ABSTRACT:
The mangrove is a coastal ecosystem that is present in different parts of the world. It provides various ecosystem services from food supply to the influence of climate change. Due to the development of society, this ecosystem has been subjected to significant impacts from anthropogenic activities. Therefore, the objective of this research was to evaluate the environmental impacts caused in mangrove areas that have undergone modifications as a result of anthropic activities (agricultural cultivation, deforestation, civil construction) compared with those of conserved mangrove areas. This research took place through the analysis of the temporal sequence of aerial images (Google Earth) and soil quality analysis through field collections to evaluate the chemical and biological indicators in the different land use systems. As these are permanent changes that affect the type of soil and its coverage, significant differences were obtained between the chemical and biological characteristics of the four environments, with different usage systems. The mangrove has been negatively impacted by inadequate management and land occupation. Continuity of anthropic intervention in the mangrove will promote the disappearance of this ecosystem in the long term. Among the chemical and biological attributes used for the analyses that were performed, aluminum and edaphic organisms were the ones that allowed the greatest contribution of distinction from the degree of disturbance in areas of agricultural cultivation, deforestation and civil construction/mangrove transition.
Key words:
degradation; coastal ecosystem; environmental impact
RESUMO:
O manguezal é um ecossistema costeiro, presente em diversas partes do mundo, provedor de diversos serviços ecossistêmicos desde a provisão de alimentos a influência das mudanças climáticas. Devido ao desenvolvimento da sociedade, este ecossistema tem sido submetido a significativos impactos proveniente das atividades antropogênicas. Diante disso, o objetivo deste trabalho foi avaliar os impactos ambientais ocasionados em áreas de mangue que sofreram modificações resultantes de atividades antrópicas (cultivo agrícola, desmatamento, construção civil) comparando com área de mangue conservado. Esta pesquisa se deu através da análise da sequência temporal de imagens aéreas (Google Earth), e análise da qualidade do solo mediante coletas em campo para avaliação dos indicadores químicos e biológicos nos diferentes sistemas de uso do solo. Por se tratarem de alterações permanentes e que afetaram o tipo do solo e a cobertura do mesmo, foram obtidas diferenças significativas entre as características químicas e biológicas dos quatro ambientes, com os diferentes sistemas de uso. O manguezal tem sofrido impactos negativos pelo manejo inadequado e ocupação do solo. A continuidade da intervenção antrópica no mangue dará prosseguimento ao desaparecimento deste ecossistema a longo prazo. Dentre os atributos químicos e biológicos utilizados para as análises realizadas, o alumínio e os organismos edáficos foram os que permitiram maior contribuição para descriminação do grau de perturbação das áreas de cultivo agrícola, desmatada e transição construção civil/manguezal.
Palavras-chave:
degradação; ecossistema costeiro; impacto ambiental
INTRODUCTION:
Mangroves are coastal environments present in tropical and subtropical regions of the globe and have great diversity of fauna and flora (SEMADS, 2001SEMADS. Secretaria de Estado de Meio Ambiente e Desenvolvimento Sustentável. MANGROVES EDUCATE TO PROTECT. SEA STUDIES FOUNDATION, 2001. Available from: <Available from: https://www.mma.gov.br/estruturas/sqa_pnla/_arquivos/manguezais.pdf >. Accessed: Nov. 02, 2019.
https://www.mma.gov.br/estruturas/sqa_pn...
). It contributes to the community in providing food and water, maintaining the climate, stabilizing the soil, and controlling erosion, and nutrient cycling. Among others, it reduces the vulnerability of coastal regions to floods and storms (GASPARINETTI et al., 2018GASPARINETTI, P.; The values of ecosystem services of Brazilian mangroves, economic instruments for their conservation and the case study of Salgado Paraense. Conservation Strategy Fund (CSF), 2018. Available from: <Available from: https://www.conservation-strategy.org/sites/default/files/field-file/PORT_DP_Os_valores_dos_servicos_ecossistemicos_dos_manguezais_brasileiros_Abr_2018.pdf >. Accessed: Nov. 02, 2019.
https://www.conservation-strategy.org/si...
).
Mangrove forests possess a vast worldwide extension of approximately 14 million hectares (140,000 km²) (data from 2000), distributed in 118 countries and territories (GIRI et al., 2010GIRI, C. et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography, v.20, n.1, p.154-159, 2010. Available from: <Available from: https://doi.org/10.1111/j.1466-8238.2010.00584.x >. Accessed: Mar. 05, 2019. doi: 10.1111/j.1466-8238.2010.00584.x
https://doi.org/10.1111/j.1466-8238.2010...
). In Brazil, there are 17,287 km² of this estuarine environment, from the coast of state of Amapá to Santa Catarina (BIBI et al., 2019BIBI, SN. Et al. Ethnopharmacology, Phytochemistry, and global distribution of mangroves - A comprehensive Review. Marine Drugs, 2019, 17, 231. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pubmed/31003533 >. Accessed: Jun. 10, 2019. doi: 10.3390/md17040231.
https://www.ncbi.nlm.nih.gov/pubmed/3100...
; SEMADS, 2001SEMADS. Secretaria de Estado de Meio Ambiente e Desenvolvimento Sustentável. MANGROVES EDUCATE TO PROTECT. SEA STUDIES FOUNDATION, 2001. Available from: <Available from: https://www.mma.gov.br/estruturas/sqa_pnla/_arquivos/manguezais.pdf >. Accessed: Nov. 02, 2019.
https://www.mma.gov.br/estruturas/sqa_pn...
). The state of Alagoas covers 5,535.27 hectares of mangroves (around 55.3527 km²) (MMA and ICMBIO, 2018MINISTÉRIO DO MEIO AMBIENTE (MMA); INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE (ICMBIO). THE BRAZILIAN MANGROVES ATLAS. Brasília: [s.n.], p.43-88, 2018.).
Despite their important provisional services to society, mangroves have been suffering from threats of human activities such as tourism and resource extraction. Results consequently are pollution and land occupation. The Brazilian Northeast is between the two regions that present a critical scenario (Northeast and Southeast, among the five regions of Brazil), where around 40% of the existing mangroves have been extinct (MMA and ICMBIO, 2018MINISTÉRIO DO MEIO AMBIENTE (MMA); INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE (ICMBIO). THE BRAZILIAN MANGROVES ATLAS. Brasília: [s.n.], p.43-88, 2018.).
