ABSTRACT:
Meloidogyne javanica is a plant-parasitic nematode that infects a wide range of vegetables. Its negative effects on crop yield and value are well documented. However, few studies have investigated the impact of the parasite on the nutritional value of vegetables. This study aimed to assess the effect of M. javanica parasitism on the vegetative characteristics, nematological parameters, chemistry composition and antioxidant activity of carrots. Seedlings were inoculated with 0 (control), 1000, 2500, or 5000 eggs and eventual second-stage juveniles (J2) of M. javanica. At 60 days after inoculation, plants were harvested and evaluated. Plants inoculated with 2500 eggs and J2 of M. javanica had higher root and tuber fresh weight than the control. Gall number increased with increasing inoculum density. The number of nematodes in the roots increased until 3000 specimens, decreasing thereafter. Proximate analysis revealed that plants inoculated with 1000 eggs and J2 of M. javanica or more had higher protein content in roots. In contrast, inoculation with 1775 nematodes or more resulted in a decrease in carotenoid content. There was no effect of inoculation on total phenolic content or antioxidant activity. Although, M. javanica infection did not have a marked impact on the nutritional quality of carrots, gall formation resulted in deformed roots of low commercial value.
Key words:
Daucus carota; root-knot nematodes; phenolic compounds; carotenoids; proteins.
RESUMO:
Meloidogyne javanica é um nematoide parasita de plantas que infecta uma grande variedade de vegetais. Seus efeitos negativos sobre o rendimento e o valor das culturas estão bem documentados. No entanto, poucos estudos investigaram o impacto do parasita no valor nutricional dos vegetais. Este estudo teve como objetivo avaliar o efeito do parasitismo por M. javanica sobre as características vegetativas, parâmetros nematológicos, composição química e atividade antioxidante de cenouras. As plântulas foram inoculadas com 0 (controle), 1000, 2500 ou 5000 ovos e eventuais juvenis (J2) de M. javanica no segundo estágio. Aos 60 dias após a inoculação, as plantas foram colhidas e avaliadas. As plantas inoculadas com 2500 ovos e J2 de M. javanica apresentaram maior peso fresco das raízes e tubérculos que o controle. O número de galhas aumentou com o aumento da densidade do inóculo. Verificou-se aumento do número de nematoides nas raízes até 3000 espécimes, diminuindo posteriormente. A análise imediata revelou que plantas inoculadas com 1000 ovos e J2 de M. javanica ou mais tinham maior teor de proteína nas raízes. Por outro lado, a inoculação com 1775 nematoides ou mais resultou em diminuição no conteúdo de carotenoides. Não houve efeito da inoculação no conteúdo fenólico total ou na atividade antioxidante. Embora a infecção por M. javanica não tenha impactado significativamente a qualidade nutricional das cenouras, a formação de galhas resultou em raízes deformadas e de baixo valor comercial.
Palavras-chave:
Daucus carota; nematoides das galhas; compostos fenólicos; carotenoides; proteínas
INTRODUCTION:
Carrot (Daucus carota L.) is a root vegetable with great economic importance worldwide (BONTEMPO et al., 2017BONTEMPO, A.F. et al. Dose-response effect of Pochonia chlamydosporia against Meloidogyne incognita on carrot under field conditions. Revista Caatinga, Mossoró, v.30, n.1, p.258-262, 2017. Available from: <Available from: http://www.scielo.br/pdf/rcaat/v30n1/1983-2125-rcaat-30-01-00258.pdf
>. Accessed: Mar. 03, 2017. doi: 10.1590/1983-21252017v30n129rc.
http://www.scielo.br/pdf/rcaat/v30n1/198...
; GRABAU et al., 2017GRABAU, Z.J. et al. Effects of Cover Crops on Pratylenchus penetrans and the Nematode Community in Carrot Production. Journal of Nematology, Loudonville, v.49, n.1, p.114-123, 2017. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5411247/pdf/114.pdf
>. Accessed: Mar. 06, 2017.
https://www.ncbi.nlm.nih.gov/pmc/article...
). In Brazil, it is one of the most produced and consumed vegetables (BONTEMPO et al., 2017). Carrots provide many health benefits because of their high content of carotenoids (0.51 g kg−1 dry weight), which are important vitamin A precursors (ZHANG & HAMAUZU, 2004ZHANG, D.; HAMAUZU, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). Journal of Food Agriculture and Environmental, Helsinki, v.2, p.95-100, 2004. Available from: <Available from: https://doi.org/10.1234/4.2004.102
>. Accessed: Mar. 12, 2017. doi: 10.1234/4.2004.102.
https://doi.org/10.1234/4.2004.102...
; RODRIGUEZ-CONCEPCION & STANGE, 2013RODRIGUEZ-CONCEPCION, M.; STANGE, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Archives of Biochemistry and Biophysics, New York, v.539, n.2, p.110-116, 2013. Available from: <Available from: https://doi.org/10.1016/j.abb.2013.07.009
>. Accessed: Mar. 09, 2017. doi: 10.1016/j.abb.2013.07.009.
https://doi.org/10.1016/j.abb.2013.07.00...
; HIRANVARACHAT & DEVAHASTIN, 2014HIRANVARACHAT, B.; DEVAHASTIN, S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. Journal of Food Engineering, Berkeley, v.126, p.17-26, 2014. Available from: <Available from: https://doi.org/10.1016/j.jfoodeng.2013.10.024
>. Accessed: Feb. 25, 2017. doi: 10.1016/j.jfoodeng.2013.10.024.
https://doi.org/10.1016/j.jfoodeng.2013....
), and phenolic compounds, which have high antioxidant activity. The tuberous root is also a source of proteins, carbohydrates, fibers, and minerals, particularly potassium, sodium, phosphorus, calcium, and magnesium (USDA, 2019UNITED STATES DEPARTMENT OF AGRICULTURE (USDA). Agricultural Research Service. Available at: <Available at: http://www.usda.gov
>. Accessed: Mar. 20, 2019.
http://www.usda.gov...
). Carrots are typically consumed raw or cooked and can be processed into canned foods, juices, and baby foods.
The yield, nutritional quality, and commercial value of the vegetable are influenced by many factors, such as exposure to insects, pathogens, and ultraviolet radiation in the field (SELJASEN et al., 2013SELJASEN, R. et al. Quality of carrots as affected by pre-and postharvest factors and processing. Journal of the Science of Food and Agriculture, Oxford, v.93, n.11, p.2611-2626, 2013. Available from: <Available from: https://doi.org/10.1002/jsfa.6189
>. Accessed: Feb. 26, 2017. doi: 10.1002/jsfa.6189.
https://doi.org/10.1002/jsfa.6189...
