrapa Cerrados. 208p, 2011.
- FORNER-GINER, M.A. et al. Nutritional responses of citrus rootstocks to salinity: performance of new hybrids Forner-Alcaide 5 and Forner-Alcaide 13 Journal of Plant Nutrition, v.34, p.1437-1452, 2011. Available from: <Available from: http://dx.doi.org/10.1080/01904167.2011.585202 >. Accessed: Mar. 10, 2016. doi: 10.1080/01904167.2011.585202.
» https://doi.org/10.1080/01904167.2011.585202.» http://dx.doi.org/10.1080/01904167.2011.585202 - GANDONOU, C.H. et al. Effects of NaCl on growth and ion and proline accumulation in sugarcane (Saccharum sp.) callus culture. Belgian Journal of Botany, v.138, n.2, p.173-180, 2015. Available from: <Available from: http://www.jstor.org/stable/20794582 >. Accessed: Apr.15, 2016.
» http://www.jstor.org/stable/20794582 - GREENWAY, H.; MUNS, R. Mechanism of salt tolerance in nonhalophytes. Annual Review of Plant Physiology, v.31, p.149-190, 1980. Available from: :<Available from: :http://dx.doi.org/10.1146/annurev.pp.31.060180.001053 >. Accessed: Dec. 08, 2015. doi: 10.1146/annurev.pp.31.060180.001053.
» https://doi.org/10.1146/annurev.pp.31.060180.001053.» http://dx.doi.org/10.1146/annurev.pp.31.060180.001053 - GUPTA, B.; HUANG, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, v. 1, p. 1-18, 2014. Available from: Available from: https://doi.org/10.1155/2014/701596 Accessed: Oct. 13, 2018. doi: 10.1155/2014/701596.
» https://doi.org/10.1155/2014/701596.» https://doi.org/10.1155/2014/701596 - HASANUZZAMAN, M et al. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Research International, v. 2014, p. 757219, 2014. Avaiable: < Avaiable: http://dx.doi.org/10.1155/2014/757219 >. Acessed: Oct. 13, 2018. doi: 0.1155/2014/757219.
» https://doi.org/0.1155/2014/757219.» http://dx.doi.org/10.1155/2014/757219 - KANAWAPEE, N. et al. Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil, v.358, p.235-249, 2012. Available from: <Available from: http://dx.doi.org/10.1007/s11104-012-1179-6 >. Accessed: May, 08, 2016. doi: 10.1007/s11104-012-1179-6.
» https://doi.org/10.1007/s11104-012-1179-6.» http://dx.doi.org/10.1007/s11104-012-1179-6 - KHENIFI, M.L. et al. Effects of salt stress on micropropagation of potato (Solanum tuberosum L.). African Journal of Biotechnology, v.10, n.40, p.7840-7845, 2011. Available from: <Available from: http://dx.doi.org/10.5897/AJB10.982 >. Accessed: Sep. 12, 2016. doi: 10.5897/AJB10.982.
» https://doi.org/10.5897/AJB10.982.» http://dx.doi.org/10.5897/AJB10.982 - KHOUSHBAKHT, D. et al. Effect of salinity on growth parameters of 9 citrus rootstocks. Iran Journal of Agricultural Science, v.40, p.71-81, 2010. Available from: <Available from: http://en.journals.sid.ir/ViewPaper.aspx?ID=179101 >. Accessed: May 10, 2016.
» http://en.journals.sid.ir/ViewPaper.aspx?ID=179101 - KIBRIA et al. Antioxidant Defense Mechanisms of Salinity Tolerance in Rice Genotypes. Rice Science, v. 24, n. 3, p. 155-162, 2017. Available: <Available: https://doi.org/10.1016/j.rsci.2017.05.001 >. Acessed: Oct. 13, 2018. doi: 10.1016/j.rsci.2017.05.001.
» https://doi.org/10.1016/j.rsci.2017.05.001.» https://doi.org/10.1016/j.rsci.2017.05.001 - LEMA-RUMIŃSKA, J.; KULUS, D. Micropropagation of cacti - a Review. Haseltonia, v.19, p.46-63, 2014. Available from: <Available from: http://dx.doi.org/10.2985/026.019.0107 >. Accessed: Sept. 16, 2016. doi: 10.2985/026.019.0107.
» https://doi.org/10.2985/026.019.0107.» http://dx.doi.org/10.2985/026.019.0107 - LIMA, G.P. et al. Peroxidase activity and carboydrate content alteractions in cassava cultivated in vitro under saline stress. Scientia Agricola, v.55, n.3, p.413-417, 1998. Available from: <Available from: http://dx.doi.org/10.1590/S0103-90161998000300009 >. Accessed: Dec. 22, 2016. doi: 10.1590/S0103-90161998000300009.