In view of this context and a few studies undertaken in the region of Alagoas, the objective of this study was to assess the environmental impacts caused in mangrove areas of the Mundaú / Manguaba Estuarine Lagoon Complex (CELMM) due to the changes resulting from anthropic activities (cultivation agriculture, deforestation, civil construction) contrasting with the conserved mangrove.
MATERIALS AND METHODS:
Study area
The research was carried out in the municipality of Marechal Deodoro/Alagoas (Figure 1), located in the Metropolitan Region of Maceió, a tropical climate territory, with a dry and a rainy season during the year and an average annual rainfall of 1,648.1 mm (SEMARH, 2014SEMARH. Secretaria de Estado do Meio Ambiente e dos Recursos Hídricos. Monthly Precipitation Data -2014. 2014. Available from: <Available from: http://dados.al.gov.br/dataset/7255537c-bc40-4c79-841d-e3977f6ab2f9/resource/16d07384-32a5-4896-926d-57c7bb6ab747/download/dadosmensaisdez2014.pdf >. Accessed: Oct. 31, 2019.
http://dados.al.gov.br/dataset/7255537c-...
). The study environments are located in the Barra Nova neighborhood, belonging to the CELMM.
Location of collection areas (Marechal Deodoro/Alagoas/Brazil, 2019) (CNES/Airbus, Maxar Technologies, 2019). Source: Researchdata.
The research took place in two moments: temporal analysis of the areas (between the years 2002 and 2019) carried out through the Google Earth application, through historical images obtained from satellites, and analysis of the soil indicators (chemical and biological, conducted over the period of April, May and June 2019).
Satellite images evaluation
Utilizing the Google Earth application and on-site visits, four experimental areas were selected: agricultural cultivation (Area 1 - 9°42’28.9”S, 35°48’27.02”W), deforested (Area 2 - 9°42’22.39”S, 35°48’17.23”W), civil construction/mangrove transition (Area 3 - 9°42’04.78”S, 35°48’04.16”W) and conserved mangrove (Area 4 - 9°41’56.55”S, 35°47’52.13”W).
Soil samples (sampling, preparation and analyses)
In each study area within a period of three months, soil collections were performed at a depth of 0 to 20 cm by making up simple samples (ten points from each study area) to obtain the composite samples (four samples per area) to determine the chemical indicators (pH: hydrogen potential, Na: Sodium, P: Phosphorus, K: Potassium, Ca: Calcium, Mg: Magnesium, Al: Aluminum, H: hydrogen, S: Sum of Bases, CTC: Cation Exchange Capacity, V: Ind de Sat. de Bases, MO: Organic Matter, C: carbon), according to EMBRAPA’S METHODOLOGY (1997)EMBRAPA. Manual of soil analysis methods. 2 ed. Rio de Janeiro: Revista atual, 1997. .
For biological indicators, collections of macrofauna and edaphic mesofauna were carried out in all the different study areas. As a means of capturing the macrofauna organisms, ten Provid-type traps were installed per area over the course of four days - made from a 2L plastic bottle containing 200 mL of 5% neutral detergent solution and 12 drops of formaldehyde. The mesofauna was collected close to the collection points of the macrofauna, using metallic rings with a diameter of 4.8 cm and a height of 5 cm. Identification and counting took place in the laboratory by evaluating organisms between 0.2 and 2.0 mm in length for mesofauna and > 2 mm for macrofauna. Quantitative and qualitative assessments were carried out using the Shannon (H) and Pielou (e) Diversity Indices. Also the constancy of the groups was calculated according to SILVEIRA NETO et al. (1976SILVEIRA NETO, S. et al. Insect Ecology Handbook. São Paulo: Ceres, 1 ed., 1976. 429 p.).
Statistic analyses
In order to identify the differences between the diverse collective points of the different environments, in relation to all the variables studied, the data obtained were subjected to cluster analyses such as the Scott-Knott test, and Tocher’s optimization method. This was done by using the matrix of average Euclidean distance and with standardized data as a measure of dissimilarity and principal component analysis. The GENES program (CRUZ, 2013CRUZ, C. D. Genes - a software package for analysis in experimental statistics and quantitative genetics. Acta Scientiarum. Agronomy, v.35, n.3, p.271-276, 2013. Available from: <Available from: https://doi.org/10.4025/ actasciagron.v35i3.21251 >. Accessed: Jun. 10, 2019.
https://doi.org/10.4025/ actasciagron.v3...
; CRUZ, 2016CRUZ, C. D. Genes Software-extended and integrated with the R, Matlab and Selegen. Acta Scientiarum. Agronomy, v.38, n.4, p.547-552, 2016.) and Action Stat were used to perform the statistical procedures.
RESULTS AND DISCUSSION:
Time series analysis
Through the analysis of the temporal sequence of aerial images of the Google Earth application (Figure 2), it was possible to make comparisons of the coverage of the areas of collection of this research in a seventeen-year interval (2002 to 2019).
Collection areas 1 to 4 in the years 2002, 2007, 2013 and 2019, respectively (CNES/Airbus, Maxar Technologies, 2019).
Source: Google Earth.
In the first image (year 2002) (Figure 2), the vegetation cover common to the four areas is observed, which until then were indistinct. However, occupation was already underway since 1979 when the AL 101 South Highway was built. This consequently opened clearings along the margins of the highway, as well as the formation of corridors connecting the highway to banks of the Mundaú Lagoon.
In 2007, areas 1, 2 and 4 remained with natural coverage of the environment, while area 3 suffered intervention from civil construction and the incorporation of a real estate project in the region. The residential complex in area 3 had its inauguration mark with the subdivision process started in 2006.
In 2013, the development of construction in relation to the previous record (2007) and the beginning of the modification of areas 1 and 2 could be seen. Until 2019, Area 1 had been converted into an agricultural area leased for family farming through low intensity soil management with cultivation of okra, cackrey and cowpeas. The usage of fertilizers and pesticides such as insecticides and herbicides were applied as crop management.