). Variation in composition can alter the color and flavor characteristics of carrots (TALCOTT & HOWARD, 1999TALCOTT, S.T.; HOWARD, L.R. Chemical and sensory quality of processed carrot puree as influenced by stress-induced phenolic compounds. Journal of Agricultural and Food Chemistry, Washington, v.47, n.4, p.1362-1366, 1999. Available from: <Available from: https://doi.org/10.1021/jf981135f
>. Accessed: Mar. 26, 2017. doi: 10.1021/jf981135f.
https://doi.org/10.1021/jf981135f...
).
Root-knot nematodes, such as Meloidogyne javanica and M. incognita, are one of the major limiting factors to carrot crop yield, leading, in some cases, to total production loss (COLLANGE et al., 2011COLLANGE, B. et al. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Protection, Wheinheim, v.30, n.10, p.1251-1262, 2011. Available from: <Available from: https://doi.org/10.1016/j.cropro.2011.04.016
>. Accessed: Feb. 27, 2017. doi: 10.1016/j.cropro.2011.04.016.
https://doi.org/10.1016/j.cropro.2011.04...
; VIGGIANO et al., 2014VIGGIANO, J.R. et al. Use of Pochonia chlamydosporia to control Meloidogyne javanica in cucumber. Biological Control, Bangalore, v.69, p.72-77, 2014. Available from: <Available from: https://doi.org/10.1016/j.biocontrol.2013.11.004
>. Accessed: Mar. 06, 2017. doi: 10.1016/j.biocontrol.2013.11.004.
https://doi.org/10.1016/j.biocontrol.201...
). During feeding, nematodes inject esophageal secretions into plant tissues, causing hypertrophy and hyperplasia of cortical cells of the host root. This process leads to the formation of root galls, which alter root shape, affect water and nutrient transport, and; consequently, reduce vegetative growth (HUSSAIN et al., 2016HUSSAIN, M. et al. Response of selected okra cultivars to Meloidogyne incognita. Crop Protection, Wheinheim, v.82, p.1-6, 2016. Available from: <Available from: https://doi.org/10.1016/j.cropro.2015.12.024
>. Accessed: Mar. 05, 2017. doi: 10.1016/j.cropro.2015.12.024.
https://doi.org/10.1016/j.cropro.2015.12...
). Changes caused by root-knot nematode infection, even when at low levels, are responsible for reduced commercial value (GUGINO et al., 2006GUGINO, B.K. et al. Damage and management of Meloidogyne hapla using oxamyl on carrot in New York. Journal of Nematology, Loudonville, v.38, n.4, 2006p.483-490. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2586463/
>. Accessed: Jun. 09, 2020.
https://www.ncbi.nlm.nih.gov/pmc/article...
). Affected carrots; albeit being unsuitable for the consumer market, can be used in the food industry for compound extraction or processing. However, studies on tomato and bean have shown that stress caused by nematodes can alter the chemical and nutritional composition of vegetables (AHMED et al., 2009AHMED, N. et al. Physiological changes in leaves of mung bean plants infected with Meloidogyne javanica. Phytopathologia Mediterranea, Bologna v.48, n.2 p.262-268, 2009. Available from: <Available from: http://www.fupress.net/index.php/pm/article/view/3156
>. Accessed: Feb. 23, 2017. doi: 10.14601/Phytopathol_Mediterr-3156.
http://www.fupress.net/index.php/pm/arti...
; ATKINSON et al., 2011ATKINSON, N.J. et al. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). Journal of Agricultural and Food Chemistry, Washington, v.59, n.17, p.9673-9682, 2011. Available from: <Available from: https://pubs.acs.org/doi/pdf/10.1021/jf202081t?rand=8dgfzemm
>. Accessed: Feb. 21, 2017. doi: /10.1021/jf202081t.
https://pubs.acs.org/doi/pdf/10.1021/jf2...
).
Research on the effects of nematodes on carrot plants is limited to susceptibility analyses. There is little information about the influence of such parasitism on the proximate composition and nutritional quality of the vegetable. This study aimed to fill this research gap by assessing and quantifying the nutritional loss and physical damage caused by root-knot nematode infection in carrots and presenting alternative uses for affected roots.
MATERIALS AND METHODS:
The experiment was conducted in a greenhouse using a completely randomized design with four treatments, four replications for vegetative parameters and nematode population density, and three replications for proximate composition and antioxidant activity. Treatments consisted of the following inoculum levels: 0 (T1, control), 1000 (T2), 2500 (T3), and 5000 (T4) eggs and eventual second-stage juveniles (J2) of M. javanica plant−1.
Carrot cv. Carandai (Horticeres®) was sown in pots filled with 2.8 L of a 2:1:1 mixture of sandy soil, commercial substrate (sphagnum peat, coconut fiber, rice husk, pine bark, vermiculite, and nutrients; Bioplant Agrícola Ltda., Nova Ponte, Brazil), and sand. The mixture was previously homogenized and sterilized in an autoclave for 2 h at 120 °C. The soil was fertilized with a granular fertilizer containing N (40 g), P2O5 (140 g), and K2O (80 g) (Dimy, Cajamar, Brazil).
Thirty days after planting, carrot seedlings were thinned to one per pot and inoculated with 4 mL of a suspension containing different levels of M. javanica eggs and second-stage juveniles (J2). Eggs and J2 were extracted according to the method of HUSSEY & BARKER (1973) adapted by BONETI & FERRAZ (1981BONETI, J.I.S.; FERRAZ, S. Modification of the Hussey & Barker method for extracting Meloidogyne exigua eggs from coffee roots. Fitopatologia Brasileira, Brasília, v.6, n.3, p.553, 1981.) and counted using a Peters chamber under a light microscope (BA210 Binocular, Motic, Hong Kong, China). Irrigation was performed daily, and foliar fertilization was conducted every 15 days using 5 g L−1 Nutrijá® (380 g of N, 380 g of P2O5, and 380 g of K2O; Agrária, Jardinópolis, Brazil).