» https://doi.org/10.1590/S0103-90161998000300009.» http://dx.doi.org/10.1590/S0103-90161998000300009 - MARTINEZ, C.A. et al. In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance. Plant Science, v.116, p.117-184, 1996. Available from: <Available from: http://dx.doi.org.ez20.periodicos.capes.gov.br/10.1016/0168-9452(96)04374-9 >. Accessed: May, 20, 2016. doi: 10.1016/0168-9452(96)04374-9.
» https://doi.org/10.1016/0168-9452(96)04374-9.» http://dx.doi.org.ez20.periodicos.capes.gov.br/10.1016/0168-9452(96)04374-9 - MOLINARI, H. B. C. et al. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.) osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiologia Plantarum, v. 130, p. 218-229, 2007. Avaiable from: < Avaiable from: https://doi.org/10.1111/j.1399-3054.2007.00909 >. Accessed: Oct. 13, 2018. doi: 10.1111/j.1399-3054.2007.00909.
» https://doi.org/10.1111/j.1399-3054.2007.00909.» https://doi.org/10.1111/j.1399-3054.2007.00909 - MONTEIRO, J.G. et al. Crescimento e conteúdo de prolina em plântulas de guandu submetidas a estresse osmótico e à putrescina exógena. Pesquisa Agropecuária Brasileira, v.49, n.1, p.18-25, 2014. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2014000100003 >. Accessed: Oct. 18, 2016. doi: 10.1590/S0100-204X2014000100003.
» https://doi.org/10.1590/S0100-204X2014000100003.» http://dx.doi.org/10.1590/S0100-204X2014000100003 - MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, v.15, n.3, p.437-497, 1962. Available from: <Available from: http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x >. Accessed: Jul. 20, 2016. doi: 10.1111/j.1399-3054.1962.tb08052.x.
» https://doi.org/10.1111/j.1399-3054.1962.tb08052.x.» http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x - NOBREGA, J.A. et al. Crescimento do pinhão-manso sob irrigação com água salina e adubação orgânica em condições de campo. Revista Verde, v.7, n.1, p.60-61, 2012. Availablefrom:<Availablefrom:http://www.gvaa.com.br/revista/index.php/RVADS/article/view/1169/1124 >. Accessed: Jun. 15, 2016.
» http://www.gvaa.com.br/revista/index.php/RVADS/article/view/1169/1124 - OLIVEIRA, L.A.R et al. Effects of in vitro Drought Stress on Growth, Proline Accumulation and Antioxidant Defense in Sugarcane. Journal of Agricultural Science; v. 10, n. 5, 2018. Available from: <Available from: https://doi.org/10.5539/jas.v10n5p135 >. Accessed: Oct. 13, 2018. doi: 10.5539/jas.v10n5p135.
» https://doi.org/10.5539/jas.v10n5p135.» https://doi.org/10.5539/jas.v10n5p135 - PONTE, L.F.A. et al. Variabilidade de indicadores fisiológicos de resistência à salinidade entre genótipos de cajueiro-anão e gigante. Pesquisa Agropecuária Brasileira, v.46, n.1, p.1-8, 2011. Available from: <Available from: http://dx.doi.org/10.1590/S0100-204X2011000100001 >. Accessed: June 15, 2016. doi: 10.1590/S0100-204X2011000100001.
» https://doi.org/10.1590/S0100-204X2011000100001.» http://dx.doi.org/10.1590/S0100-204X2011000100001 - RHOADES, J.D. et al. Uso de águas salinas para a produção agrícola. In: GHEYI, H.R.; et al. (Eds.) Estudos FAO de irrigação e drenagem. Campina Grande, UFPB. 2000. p.40-48.
- SHARMA, L.K. et al. Evaluation of rough lemon (Citrus jambhiri Lush.) as rootstock for salinity tolerance at seedling stage under in vitro conditions. African Journal of Biotechnology, v.12, n.44, p.6267-6275, 2013. Available from: <Available from: http://dx.doi.org/10.5897/AJB2013.12994 >. Accessed: Mar. 30, 2016. doi: 10.5897/AJB2013.12994.
» https://doi.org/10.5897/AJB2013.12994.» http://dx.doi.org/10.5897/AJB2013.12994 - SILVEIRA, J. A. G. et al. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. Journal of Plant Physiology, v. 160, p. 115-123, 2003. Available: <Available: https://doi.org/10.1078/0176-1617-00890 >. Acessed: Oct. 13, 2018. doi: 10.1078/0176-1617-00890.
» https://doi.org/10.1078/0176-1617-00890.» https://doi.org/10.1078/0176-1617-00890
-
CR-2017-0175.R5
Publication Dates
-
Publication in this collection
16 May 2019 -
Date of issue
2019
History
-
Received
16 Mar 2017 -
Accepted
25 Mar 2019 -
Reviewed
24 Apr 2019