In the current year, 2019, the degree of devastation of the first three areas was notable respectively from agriculture, deforestation and real estate expansion. It was a slow and sequential process that involved routine burning, construction landfills, real estate pressure for the construction of high standard condominiums, and infrastructure associated with the AL 101 South Highway.
The occupation process of areas 1, 2 and 3 took into account a historical follow-up that was intensified with the opening of the AL 101 South Highway (1979) and its duplication (2012) which was the process that resulted from the occupation of the margins and the formation of real estate developments of high standards condominiums.
Through satellite images, information about areas and changes in mangroves has become more accessible (VALIELA et al., 2001VALIELA, I. et al. Mangrove forests: one of the world’s threatened major tropical environments. BioScience, v.51, n.10, p.807-815, 2001. Available from: <Available from: https://doi.org/10.1641/0006-3568(2001)051[0807:MFOOTW]2.0.CO;2 >. Accessed: Sep. 07, 2019.
https://doi.org/10.1641/0006-3568(2001)0...
). Studies carried out in different parts of the world, including Brazil, have shown the reduction of mangrove areas in some regions over the years (THOMAS et al., 2017THOMAS, N. et al. Distribution and drivers of global mangrove forest change, 1996-2010. PLoS ONE, v.12, n.6, e0179302, 2017. Available from: <Available from: https://doi.org/10.1371/journal.pone.0179302 . Accessed: Oct. 15, 2018.
https://doi.org/10.1371/journal.pone.017...
; MONDAL et al., 2018MONDAL, P. et al. Landsat-derived estimates of mangrove extents in the Sierra Leone Coastal landscape complex during 1990-2016. Sensors, 2018, 18, 12. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pubmed/29267247 >. Accessed: Oct. 10, 2019. doi: 10.3390/s18010012.
https://www.ncbi.nlm.nih.gov/pubmed/2926...
; MATIAS and SILVA, 2017MATIAS, L.; SILVA, M. D. da. Monitoring and analysis of mangrove vegetation on the south coast of Alagoas. Journal of Environmental Analysis and Progress, v.02, n.03, p.312-319, 2017. Available from: <Available from: http://dx.doi.org/10.24221/jeap.2.3.2017.1447.312-319 >. Accessed: Oct. 05, 2019.
http://dx.doi.org/10.24221/jeap.2.3.2017...
and GURGEL et al., 2015GURGEL, D. de F. et al. Assessment of Mangrove Areas with Google Earth Image Support: the case of the Environmental Protection Zone Mangrove Ecosystem and Potengi / Jundiaí Estuary. In: XXVI SIMPÓSIO DE GEOLOGIA DO NORDESTE, Natal-RN, 2015.).
A decrease in the global extension of mangroves was observed in the period 1996-2010, where the changes occurred due to anthropic practices, as well as natural aspects (THOMAS et al., 2017THOMAS, N. et al. Distribution and drivers of global mangrove forest change, 1996-2010. PLoS ONE, v.12, n.6, e0179302, 2017. Available from: <Available from: https://doi.org/10.1371/journal.pone.0179302 . Accessed: Oct. 15, 2018.
https://doi.org/10.1371/journal.pone.017...
). Changes caused by natural phenomena are short-lived. They briefly resume the structure from before on account of them being proportionately small, though the changes due to anthropic practices are long-term (MATIAS and SILVA, 2017MATIAS, L.; SILVA, M. D. da. Monitoring and analysis of mangrove vegetation on the south coast of Alagoas. Journal of Environmental Analysis and Progress, v.02, n.03, p.312-319, 2017. Available from: <Available from: http://dx.doi.org/10.24221/jeap.2.3.2017.1447.312-319 >. Accessed: Oct. 05, 2019.
http://dx.doi.org/10.24221/jeap.2.3.2017...
). Consequences such as the emission of gases and the alteration of the biological diversity of the region due to the decrease of water in the soil and biomass, come from these modifications (ALBUQUERQUE et al., 2017ALBUQUERQUE, E. Z. et al. Structure of ground-dwelling ant communities in burned and unburned areas in Brazilian subtropical grasslands. Entomological Science, v.20, n.01, p.427-436, 2017. Available from: <Available from: https://doi.org/10.1111/ens.12270 >. Accessed: Dec. 12, 2019. doi: 10.1111/ens.12270
https://doi.org/10.1111/ens.12270...
).
Chemical soil indicators
The chemical parameters of the different environments are shown in Table 1. It appears that there was significant difference between environments for all indicators, evidencing the interference of anthropic actions.
Analyzing the levels of chemical elements in the areas, greater changes were observed in the deforested area compared to the conserved mangrove area. According to some studies, fire with dead vegetation provides effects that enhance the levels of soil nutrients. This is due to ash deposition, with the record, for example, of increased phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) (BATISTA et al., 1997BATISTA, A. C. et al. Effects of prescribed burning on soil chemical properties in a loblolly pine plantation in Sengés - PR. Floresta, v.27, n.1/2, p.59-70, 1997. Available from: <Available from: http://dx.doi.org/10.5380/rf.v27i12.2298 >. Accessed: Jan. 20, 2018.
http://dx.doi.org/10.5380/rf.v27i12.2298...
; RHEINHEIMER et al., 2003RHEINHEIMER, D. dos S. et al. Changes of chemical attributes of a soil after burning its native permanent pasture. Ciência Rural, v.33, n.1, p.49-55, 2003. ISSN 0103-8478. Available from: <Available from: https://doi.org/10.1590/S0103-84782003000100008 >. Accessed: Nov. 11, 2019.
https://doi.org/10.1590/S0103-8478200300...
). Contrary values were observed in this research when lower levels of P, K, Ca and Mg were obtained in this environment. One could admit the percolation of nutrients resulting from the rain within the given period in which the research was developed, as well as the occurrence of successive fires that result in the impoverishment of the soil by including the reduction of its organic matter (REDIN et al., 2011REDIN, M. et al. Impacts of burning on chemical, physical and biological attributes of soil. Ciência Florestal, Santa Maria, v.21, n.2, p.381-392, 2011. ISSN 0103-9954. Available from: <Available from: https://doi.org/10.5902/198050983243 >. Accessed: Sep. 23, 2019.
https://doi.org/10.5902/198050983243...