Plants were collected 60 days after inoculation, and the material was separated into shoots and roots (tuber + secondary roots). The following vegetative parameters were assessed: shoot fresh weight, shoot dry weight, shoot height, root fresh weight, tuber fresh weight, and tuber length. Shoot height was measured with a millimeter ruler and tuber length was measured using a Pantec® digital caliper. Shoot dry weight was determined after drying in a forced air-oven at 65 °C for 3 days.
Nematodes were extracted from roots and tubers following the method of CHARCHAR et al. (2006CHARCHAR, J.M. et al. Extraction of Meloidogyne specimens from tomato roots using the liquidizer technique. Nematologia Brasileira, Brasília, v.30, n.3, p.245-250, 2006. Available from: <Available from: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/23644/1/charchar-extracao.pdf
>. Accessed: Feb. 26, 2017.
https://ainfo.cnptia.embrapa.br/digital/...
), with modifications. Roots and tubers were peeled to a depth of about 3 mm, cut into 1-2 cm pieces, and homogenized with 0.5% hypochlorite solution in a blender for 30 s at high speed. The suspension was sieved through 60- (0.250 mm) and 500-mesh (0.025 mm) sieves. The material retained in the bottom sieve was washed with water to obtain the nematode extract. Eggs and J2 were counted using a Peters counting chamber under a light microscope (BA210 Binocular, Motic, Hong Kong, China). The total number of nematodes was divided by the root fresh weight to obtain the nematode population density (nematodes g−1 root). The reproduction factor (RF) was calculated as the ratio of total population to inoculum level (OOSTENBRINK, 1966OOSTENBRINK, M. Major characteristics of the relation between nematodes and plants. Mededeelingen der Landbouw-Hoogeschool, Wageningen, 1966. v.66, p.1-46.).
Moisture, ash, and protein contents were determined according to AOAC methods 925.09, 923.03, and 920.87, respectively (HORWITZ & LATIMER, 2005HORWITZ, W.; LATIMER, G. Official methods of analysis of AOAC International. 18th ed. AOAC International: Gaithersburg, Maryland, 2005. 1094p.). Moisture content was determined by oven-drying (SL-102, Solab, Piracicaba, Brazil) samples at 105 °C to constant weight. Ash content was determined by burning samples at 550 °C in a muffle furnace (Q318M, Quimis, Diadema, Brazil). Proteins were quantified by the Kjeldahl method (HORWITZ & LATIMER, 2005).
Carotenoids were extracted by maceration of 3 g of freshly harvested carrots with 30 mL of acetone. The mixture was filtered, and the liquid fraction was transferred to a separatory funnel containing 60 mL of petroleum ether. The organic phase was washed three times with 300 mL of distilled water to remove the acetone and used as carotenoid extract. Absorbance was read spectrophotometrically (700Plus, Femto, São Paulo, Brazil) at 453 nm. Carotenoid content (C, µg g−1) was calculated as follows: C = A × V × 104 ÷ E × W, where A is the absorbance, V is the total volume of carotenoid extract, E is the extinction coefficient of β-carotene in petroleum ether, and W is the weight of sample used to prepare the extract (RODRIGUEZ et al., 1976RODRIGUEZ, D.B. et al. Carotenoid pigment changes in ripening Momordica charantia fruits. Annals of Botany, Rome, v.40, n.3, p.615-624, 1976. Available from: <Available from: https://doi.org/10.1093/oxfordjournals.aob.a085171
>. Accessed: Feb. 21, 2017. doi: 10.1093/oxfordjournals.aob.a085171.
https://doi.org/10.1093/oxfordjournals.a...
).
Antioxidant compounds were extracted according to RAVICHANDRAN et al. (2013RAVICHANDRAN, K. et al. Impact of processing of red beet on betalain content and antioxidant activity. Food Research International, Edmonton, v.50, n.2, p.670-675, 2013. Available from: <Available from: https://doi.org/10.1016/j.foodres.2011.07.002
>. Accessed: Mar. 11, 2017. doi: 10.1016/j.foodres.2011.07.002.
https://doi.org/10.1016/j.foodres.2011.0...
), with minor modifications. Fresh samples were dissolved in 20 mL of 50% ethanol, stirred for 4 h on an orbital shaker, and centrifuged (MTD III plus, Metroterm, Porto Alegre, Brazil) at 3000 rpm for 10 min. The supernatant was collected, and the extraction process was repeated twice more with 5 mL of 50% ethanol. The ethanolic extract was used for measuring total phenolic content (TPC) and antioxidant activity.
The TPC was determined by the Folin-Ciocalteu method, according to CHEN et al. (2015CHEN, M. et al. Optimization of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chemistry, Berlin, v.172, p.543-550, 2015. Available from: <Available from: https://doi.org/10.1016/j.foodchem.2014.09.110
>. Accessed: Mar. 05, 2017. doi: 10.1016/j.foodchem.2014.09.110.
https://doi.org/10.1016/j.foodchem.2014....
). Absorbance was read spectrophotometrically at 765 nm (700Plus, Femto). A standard curve of gallic acid was constructed, and the results are expressed in mg gallic acid equivalents (GAE) g−1 sample.
Antioxidant activity was assessed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and the ferric reducing antioxidant potential (FRAP) assays. Briefly, the DPPH assay was performed by mixing 3.80 mL of DPPH solution (0.011 g mL−1) with 0.20 mL of ethanolic extract, incubating the solution for 30 min at room temperature, and measuring the absorbance at 515 nm (700Plus spectrophotometer, Femto) (RAVICHANDRAN et al., 2012RAVICHANDRAN, K. et al. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Research International, Edmonton, v.48, n.1, p.16-20, 2012. Available from: <Available from: https://doi.org/10.1016/j.foodres.2012.01.011
>. Accessed: Mar. 13, 2017. doi: 10.1016/j.foodres.2012.01.011.
https://doi.org/10.1016/j.foodres.2012.0...
). For the FRAP assay, an aliquot of ethanolic extract was mixed with water and a solution containing 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPZT), and 40 mM HCL. After 30 min of incubation at 37 °C, the absorbance was read at 593 nm (HIRANVARACHAT & DEVAHASTIN, 2014HIRANVARACHAT, B.; DEVAHASTIN, S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. Journal of Food Engineering, Berkeley, v.126, p.17-26, 2014. Available from: <Available from: https://doi.org/10.1016/j.jfoodeng.2013.10.024
>. Accessed: Feb. 25, 2017. doi: 10.1016/j.jfoodeng.2013.10.024.
https://doi.org/10.1016/j.jfoodeng.2013....
). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was used as standard. DPPH and FRAP results are expressed in µmol Trolox g−1 sample.
Data were subjected to analysis of variance and regression analysis at the 5% and 10% significance levels using the Sisvar software version 5.6. Nematode and vegetative growth data were transformed to to meet normality assumptions based on the Shapiro-Wilk test. Pearson correlation analysis was performed at the 5% significance level using Statistica version 8.
RESULTS AND DISCUSSION:
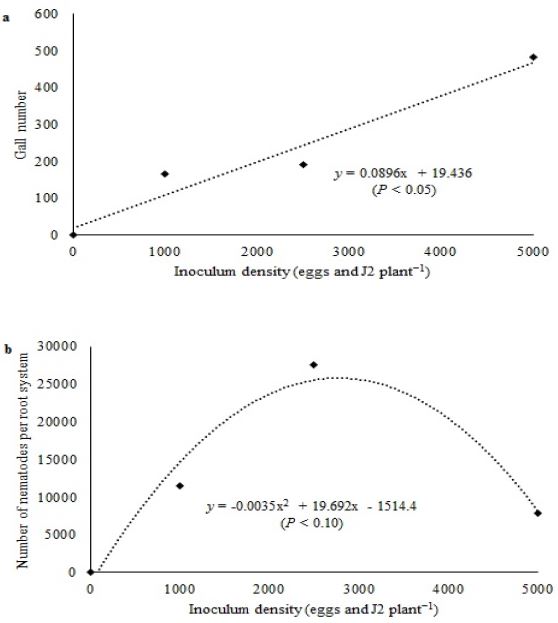
Gall number increased with increasing inoculum density (Figure 1a), as also observed in parsley and spring onion exposed to 0, 760, and 5700 M. incognita eggs + J2 (WALKER, 2002WALKER, J.T. Susceptibility of eight herbs to common root-knot nematodes. Journal of Environmental Horticulture, Washington, v.20, n.2, p.101-103, 2002. Available from: <Available from: https://hrijournal.org/doi/pdf/10.24266/0738-2898-20.2.101
>. Accessed: Feb. 23, 2017. doi: 10.24266/0738-2898-20.2.101.
https://hrijournal.org/doi/pdf/10.24266/...
) and cucumber inoculated with M. incognita (KAYANI et al., 2017KAYANI, M.Z. et al. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Protection, Wheinheim, v.92, p.207-212, 2017. Available from: <Available from: https://doi.org/10.1016/j.cropro.2016.09.007
>. Accessed: Feb. 23, 2017. doi: 10.1016/j.cropro.2016.09.007.
https://doi.org/10.1016/j.cropro.2016.09...
). The number of nematodes in the roots increased with inoculum density up to 2500 nematodes plant-1 but decreased thereafter (Figure 1b). Carrot plants were susceptible to all inoculum densities (RF > 1), but multiplication was affected by high initial population densities. At an inoculum density of 1000 nematodes plant−1, the RF was 11.47, whereas at 5000 nematodes plant-1, the RF was reduced to 1.57 (Figure 1b). This result is likely due to competition between nematodes, as previously observed in other plant pathosystems (HUSSAIN et al., 2016HUSSAIN, M. et al. Response of selected okra cultivars to Meloidogyne incognita. Crop Protection, Wheinheim, v.82, p.1-6, 2016. Available from: <Available from: https://doi.org/10.1016/j.cropro.2015.12.024
>. Accessed: Mar. 05, 2017. doi: 10.1016/j.cropro.2015.12.024.
https://doi.org/10.1016/j.cropro.2015.12...
). No differences (P > 0.10) were observed between treatments in nematode density, ranging from 1133 to 1726 nematodes g−1 root.
Relationship of inoculum density with gall number (a) and number of nematodes per root system (b) in carrots at 60 days after inoculation with Meloidogyne javanica.
Shoot dry weight (2.21-2.85 g) and tuber length (10.97-12.15 cm) were not affected by M. javanica inoculum levels. Conversely, shoot height, shoot fresh weight, root fresh weight, and tuber fresh weight increased (P < 0.05) up to inoculum densities of 4000, 2700, 2125, and 2250 nematodes plant-1, respectively (Figure 2). Although, nematodes stunt the growth of the root system by inducing necrotic lesions and galls (AFSHAR et al., 2014AFSHAR, F.J. et al. Effects of the root knot nematodes Meloidogyne incognita and M. javanica on olive plants growth in glasshouse conditions. Helminthologia, Kosice, v.51, n.1, p.46-52, 2014. Available from: <Available from: https://www.researchgate.net/publication/270265976_Effects_of_the_rootknot_nematodes_Meloidogyne_incognita_and_M_javanica_on_olive_plants_growth_in_glasshouse_conditions
>. Accessed: Feb. 21, 2017. doi: 10.2478/s11687-014-0207-x.
https://www.researchgate.net/publication...
; HUNT & HANDOO, 2009HUNT, D.J.; HANDOO, Z.A. Root-Knot Nematodes. In: PERRY, R. N., MOENS, M., STARR, J. L. (Ed). Taxonomy, Identification and Principal Species CABI International, Cambridge, MA, USA, 2009, pp.55-97.), root and tuber weight can increase with gall formation (KAYANI et al., 2017KAYANI, M.Z. et al. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Protection, Wheinheim, v.92, p.207-212, 2017. Available from: <Available from: https://doi.org/10.1016/j.cropro.2016.09.007
>. Accessed: Feb. 23, 2017. doi: 10.1016/j.cropro.2016.09.007.
https://doi.org/10.1016/j.cropro.2016.09...
). Galls form from the hypertrophy and hyperplasia of vascular parenchyma in root cells affected by secretions of the dorsal esophageal glands of nematodes. Root galls hamper the formation of secondary roots, reduce water and nutrient absorption, and impair plant growth (ESCOBAR et al., 2015ESCOBAR, C. et al. Overview of root-knot nematodes and giant cells. In: ESCOBAR, C.; FENOLL, C. Plant Nematode interactions - A view on compatible interrelationships. Advances in Botanical Research, Cambridge, v.73, p. 1-32, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0065229615000142
>. Accessed: Nov. 29, 2019. doi: https://doi.org/10.1016/bs.abr.2015.01.001.
https://www.sciencedirect.com/science/ar...