; SOUZA et al., 2019SOUZA, M. A. et al. Impact of deforestation and use of fire on soil mesofauna. Braz. J. Anim. Environ. Res., Curitiba, v.2, n.6, p.1901-1906, 2019. ISSN 2595-573X. Available from: <Available from: http://www.brazilianjournals.com/index.php/BJAER/article/view/5390 >. Accessed: Jan. 04, 2020.
http://www.brazilianjournals.com/index.p...
).
The soil in the deforested area showed low pH, displaying acidity, which according to SOBRAL et al. (2015SOBRAL, L. F. et al. Practical Guide for Interpreting Soil Analysis Results. Aracaju: Embrapa Tabuleiros Costeiros, 2015.) is an indicative factor of the presence of exchangeable aluminum as the presence that was also found in this research (Table 1). The existence of exchangeable aluminum in the soil can hinder radicular growth and affect the availability of nutrients and processes, such as the mineralization of organic matter (SOBRAL et al., 2015) which also was observed in the present study.
Obtained sodium and magnesium values from the preserved mangrove indicated the characteristic salinity of this ecosystem, whose levels are higher than other environments. This characteristic affects fertility in certain soils, such as the decrease in sodium values between areas may be related to presence of fresh water in these (EBELING et al., 2008EBELING, A. G. et al. Relationship between acidity and other chemical attributes of soils with high organic matter content. Bragantia, v.67, n.2, p.429-439, 2008. Available from: <Available from: https://doi.org/10.1590/S0006-87052008000200019 >. Accessed: May, 27, 2019.
https://doi.org/10.1590/S0006-8705200800...
; FIRME, 2003FIRME, L. P. Physical-chemical characterization of mangrove soils and assessment of their contamination by domestic sewage via faecal tracers. 2003. 82 f. Thesis (Master’s degree in Agronomy) - Escola Superior de Agricultura Luiz de Queiroz, Piracicaba.).
However, when comparing the quantitative of the chemical attributes of the study with the data of PRADA-GAMERO et al. (2004PRADA-GAMERO, R. M. et al. Mineralogy and physical chemistry of mangrove soils from Iriri River at the Bertioga channel (Santos, São Paulo State, Brazil). R. Bras. Ci. Solo, vol. 28, p.233-243, 2004. Available in: <Available in: https://doi.org/10.1590/S0100-06832004000200002 >. Accessed: Sep. 22, 2019.
https://doi.org/10.1590/S0100-0683200400...
) of the mangrove study located on the margins of the Rio-Santos Highway, km 93, great variations were reportedin respect to pH, phosphorus, sodium, calcium, aluminum, and CTC. In regard to organic carbon, a value of 1.56 was obtained by this research similarly to what was obtained by BARBOSA et al. (2015BARBOSA, I. C. C. et al. Chemical composition of mangrove sediment from the Bragantino Estuary (PA) - Brazil. Rev. Virtual Quim., v.7, n.4, p. 1087-1101, 2015. Available from: <Available from: http://static.sites.sbq.org.br/rvq.sbq.org.br/pdf/v7n4a04.pdf >. Accessed: Oct. 07, 2019. doi: 10.5935/1984-6835.20150060
http://static.sites.sbq.org.br/rvq.sbq.o...
), in his research on the chemical composition of mangrove sediment from the Bragantino estuary (PA) - Brazil.
The mangrove, despite the interference of civil construction in the environment, is shown to be resilient. The construction industry impacted the existing ecosystem in interfering in the natural environment and preventing its expansion by promoting a physical barrier through the construction of the condominium. The civil construction sector causes negative disturbance to the environment from the conception of a project until after the completion of the construction due to factors such as the extraction of natural resources, topographic alteration, changes in water courses, flora and fauna, and disposition of waste (ROTH and GARCIAS, 2009ROTH, C. das G.; GARCIAS, C. M. Civil construction and environmental degradation. DESENVOLVIMENTO EM QUESTÃO, ano 7, n.13, p.111-128, 2009. Available from: <Available from: https://doi.org/10.21527/2237-6453.2009.13.111-128 >. Accessed: Jul. 17, 2019.
https://doi.org/10.21527/2237-6453.2009....
). Such factors are responsible for changes in parameters such as high organic matter content (Table 1), through the release of domestic sewage. Consequently, there are higher phosphorus values due to the possible correlation between parameters (FIRME, 2003FIRME, L. P. Physical-chemical characterization of mangrove soils and assessment of their contamination by domestic sewage via faecal tracers. 2003. 82 f. Thesis (Master’s degree in Agronomy) - Escola Superior de Agricultura Luiz de Queiroz, Piracicaba.).
Biological indicators
Analyzing the organisms present in the soil as to the degree of disturbance due to anthropic actions in the areas, a variability between them with a greater number of organisms in the soil (macro and mesofauna) of the deforested area was observed (Table 2). Due to the richness and diversity of the fauna groups, close values were reported between the areas of agricultural cultivation, deforestation and transition that demonstrated the biodiversity of the fauna communities (Araneae, Acarina, Collembola, Coleoptera, Diptera, Diplopoda, Hymenoptera, Orthoptera, etc.), with dominance of the Hymenoptera (macrofauna) and Acarina (mesofauna) groups in the deforested and burned areas.
Number of Individuals (NI), richness of fauna groups, Shannon’s diversity indexes (H’) and Pielou’s equability (e) in agricultural, deforested, transition (civil construction / mangrove) and mangrove areas.
These results are in opposition to those presented by REZENDE et al. (2017REZENDE, L. P. et al. Identification of soil macrofauna on pasture of Panicum Maximum Jacq. and area under submitted in the municipality of Sambaíba-Ma. Biodiversidade, v.16, n.1, p.155-166, 2017. Available from: <Available from: http://periodicoscientificos.ufmt.br/ojs/index.php/biodiversidade/article/view/4 980/3358 >. Accessed: Oct. 12, 2019.
http://periodicoscientificos.ufmt.br/ojs...
) and FERNANDES et al. (2009FERNANDES, R. A. et al. Iimpacts of burning to the soil properties in the semi arid of Paraíba. In: VI Congresso de Iniciação Científica da Universidade Federal de Campina Grande. Anais v.02. Pombal: UFCM, 2009.), in which they claim that the practice of burning forests brings about the loss of individuals belonging to the macrofauna. The large number of individuals in this area is justified by the resistance to the practice of burning by groups such as Acarina and Collembola, and by the presence of Hymenoptera. They are characterized as organisms that frequent environments without adequate survival conditions (SILVA et al., 2011SILVA, R. F. da et al. Impactof fire in edaphic fauna community in Eucalyptus grandis AND Pinus taeda FORESTS. R. Bras. Agrociência, Pelotas, v.17, n.2-4, p.234-241, 2011. Available from: <Available from: http://dx.doi.org/10.18539/cast.v17i2.2054 >. Accessed: Jun. 04, 2019.
http://dx.doi.org/10.18539/cast.v17i2.20...
).
Similar results were obtained by LUDWIG et al. (2012LUDWIG, R. L. et al. Effect of different systems of soil use on diversity of edaphic fauna on the central region of the Rio Grande Do SuL. ENCICLOPÉDIA BIOSFERA, Centro Científico Conhecer - Goiânia, v.8, n.14; p.485-495, 2012. Available from: <Available from: http://www.conhecer.org.br/enciclop/2012a/agrari as/efeitos%20de%20diferentes.pdf >. Accessed: Oct. 05, 2019
http://www.conhecer.org.br/enciclop/2012...
) who also assessed the diversity of edaphic fauna in different environments which included land use for agricultural activities. The same methodology was used, in which ten taxonomic groups were present (Hymenoptera, Diptera, Coleoptera, Collembola, Chilopoda, Orthoptera, Isoptera, Acarina, Araneae and Hemiptera), in addition to the predominance of the Hymenoptera and Collembola groups in the areas.
When analyzing the constancy of the fauna groups of the macrofauna and soil mesofauna, a low number of groups considered as constant (reported in more than 50% of the collection points) and a greater number of groups considered accidental (present in less than 25% of collections) is observed. This factor pointed to the need and importance of studies to monitor and map these environments to determine the groups naturally belonging to them, as well as the explanation of the occurrence of their presence due to seasonality or other factors.
However, it was reported that the dominant groups (Acarina and Collembola) are in agreement with other studies, being exposed as the most abundant organisms of the mesofauna. In the vast majority of soil types, they are considered as parameters of the biological quality of the soil (SILVA et al., 2013SILVA, A. C. F. da et al. Edaphic mesofauna in mangrove ecosystem on the south coast of Rio Grande do Norte. In: XXXIV CONGRESSO BRASILEIRO DE CIÊNCIA DO SOLO, Florianópolis-SC, 2013.). In surveys in other cultivation areas (tobacco, native field, native or reforested areas) the Acarina group has isolated appearances, while Hymenoptera, Coleoptera and Collembola are the ones with the highest occurrences (GIRACCA et al., 2003GIRACCA, E. M. N. et al. Survey of soil meso and macrofauna in arroio lino watershed, Agudo/RS. R. Bra. Agrociência, v.9, n.3, p.257-261, 2003. Available from: <Available from: http://dx.doi.org/10.18539/cast.v9i3.604 >. Accessed: Oct. 05, 2019.
http://dx.doi.org/10.18539/cast.v9i3.604...
).
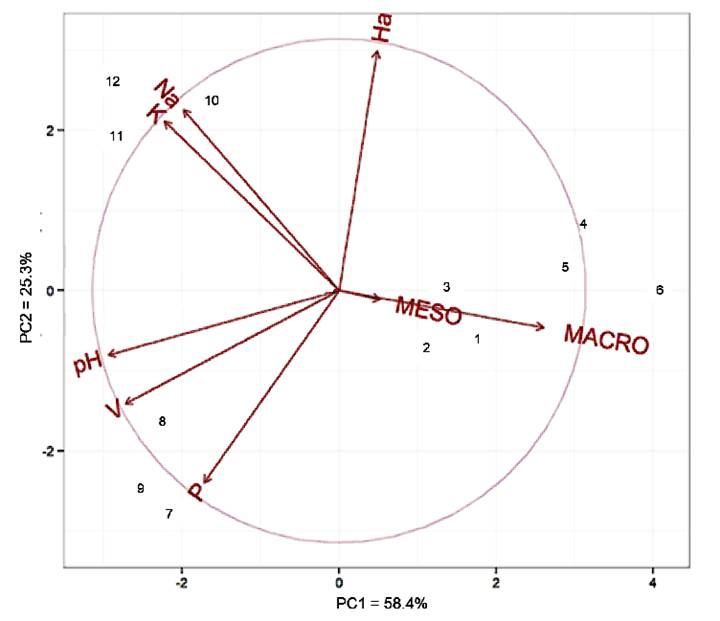
The principal component analysis showed that the first two components were explained with 83.7% of the total variance, with 58.4% on PC1 (horizontal axis) and 25.3% on PC2 (vertical axis) (Figure 3). This result can be considered satisfactory, considering that total variations above 80% obtained with the first two or three main components make it possible to analyze groups using scatter plots (CRUZ AND REGAZZI, 2001CRUZ, C. D.; REGAZZI, A. J. Biometric models applied to genetic improvement. Viçosa, MG: UFV, 2001. 390p. 2. ed. rev.). At least 70% of the total variance must be explained by the first and the second main components (RENCHER, 2005RENCHER, A. C. A review of “Methods of Multivariate Analysis”. 2005. Available in: <Available in: https://doi.org/10.1080/07408170500232784 >. Accessed: Oct. 12, 2019.
https://doi.org/10.1080/0740817050023278...