). It is possible that the 60-day experimental period was not long enough for the effects of M. javanica parasitism on shoot development to become evident. Moreover, plants may still develop when exposed to small nematode populations, as new roots can emerge (OLTHOF & POTTER, 1977OLTHOF, T.A.; POTTER, J.W. Effects of population densities of Meloidogyne hapla on growth and yield of tomato. Journal of Nematology, Loudonville, v.9, n.4, p.296-300, 1977. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620265/pdf/296.pdf
>. Accessed: Feb. 24, 2017.
https://www.ncbi.nlm.nih.gov/pmc/article...
). This could explain the high root fresh weight and tuber fresh weight of plants inoculated with up to 2125 and 2250 nematodes, respectively, compared with plants inoculated with higher nematode densities (Figure 2c and 2d).
Effect of Meloidogyne javanica on shoot height (a), shoot fresh weight (b) root fresh weight (c), and tuber fresh weight (d) in carrots at 60 days after inoculation.
Moisture and ash contents did not differ significantly between treatments, ranging from 852.36 to 853.86 g kg−1 and from 11.40 to 11.86 g kg−1 in T1 and T4, respectively. Protein levels increased with inoculum densities greater than 1000 nematodes plant-1 (Figure 3a). This result can be attributed to the plant’s response to stress caused by nematode infection (ROCHA et al., 2015ROCHA, R.O. et al. Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita. Journal of Agricultural and Food Chemistry, Washington, v.63, n.22, p.5335-5343, 2015. Available from: <Available from: https://doi.org/10.1021/acs.jafc.5b01109
>. Accessed: Mar. 10, 2017. doi: 10.1021/acs.jafc.5b01109.
https://doi.org/10.1021/acs.jafc.5b01109...
). Plants have a wide array of mechanisms to respond to biotic and abiotic stresses (ATKINSON et al., 2011ATKINSON, N.J. et al. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). Journal of Agricultural and Food Chemistry, Washington, v.59, n.17, p.9673-9682, 2011. Available from: <Available from: https://pubs.acs.org/doi/pdf/10.1021/jf202081t?rand=8dgfzemm
>. Accessed: Feb. 21, 2017. doi: /10.1021/jf202081t.
https://pubs.acs.org/doi/pdf/10.1021/jf2...
), including metabolic activation, phytoalexin biosynthesis, phenolic compound accumulation, and increase in peroxidase, catalase, phenylalanine ammonia-lyase, and β-1,3-glucanase activities (DIAS-ARIEIRA et al., 2013DIAS-ARIEIRA, C.R. et al. Induced resistance in the nematodes control. African Journal of Agricultural Research, Johannesburg, v.8, n.20, p.2312-2318, 2013. Available from: <Available from: https://www.researchgate.net/publication/307721565_Induced_resistance_in_the_nematodes_control
>. Accessed: Mar. 06, 2017. doi: 10.5897/AJARx12.012.
https://www.researchgate.net/publication...
). Thus; although the current study assessed the level of total proteins rather than that of pathogenesis-related proteins, it is likely that protein-related defense mechanisms were activated by high inoculum densities. A previous study showed that nematodes parasitism can alter the protein profile of plants (GHEYSEN & FENOLL, 2002GHEYSEN, G.; FENOLL, C. Gene expression in nematode feeding site. Annual Review of Phytopathology, Palo Alto, v.40, p.191-219, 2002. Available from: <Available from: https://www.annualreviews.org/doi/pdf/10.1146/annurev.phyto.40.121201.093719
>. Accessed: Dec. 03, 2019. doi: 10.1146/annurev.phyto.40.121201.093719.
https://www.annualreviews.org/doi/pdf/10...
). These effects should be further studied.
Protein (a) and carotenoid (b) contents in carrots inoculated with different inoculum levels of Meloidogyne javanica.
Carotenoid content increased up to inoculum densities of 1775 nematodes plant-1, decreasing thereafter (Figure 3b). A similar effect was observed in mung bean (Vigna radiata) inoculated with 2000 M. javanica eggs + J2. The reduction in carotenoid content was attributed to a reduction in leaf area (AHMED et al., 2009AHMED, N. et al. Physiological changes in leaves of mung bean plants infected with Meloidogyne javanica. Phytopathologia Mediterranea, Bologna v.48, n.2 p.262-268, 2009. Available from: <Available from: http://www.fupress.net/index.php/pm/article/view/3156
>. Accessed: Feb. 23, 2017. doi: 10.14601/Phytopathol_Mediterr-3156.
http://www.fupress.net/index.php/pm/arti...
). Oxidation is the main cause of carotenoid degradation. The compound is easily oxidized because of its large number of double bonds. Carotenoids are protected from oxidation in intact tissues; however, tissue damage increases susceptibility to oxidation (SAINI et al., 2015SAINI, R.K. et al. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, Essex, v.76, p.735-750, 2015. Available from: <Available from: https://doi.org/10.1016/j.foodr es.2015.07.047
>. Accessed: Jun. 9, 2020. doi: 10.1016/j.foodr es.2015.07.047.
https://doi.org/10.1016/j.foodr es.2015....
). Activation of plant defense responses to nematode infection probably increased the activity of enzymes such as peroxidase (DIAS-ARIEIRA et al., 2013DIAS-ARIEIRA, C.R. et al. Induced resistance in the nematodes control. African Journal of Agricultural Research, Johannesburg, v.8, n.20, p.2312-2318, 2013. Available from: <Available from: https://www.researchgate.net/publication/307721565_Induced_resistance_in_the_nematodes_control
>. Accessed: Mar. 06, 2017. doi: 10.5897/AJARx12.012.
https://www.researchgate.net/publication...
), promoting carotenoid degradation (UENOJO et al., 2007UENOJO, M. et al. Carotenoides: propriedades, aplicações e biotransformação para formação de compostos de aroma. Química Nova, São Paulo, v.30, n.3, p.616-622, 2007. Available from: <Available from: http://dx.doi.org/10.1590/S0100-40422007000300022
>. Accessed: Mar. 21, 2017. doi: 10.1590/S0100-40422007000300022.
http://dx.doi.org/10.1590/S0100-40422007...
). There was a negative correlation (−0.60, P < 0.05) between total carotenoid and protein contents, suggesting that the increase in protein content affected carotenoid accumulation in roots.