). Therefore, in thisresearch, only the first two components were used, as they are considered sufficient to explain the data and also because it facilitates the interpretation of the graph in two dimensions (GOMES et al., 2004GOMES, J. B. V. et al. Principal component analysis of physical, chemical, and mineralogical attributes of the cerrado biome soils. Revista Brasileira de Ciência do Solo, v.28, n.1, p.137-154, 2004. Available from: <Available from: https://doi.org/10.1590/S0100-06832004000100014 >. Accessed: Nov. 05, 2019.
https://doi.org/10.1590/S0100-0683200400...
).
Relationship between the main components (PC1 and PC2) and land use systems and bioindicators.
V: base saturation index, Na: sodium, P: phosphorus, K: potassium, pH: hydrogenionic potential, Ha: H + Al (hydrogen + aluminum), MESO: mesofauna, MACRO: macrofauna.
1, 2 and 3: area 1 / 4, 5 and 6: area 2 / 7, 8 and 9: area 3 / 10, 11 and 12: area 4.
Source: Research data.
The bioindicators of the edaphic fauna (mesofauna and macrofauna) proved to be of great relevance for the assessment of disturbances in the environments by presenting themselves close to the horizontal axis of the right (positive) quadrant. This results in being the axis of greatest value of the main component. This result was also demonstrated by BARTZ et al. (2014BARTZ, M. L. C. et al. The influence of land use systems on soil and surface litter fauna in the western region of Santa Catarina. Revista Ciência Agronômica, v.45, n.5 (Especial), p. 880-887, 2014. Available from: <Available from: https://doi.org/10.1590/S1806-66902014000500003 >. Accessed: Oct. 07, 2019.
https://doi.org/10.1590/S1806-6690201400...
) in his research, where he stated that the existence, plurality and abundance of edaphic fauna organisms are influenced by the use of land systems due to their sensitivity and being able to act as quality parameters.
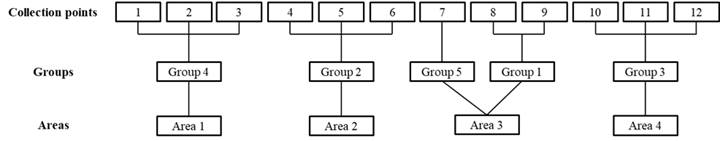
The result of the grouping using Tocher’s method is shown in Figure 4. The chemical (pH, Na, P, K, Ca, Mg, Al, H, S, CTC, V, MO and C) and biological (meso and macrofauna) attributes of the soil based on Tocher’s method, allowed the points and environments studied to be divided into five groups: the first formed by points 8 and 9 belonging to environment 3 (transition); the second through points 4, 5 and 6 belonging to environment 2 (deforested); the third group formed by points 10, 11 and 12 belonging to environment 4 (mangrove); the fourth group formed by points 1, 2 and 3 (agricultural cultivation) and the fifth group formed by point 7 (transition). It appears that only point 7 was outside the expected, forming an isolated group when it should have been part of group 1 (transition). It was reported that the variables mesofauna, aluminum, and macrofauna were the ones that contributed the most to the distinction of groups (environments) with 16.7%, 18.2% and 19.7% respectively. The groups formed using Tocher’s method were, in part, confirmed through the ACP.
Clusters between the twelve points from the four areas, obtained by the Tocher method, based on the standardized average Euclidean distance, considering all variables related to soil quality1. 1pH, sodium, phosphorus, potassium, calcium, magnesium, aluminum, hydrogen, sum of bases, cation exchange capacity, ind. of sat. of bases, organic matter, carbon, meso and macrofauna. Source: Research data.
CONCLUSION:
Anthropic actions caused successive changes in the environment. As these are permanent changes that affect the type of soil and its coverage. Significant differences were obtained between the chemical and biological characteristics of the four environments, with the use of different systems in which a few years ago belonged to the mangrove as their singular ecosystem.
The mangrove has suffered negative impacts due to inadequate management and soil occupation, demonstrated by the chemical alteration of the soil and modification of the landscape. This has lead to the degradation process. The continuity of anthropic intervention in the mangrove will promote the disappearance of this ecosystem in the long term.
Among the chemical and biological attributes used for the performed analyses, aluminum and edaphic organisms were the ones that allowed the greatest contribution to discriminate the degree of disturbance in areas of agricultural cultivation, deforestation and civil construction/mangrove transition.
ACKNOWLEDGEMENTS
To the O Laboratório de Ecogeografia e Sustentabilidade Ambiental (LabESA) of the Universidade Federal do Alagoas (UFAL), to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) and to the Graduate Programa de Pós-graduação em Análise de Sistemas Ambientais (PPGASA) of Centro Universitário Cesmac.
REFERENCES
- ALBUQUERQUE, E. Z. et al. Structure of ground-dwelling ant communities in burned and unburned areas in Brazilian subtropical grasslands. Entomological Science, v.20, n.01, p.427-436, 2017. Available from: <Available from: https://doi.org/10.1111/ens.12270 >. Accessed: Dec. 12, 2019. doi: 10.1111/ens.12270
» https://doi.org/10.1111/ens.12270» https://doi.org/10.1111/ens.12270 - BARBOSA, I. C. C. et al. Chemical composition of mangrove sediment from the Bragantino Estuary (PA) - Brazil. Rev. Virtual Quim., v.7, n.4, p. 1087-1101, 2015. Available from: <Available from: http://static.sites.sbq.org.br/rvq.sbq.org.br/pdf/v7n4a04.pdf >. Accessed: Oct. 07, 2019. doi: 10.5935/1984-6835.20150060
» https://doi.org/10.5935/1984-6835.20150060» http://static.sites.sbq.org.br/rvq.sbq.org.br/pdf/v7n4a04.pdf - BARTZ, M. L. C. et al. The influence of land use systems on soil and surface litter fauna in the western region of Santa Catarina. Revista Ciência Agronômica, v.45, n.5 (Especial), p. 880-887, 2014. Available from: <Available from: https://doi.org/10.1590/S1806-66902014000500003 >. Accessed: Oct. 07, 2019.
» https://doi.org/10.1590/S1806-66902014000500003 - BATISTA, A. C. et al. Effects of prescribed burning on soil chemical properties in a loblolly pine plantation in Sengés - PR. Floresta, v.27, n.1/2, p.59-70, 1997. Available from: <Available from: http://dx.doi.org/10.5380/rf.v27i12.2298 >. Accessed: Jan. 20, 2018.
» http://dx.doi.org/10.5380/rf.v27i12.2298 - BIBI, SN. Et al. Ethnopharmacology, Phytochemistry, and global distribution of mangroves - A comprehensive Review. Marine Drugs, 2019, 17, 231. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pubmed/31003533 >. Accessed: Jun. 10, 2019. doi: 10.3390/md17040231.
» https://doi.org/10.3390/md17040231.» https://www.ncbi.nlm.nih.gov/pubmed/31003533 - CRUZ, C. D. Genes - a software package for analysis in experimental statistics and quantitative genetics. Acta Scientiarum. Agronomy, v.35, n.3, p.271-276, 2013. Available from: <Available from: https://doi.org/10.4025/ actasciagron.v35i3.21251 >. Accessed: Jun. 10, 2019.
» https://doi.org/10.4025/ actasciagron.v35i3.21251 - CRUZ, C. D. Genes Software-extended and integrated with the R, Matlab and Selegen. Acta Scientiarum. Agronomy, v.38, n.4, p.547-552, 2016.
- CRUZ, C. D.; REGAZZI, A. J. Biometric models applied to genetic improvement. Viçosa, MG: UFV, 2001. 390p. 2. ed. rev.
- EBELING, A. G. et al. Relationship between acidity and other chemical attributes of soils with high organic matter content. Bragantia, v.67, n.2, p.429-439, 2008. Available from: <Available from: https://doi.org/10.1590/S0006-87052008000200019 >. Accessed: May, 27, 2019.
» https://doi.org/10.1590/S0006-87052008000200019 - EMBRAPA. Manual of soil analysis methods. 2 ed. Rio de Janeiro: Revista atual, 1997.
- FERNANDES, R. A. et al. Iimpacts of burning to the soil properties in the semi arid of Paraíba. In: VI Congresso de Iniciação Científica da Universidade Federal de Campina Grande. Anais v.02. Pombal: UFCM, 2009.
- FIRME, L. P. Physical-chemical characterization of mangrove soils and assessment of their contamination by domestic sewage via faecal tracers. 2003. 82 f. Thesis (Master’s degree in Agronomy) - Escola Superior de Agricultura Luiz de Queiroz, Piracicaba.
- GASPARINETTI, P.; The values of ecosystem services of Brazilian mangroves, economic instruments for their conservation and the case study of Salgado Paraense. Conservation Strategy Fund (CSF), 2018. Available from: <Available from: https://www.conservation-strategy.org/sites/default/files/field-file/PORT_DP_Os_valores_dos_servicos_ecossistemicos_dos_manguezais_brasileiros_Abr_2018.pdf >. Accessed: Nov. 02, 2019.
» https://www.conservation-strategy.org/sites/default/files/field-file/PORT_DP_Os_valores_dos_servicos_ecossistemicos_dos_manguezais_brasileiros_Abr_2018.pdf - GIRACCA, E. M. N. et al. Survey of soil meso and macrofauna in arroio lino watershed, Agudo/RS. R. Bra. Agrociência, v.9, n.3, p.257-261, 2003. Available from: <Available from: http://dx.doi.org/10.18539/cast.v9i3.604 >. Accessed: Oct. 05, 2019.
» http://dx.doi.org/10.18539/cast.v9i3.604 - GIRI, C. et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography, v.20, n.1, p.154-159, 2010. Available from: <Available from: https://doi.org/10.1111/j.1466-8238.2010.00584.x >. Accessed: Mar. 05, 2019. doi: 10.1111/j.1466-8238.2010.00584.x
» https://doi.org/10.1111/j.1466-8238.2010.00584.x» https://doi.org/10.1111/j.1466-8238.2010.00584.x - GOMES, J. B. V. et al. Principal component analysis of physical, chemical, and mineralogical attributes of the cerrado biome soils. Revista Brasileira de Ciência do Solo, v.28, n.1, p.137-154, 2004. Available from: <Available from: https://doi.org/10.1590/S0100-06832004000100014 >. Accessed: Nov. 05, 2019.
» https://doi.org/10.1590/S0100-06832004000100014 - GOOGLE. Google Earth Pro. Available in: <Available in: https://www.google.com/intl/pt-BR/earth/desktop/ >. Accessed: Feb. 10, 2019.
» https://www.google.com/intl/pt-BR/earth/desktop/ - GURGEL, D. de F. et al. Assessment of Mangrove Areas with Google Earth Image Support: the case of the Environmental Protection Zone Mangrove Ecosystem and Potengi / Jundiaí Estuary. In: XXVI SIMPÓSIO DE GEOLOGIA DO NORDESTE, Natal-RN, 2015.
- LUDWIG, R. L. et al. Effect of different systems of soil use on diversity of edaphic fauna on the central region of the Rio Grande Do SuL. ENCICLOPÉDIA BIOSFERA, Centro Científico Conhecer - Goiânia, v.8, n.14; p.485-495, 2012. Available from: <Available from: http://www.conhecer.org.br/enciclop/2012a/agrari as/efeitos%20de%20diferentes.pdf >. Accessed: Oct. 05, 2019
» http://www.conhecer.org.br/enciclop/2012a/agrari as/efeitos%20de%20diferentes.pdf - MATIAS, L.; SILVA, M. D. da. Monitoring and analysis of mangrove vegetation on the south coast of Alagoas. Journal of Environmental Analysis and Progress, v.02, n.03, p.312-319, 2017. Available from: <Available from: http://dx.doi.org/10.24221/jeap.2.3.2017.1447.312-319 >. Accessed: Oct. 05, 2019.
» http://dx.doi.org/10.24221/jeap.2.3.2017.1447.312-319 - MINISTÉRIO DO MEIO AMBIENTE (MMA); INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE (ICMBIO). THE BRAZILIAN MANGROVES ATLAS. Brasília: [s.n.], p.43-88, 2018.
- MONDAL, P. et al. Landsat-derived estimates of mangrove extents in the Sierra Leone Coastal landscape complex during 1990-2016. Sensors, 2018, 18, 12. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pubmed/29267247 >. Accessed: Oct. 10, 2019. doi: 10.3390/s18010012.