The TPC (24.77-27.97 mg GAE g−1) and antioxidant activity were not influenced by M. javanica inoculum density (P > 0.10). DPPH activity was 0.01 μmol Trolox g−1, regardless of inoculum density. According to the FRAP assay, all samples had an antioxidant activity of 0.12 μmol Trolox g−1, except for T2, which had an activity of 0.14 μmol Trolox g−1. Similar results were observed in tomato plants subjected to water and nematode stress (ATKINSON et al., 2011ATKINSON, N.J. et al. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). Journal of Agricultural and Food Chemistry, Washington, v.59, n.17, p.9673-9682, 2011. Available from: <Available from: https://pubs.acs.org/doi/pdf/10.1021/jf202081t?rand=8dgfzemm
>. Accessed: Feb. 21, 2017. doi: /10.1021/jf202081t.
https://pubs.acs.org/doi/pdf/10.1021/jf2...
). Plants accumulate phenolic compounds as protection against nematode infection (NACZK & SHAHIDI, 2004NACZK, M.; SHAHIDI, F. Extraction and analysis of phenolics in food. Journal of Chromatography A, New York, v.1054, n.1-2, p.95-111, 2004. Available from: <Available from: https://doi.org/10.1016/j.chroma.2004.08.059
>. Accessed: Mar. 04, 2017. doi: 10.1016/j.chroma.2004.08.059.
https://doi.org/10.1016/j.chroma.2004.08...
). These compounds are toxic to the parasite and limit the penetration of nematodes and other pathogenic microorganisms (TALCOTT & HOWARD, 1999TALCOTT, S.T.; HOWARD, L.R. Chemical and sensory quality of processed carrot puree as influenced by stress-induced phenolic compounds. Journal of Agricultural and Food Chemistry, Washington, v.47, n.4, p.1362-1366, 1999. Available from: <Available from: https://doi.org/10.1021/jf981135f
>. Accessed: Mar. 26, 2017. doi: 10.1021/jf981135f.
https://doi.org/10.1021/jf981135f...
).
Although, carrot tubers affected by M. javanica had presented visible damage and reduction of carotenoids, the TPC and antioxidant activity were not affected, indicating that they could still be used in the food industry. This strategy can reduce food waste. In a study by GULL et al. (2015), GULL, A. et al. Effect of millet flours and carrot pomace on cooking qualities, color and texture of developed pasta. Food Science and Technology, Campinas, v.63, p.470-474, 2015. Available from: <https://doi.org/10.1016/j.lwt.2015.03.008>. Accessed: Feb. 21, 2017. doi: 10.1016/j.lwt.2015.03.008.
https://doi.org/https://doi.org/10.1016/...
for instance, carrot pomace was used in pasta production as a partial, highly nutritional substitute for wheat.
CONCLUSION:
Although, carrot tuber length was not negatively influenced by inoculum density, tuber weight significantly decreased with inoculum densities greater than 2250 nematodes plant−1. Antioxidant activity, TPC, moisture content, and ash content were not influenced by inoculum density. The decrease in carotenoid content was associated with an increase in protein levels. Root galls caused visible changes to tuber morphology, which negatively affects the vegetable’s commercial value. Nevertheless, affected tubers may find application in the food processing industry, as they still have high nutritional quality.
ACKNOWLEDGEMENTS
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil, for granting the doctoral scholarship to Paula J. G. Débia; and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for grating the research productivity scholarship to Claudia R. Dias-Arieira (Process 304070/2016-5) and for financing the project (Process 402136/2016-0).
REFERENCES
- AFSHAR, F.J. et al. Effects of the root knot nematodes Meloidogyne incognita and M. javanica on olive plants growth in glasshouse conditions. Helminthologia, Kosice, v.51, n.1, p.46-52, 2014. Available from: <Available from: https://www.researchgate.net/publication/270265976_Effects_of_the_rootknot_nematodes_Meloidogyne_incognita_and_M_javanica_on_olive_plants_growth_in_glasshouse_conditions >. Accessed: Feb. 21, 2017. doi: 10.2478/s11687-014-0207-x.
» https://doi.org/10.2478/s11687-014-0207-x.» https://www.researchgate.net/publication/270265976_Effects_of_the_rootknot_nematodes_Meloidogyne_incognita_and_M_javanica_on_olive_plants_growth_in_glasshouse_conditions - AHMED, N. et al. Physiological changes in leaves of mung bean plants infected with Meloidogyne javanica Phytopathologia Mediterranea, Bologna v.48, n.2 p.262-268, 2009. Available from: <Available from: http://www.fupress.net/index.php/pm/article/view/3156 >. Accessed: Feb. 23, 2017. doi: 10.14601/Phytopathol_Mediterr-3156.
» https://doi.org/10.14601/Phytopathol_Mediterr-3156.» http://www.fupress.net/index.php/pm/article/view/3156 - ATKINSON, N.J. et al. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). Journal of Agricultural and Food Chemistry, Washington, v.59, n.17, p.9673-9682, 2011. Available from: <Available from: https://pubs.acs.org/doi/pdf/10.1021/jf202081t?rand=8dgfzemm >. Accessed: Feb. 21, 2017. doi: /10.1021/jf202081t.
» https://doi.org//10.1021/jf202081t.» https://pubs.acs.org/doi/pdf/10.1021/jf202081t?rand=8dgfzemm - BONETI, J.I.S.; FERRAZ, S. Modification of the Hussey & Barker method for extracting Meloidogyne exigua eggs from coffee roots. Fitopatologia Brasileira, Brasília, v.6, n.3, p.553, 1981.
- BONTEMPO, A.F. et al. Dose-response effect of Pochonia chlamydosporia against Meloidogyne incognita on carrot under field conditions. Revista Caatinga, Mossoró, v.30, n.1, p.258-262, 2017. Available from: <Available from: http://www.scielo.br/pdf/rcaat/v30n1/1983-2125-rcaat-30-01-00258.pdf >. Accessed: Mar. 03, 2017. doi: 10.1590/1983-21252017v30n129rc.
» https://doi.org/10.1590/1983-21252017v30n129rc» http://www.scielo.br/pdf/rcaat/v30n1/1983-2125-rcaat-30-01-00258.pdf - CHARCHAR, J.M. et al. Extraction of Meloidogyne specimens from tomato roots using the liquidizer technique. Nematologia Brasileira, Brasília, v.30, n.3, p.245-250, 2006. Available from: <Available from: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/23644/1/charchar-extracao.pdf >. Accessed: Feb. 26, 2017.
» https://ainfo.cnptia.embrapa.br/digital/bitstream/item/23644/1/charchar-extracao.pdf - CHEN, M. et al. Optimization of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chemistry, Berlin, v.172, p.543-550, 2015. Available from: <Available from: https://doi.org/10.1016/j.foodchem.2014.09.110 >. Accessed: Mar. 05, 2017. doi: 10.1016/j.foodchem.2014.09.110.
» https://doi.org/10.1016/j.foodchem.2014.09.110.» https://doi.org/10.1016/j.foodchem.2014.09.110 - COLLANGE, B. et al. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Protection, Wheinheim, v.30, n.10, p.1251-1262, 2011. Available from: <Available from: https://doi.org/10.1016/j.cropro.2011.04.016 >. Accessed: Feb. 27, 2017. doi: 10.1016/j.cropro.2011.04.016.
» https://doi.org/10.1016/j.cropro.2011.04.016.» https://doi.org/10.1016/j.cropro.2011.04.016 - DIAS-ARIEIRA, C.R. et al. Induced resistance in the nematodes control. African Journal of Agricultural Research, Johannesburg, v.8, n.20, p.2312-2318, 2013. Available from: <Available from: https://www.researchgate.net/publication/307721565_Induced_resistance_in_the_nematodes_control >. Accessed: Mar. 06, 2017. doi: 10.5897/AJARx12.012.
» https://doi.org/10.5897/AJARx12.012.» https://www.researchgate.net/publication/307721565_Induced_resistance_in_the_nematodes_control - ESCOBAR, C. et al. Overview of root-knot nematodes and giant cells. In: ESCOBAR, C.; FENOLL, C. Plant Nematode interactions - A view on compatible interrelationships. Advances in Botanical Research, Cambridge, v.73, p. 1-32, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0065229615000142 >. Accessed: Nov. 29, 2019. doi: https://doi.org/10.1016/bs.abr.2015.01.001.
» https://doi.org/https://doi.org/10.1016/bs.abr.2015.01.001.» https://www.sciencedirect.com/science/article/pii/S0065229615000142 - GHEYSEN, G.; FENOLL, C. Gene expression in nematode feeding site. Annual Review of Phytopathology, Palo Alto, v.40, p.191-219, 2002. Available from: <Available from: https://www.annualreviews.org/doi/pdf/10.1146/annurev.phyto.40.121201.093719 >. Accessed: Dec. 03, 2019. doi: 10.1146/annurev.phyto.40.121201.093719.
» https://doi.org/10.1146/annurev.phyto.40.121201.093719.» https://www.annualreviews.org/doi/pdf/10.1146/annurev.phyto.40.121201.093719 - GULL, A. et al. Effect of millet flours and carrot pomace on cooking qualities, color and texture of developed pasta. Food Science and Technology, Campinas, v.63, p.470-474, 2015. Available from: <https://doi.org/10.1016/j.lwt.2015.03.008>. Accessed: Feb. 21, 2017. doi: 10.1016/j.lwt.2015.03.008.
» https://doi.org/https://doi.org/10.1016/j.lwt.2015.03.008» https://doi.org/10.1016/j.lwt.2015.03.008. - GRABAU, Z.J. et al. Effects of Cover Crops on Pratylenchus penetrans and the Nematode Community in Carrot Production. Journal of Nematology, Loudonville, v.49, n.1, p.114-123, 2017. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5411247/pdf/114.pdf >. Accessed: Mar. 06, 2017.
» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5411247/pdf/114.pdf - GUGINO, B.K. et al. Damage and management of Meloidogyne hapla using oxamyl on carrot in New York. Journal of Nematology, Loudonville, v.38, n.4, 2006p.483-490. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2586463/ >. Accessed: Jun. 09, 2020.
» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2586463/ - HIRANVARACHAT, B.; DEVAHASTIN, S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. Journal of Food Engineering, Berkeley, v.126, p.17-26, 2014. Available from: <Available from: https://doi.org/10.1016/j.jfoodeng.2013.10.024 >. Accessed: Feb. 25, 2017. doi: 10.1016/j.jfoodeng.2013.10.024.
» https://doi.org/10.1016/j.jfoodeng.2013.10.024.» https://doi.org/10.1016/j.jfoodeng.2013.10.024 - HORWITZ, W.; LATIMER, G. Official methods of analysis of AOAC International. 18th ed. AOAC International: Gaithersburg, Maryland, 2005. 1094p.
- HUSSAIN, M. et al. Response of selected okra cultivars to Meloidogyne incognita Crop Protection, Wheinheim, v.82, p.1-6, 2016. Available from: <Available from: https://doi.org/10.1016/j.cropro.2015.12.024 >. Accessed: Mar. 05, 2017. doi: 10.1016/j.cropro.2015.12.024.
» https://doi.org/10.1016/j.cropro.2015.12.024.» https://doi.org/10.1016/j.cropro.2015.12.024 - HUNT, D.J.; HANDOO, Z.A. Root-Knot Nematodes. In: PERRY, R. N., MOENS, M., STARR, J. L. (Ed). Taxonomy, Identification and Principal Species CABI International, Cambridge, MA, USA, 2009, pp.55-97.
- KAYANI, M.Z. et al. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Protection, Wheinheim, v.92, p.207-212, 2017. Available from: <Available from: https://doi.org/10.1016/j.cropro.2016.09.007 >. Accessed: Feb. 23, 2017. doi: 10.1016/j.cropro.2016.09.007.
» https://doi.org/10.1016/j.cropro.2016.09.007.» https://doi.org/10.1016/j.cropro.2016.09.007 - NACZK, M.; SHAHIDI, F. Extraction and analysis of phenolics in food. Journal of Chromatography A, New York, v.1054, n.1-2, p.95-111, 2004. Available from: <Available from: https://doi.org/10.1016/j.chroma.2004.08.059 >. Accessed: Mar. 04, 2017. doi: 10.1016/j.chroma.2004.08.059.
» https://doi.org/10.1016/j.chroma.2004.08.059.» https://doi.org/10.1016/j.chroma.2004.08.059 - OLTHOF, T.A.; POTTER, J.W. Effects of population densities of Meloidogyne hapla on growth and yield of tomato. Journal of Nematology, Loudonville, v.9, n.4, p.296-300, 1977. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620265/pdf/296.pdf >. Accessed: Feb. 24, 2017.
» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620265/pdf/296.pdf - OOSTENBRINK, M. Major characteristics of the relation between nematodes and plants. Mededeelingen der Landbouw-Hoogeschool, Wageningen, 1966. v.66, p.1-46.
- RAVICHANDRAN, K. et al. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Research International, Edmonton, v.48, n.1, p.16-20, 2012. Available from: <Available from: https://doi.org/10.1016/j.foodres.2012.01.011 >. Accessed: Mar. 13, 2017. doi: 10.1016/j.foodres.2012.01.011.
» https://doi.org/10.1016/j.foodres.2012.01.011.» https://doi.org/10.1016/j.foodres.2012.01.011 - RAVICHANDRAN, K. et al. Impact of processing of red beet on betalain content and antioxidant activity. Food Research International, Edmonton, v.50, n.2, p.670-675, 2013. Available from: <Available from: https://doi.org/10.1016/j.foodres.2011.07.002 >. Accessed: Mar. 11, 2017. doi: 10.1016/j.foodres.2011.07.002.
» https://doi.org/10.1016/j.foodres.2011.07.002.» https://doi.org/10.1016/j.foodres.2011.07.002 - ROCHA, R.O. et al. Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita Journal of Agricultural and Food Chemistry, Washington, v.63, n.22, p.5335-5343, 2015. Available from: <Available from: https://doi.org/10.1021/acs.jafc.5b01109 >. Accessed: Mar. 10, 2017. doi: 10.1021/acs.jafc.5b01109.
» https://doi.org/10.1021/acs.jafc.5b01109.» https://doi.org/10.1021/acs.jafc.5b01109 - RODRIGUEZ-CONCEPCION, M.; STANGE, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Archives of Biochemistry and Biophysics, New York, v.539, n.2, p.110-116, 2013. Available from: <Available from: https://doi.org/10.1016/j.abb.2013.07.009 >. Accessed: Mar. 09, 2017. doi: 10.1016/j.abb.2013.07.009.
» https://doi.org/10.1016/j.abb.2013.07.009.» https://doi.org/10.1016/j.abb.2013.07.009 - RODRIGUEZ, D.B. et al. Carotenoid pigment changes in ripening Momordica charantia fruits. Annals of Botany, Rome, v.40, n.3, p.615-624, 1976. Available from: <Available from: https://doi.org/10.1093/oxfordjournals.aob.a085171 >. Accessed: Feb. 21, 2017. doi: 10.1093/oxfordjournals.aob.a085171.
» https://doi.org/10.1093/oxfordjournals.aob.a085171.» https://doi.org/10.1093/oxfordjournals.aob.a085171 - SAINI, R.K. et al. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, Essex, v.76, p.735-750, 2015. Available from: <Available from: https://doi.org/10.1016/j.foodr es.2015.07.047 >. Accessed: Jun. 9, 2020. doi: 10.1016/j.foodr es.2015.07.047.
» https://doi.org/10.1016/j.foodr es.2015.07.047.» https://doi.org/10.1016/j.foodr es.2015.07.047 - SELJASEN, R. et al. Quality of carrots as affected by pre-and postharvest factors and processing. Journal of the Science of Food and Agriculture, Oxford, v.93, n.11, p.2611-2626, 2013. Available from: <Available from: https://doi.org/10.1002/jsfa.6189 >. Accessed: Feb. 26, 2017. doi: 10.1002/jsfa.6189.
» https://doi.org/10.1002/jsfa.6189.» https://doi.org/10.1002/jsfa.6189 - TALCOTT, S.T.; HOWARD, L.R. Chemical and sensory quality of processed carrot puree as influenced by stress-induced phenolic compounds. Journal of Agricultural and Food Chemistry, Washington, v.47, n.4, p.1362-1366, 1999. Available from: <Available from: https://doi.org/10.1021/jf981135f >. Accessed: Mar. 26, 2017. doi: 10.1021/jf981135f.
» https://doi.org/10.1021/jf981135f.» https://doi.org/10.1021/jf981135f - UENOJO, M. et al. Carotenoides: propriedades, aplicações e biotransformação para formação de compostos de aroma. Química Nova, São Paulo, v.30, n.3, p.616-622, 2007. Available from: <Available from: http://dx.doi.org/10.1590/S0100-40422007000300022 >. Accessed: Mar. 21, 2017. doi: 10.1590/S0100-40422007000300022.
» https://doi.org/10.1590/S0100-40422007000300022.» http://dx.doi.org/10.1590/S0100-40422007000300022 - UNITED STATES DEPARTMENT OF AGRICULTURE (USDA). Agricultural Research Service. Available at: <Available at: http://www.usda.gov >. Accessed: Mar. 20, 2019.
» http://www.usda.gov - VIGGIANO, J.R. et al. Use of Pochonia chlamydosporia to control Meloidogyne javanica in cucumber. Biological Control, Bangalore, v.69, p.72-77, 2014. Available from: <Available from: https://doi.org/10.1016/j.biocontrol.2013.11.004 >. Accessed: Mar. 06, 2017. doi: 10.1016/j.biocontrol.2013.11.004.
» https://doi.org/10.1016/j.biocontrol.2013.11.004.» https://doi.org/10.1016/j.biocontrol.2013.11.004 - WALKER, J.T. Susceptibility of eight herbs to common root-knot nematodes. Journal of Environmental Horticulture, Washington, v.20, n.2, p.101-103, 2002. Available from: <Available from: https://hrijournal.org/doi/pdf/10.24266/0738-2898-20.2.101 >. Accessed: Feb. 23, 2017. doi: 10.24266/0738-2898-20.2.101.
» https://doi.org/10.24266/0738-2898-20.2.101.» https://hrijournal.org/doi/pdf/10.24266/0738-2898-20.2.101 - ZHANG, D.; HAMAUZU, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). Journal of Food Agriculture and Environmental, Helsinki, v.2, p.95-100, 2004. Available from: <Available from: https://doi.org/10.1234/4.2004.102 >. Accessed: Mar. 12, 2017. doi: 10.1234/4.2004.102.
» https://doi.org/10.1234/4.2004.102.» https://doi.org/10.1234/4.2004.102
-
CR-2019-0585.R2
Publication Dates
-
Publication in this collection
07 Aug 2020 -
Date of issue
2020
History
-
Received
07 Aug 2019 -
Accepted
20 May 2020 -
Reviewed
30 June 2020