» https://doi.org/10.3390/s18010012.» https://www.ncbi.nlm.nih.gov/pubmed/29267247 - PRADA-GAMERO, R. M. et al. Mineralogy and physical chemistry of mangrove soils from Iriri River at the Bertioga channel (Santos, São Paulo State, Brazil). R. Bras. Ci. Solo, vol. 28, p.233-243, 2004. Available in: <Available in: https://doi.org/10.1590/S0100-06832004000200002 >. Accessed: Sep. 22, 2019.
» https://doi.org/10.1590/S0100-06832004000200002 - REDIN, M. et al. Impacts of burning on chemical, physical and biological attributes of soil. Ciência Florestal, Santa Maria, v.21, n.2, p.381-392, 2011. ISSN 0103-9954. Available from: <Available from: https://doi.org/10.5902/198050983243 >. Accessed: Sep. 23, 2019.
» https://doi.org/10.5902/198050983243 - RENCHER, A. C. A review of “Methods of Multivariate Analysis”. 2005. Available in: <Available in: https://doi.org/10.1080/07408170500232784 >. Accessed: Oct. 12, 2019.
» https://doi.org/10.1080/07408170500232784 - REZENDE, L. P. et al. Identification of soil macrofauna on pasture of Panicum Maximum Jacq. and area under submitted in the municipality of Sambaíba-Ma. Biodiversidade, v.16, n.1, p.155-166, 2017. Available from: <Available from: http://periodicoscientificos.ufmt.br/ojs/index.php/biodiversidade/article/view/4 980/3358 >. Accessed: Oct. 12, 2019.
» http://periodicoscientificos.ufmt.br/ojs/index.php/biodiversidade/article/view/4 980/3358 - RHEINHEIMER, D. dos S. et al. Changes of chemical attributes of a soil after burning its native permanent pasture. Ciência Rural, v.33, n.1, p.49-55, 2003. ISSN 0103-8478. Available from: <Available from: https://doi.org/10.1590/S0103-84782003000100008 >. Accessed: Nov. 11, 2019.
» https://doi.org/10.1590/S0103-84782003000100008 - ROTH, C. das G.; GARCIAS, C. M. Civil construction and environmental degradation. DESENVOLVIMENTO EM QUESTÃO, ano 7, n.13, p.111-128, 2009. Available from: <Available from: https://doi.org/10.21527/2237-6453.2009.13.111-128 >. Accessed: Jul. 17, 2019.
» https://doi.org/10.21527/2237-6453.2009.13.111-128 - SEMADS. Secretaria de Estado de Meio Ambiente e Desenvolvimento Sustentável. MANGROVES EDUCATE TO PROTECT. SEA STUDIES FOUNDATION, 2001. Available from: <Available from: https://www.mma.gov.br/estruturas/sqa_pnla/_arquivos/manguezais.pdf >. Accessed: Nov. 02, 2019.
» https://www.mma.gov.br/estruturas/sqa_pnla/_arquivos/manguezais.pdf - SEMARH. Secretaria de Estado do Meio Ambiente e dos Recursos Hídricos. Monthly Precipitation Data -2014. 2014. Available from: <Available from: http://dados.al.gov.br/dataset/7255537c-bc40-4c79-841d-e3977f6ab2f9/resource/16d07384-32a5-4896-926d-57c7bb6ab747/download/dadosmensaisdez2014.pdf >. Accessed: Oct. 31, 2019.
» http://dados.al.gov.br/dataset/7255537c-bc40-4c79-841d-e3977f6ab2f9/resource/16d07384-32a5-4896-926d-57c7bb6ab747/download/dadosmensaisdez2014.pdf - SILVA, A. C. F. da et al. Edaphic mesofauna in mangrove ecosystem on the south coast of Rio Grande do Norte. In: XXXIV CONGRESSO BRASILEIRO DE CIÊNCIA DO SOLO, Florianópolis-SC, 2013.
- SILVA, R. F. da et al. Impactof fire in edaphic fauna community in Eucalyptus grandis AND Pinus taeda FORESTS. R. Bras. Agrociência, Pelotas, v.17, n.2-4, p.234-241, 2011. Available from: <Available from: http://dx.doi.org/10.18539/cast.v17i2.2054 >. Accessed: Jun. 04, 2019.
» http://dx.doi.org/10.18539/cast.v17i2.2054 - SILVEIRA NETO, S. et al. Insect Ecology Handbook. São Paulo: Ceres, 1 ed., 1976. 429 p.
- SOBRAL, L. F. et al. Practical Guide for Interpreting Soil Analysis Results. Aracaju: Embrapa Tabuleiros Costeiros, 2015.
- SOUZA, M. A. et al. Impact of deforestation and use of fire on soil mesofauna. Braz. J. Anim. Environ. Res., Curitiba, v.2, n.6, p.1901-1906, 2019. ISSN 2595-573X. Available from: <Available from: http://www.brazilianjournals.com/index.php/BJAER/article/view/5390 >. Accessed: Jan. 04, 2020.
» http://www.brazilianjournals.com/index.php/BJAER/article/view/5390 - THOMAS, N. et al. Distribution and drivers of global mangrove forest change, 1996-2010. PLoS ONE, v.12, n.6, e0179302, 2017. Available from: <Available from: https://doi.org/10.1371/journal.pone.0179302 Accessed: Oct. 15, 2018.
» https://doi.org/10.1371/journal.pone.0179302 - VALIELA, I. et al. Mangrove forests: one of the world’s threatened major tropical environments. BioScience, v.51, n.10, p.807-815, 2001. Available from: <Available from: https://doi.org/10.1641/0006-3568(2001)051[0807:MFOOTW]2.0.CO;2 >. Accessed: Sep. 07, 2019.
» https://doi.org/10.1641/0006-3568(2001)051[0807:MFOOTW]2.0.CO;2
-
CR-2020-0356.R2
Publication Dates
-
Publication in this collection
28 Aug 2020 -
Date of issue
2020
History
-
Received
19 Apr 2020 -
Accepted
15 June 2020 -
Reviewed
30 July 2020