ABSTRACT:
Studies on the fungal microbiota of reptiles and amphibians are necessary to better understand of host-microbe interactions and the establishment of fungal disease in these animals. However, these studies are limited. The present researchidentified yeasts from free-ranging reptiles and amphibians from the Caatinga biome andevaluated the virulence factors production, the antifungal susceptibility in planktonic and biofilm growth and the pathogenicity of Candida famata isolates. Twenty-nine isolates of the genera Candida, Cryptococcus and Rhodotorula were identified by phenotypic and/or molecular methods and production of hydrolytic enzymes in vitro by these genera of fungi was evaluated. In addition, susceptibility of planktonic cells and biofilms to azoles and amphotericin B was evaluated. The pathogenicity of C. famata, the most prevalent yeast species isolated, was evaluated using Caenorhabditis elegans model. C. famata was the most prevalent yeast in amphibian and reptilian microbiota. Phospholipase and protease production was observed in 18/29 and 11/29 of the yeast isolates, respectively, while 100% formed biofilms. Itraconazole presented high minimal inhibitory concentrations against C. famata and C. tropicalis. Amphotericin B reduced the biomass and metabolic activity of biofilms. C. famata induced the mortality of C. elegans. In conclusion, reptiles and amphibians are colonized by yeasts capable of producing important virulence factors, especially by Candida spp. that present low susceptibility to azoles which may result from imbalances in ecosystem. Finally, C. famata isolated from these animals presented high pathogenicity, showing the importance of the study of reptile and amphibians fungal microbiota.
Key words:
herpetofauna; yeasts; antifungal; virulence; pathogenicity
RESUMO:
Estudos sobre a microbiota fúngica de répteis e anfíbios são necessários para melhor compreender as interações hospedeiro-microrganismo e o estabelecimento de doenças fúngicas nesses animais. No entanto, esses estudos são limitados. O objetivo da presente pesquisa foi identificar leveduras isoladas de répteis e anfíbios do bioma Caatinga e avaliar a produção de fatores de virulência, a sensibilidade a antifúngicos no crescimento planctônico e de biofilme e a patogenicidade de Candida famata. Vinte e nove isolados dos gêneros Candida, Cryptococcus e Rhodotorula foram identificados por métodos fenotípicos e/ou moleculares e a produção de enzimas hidrolíticas in vitro por esses gêneros de fungos foi avaliada. Além disso, foi avaliada a suscetibilidade de células planctônicas e biofilmes a azólicos e anfotericina B. A patogenicidade de C. famata, a espécie de levedura isolada mais prevalente, foi avaliada usando Caenorhabditis elegans. C. famata foi a levedura mais prevalente na microbiota de anfíbios e répteis. A produção de fosfolipase e protease foi observada em 18/29 e 11/29 dos isolados de levedura, respectivamente, enquanto 100% formaram biofilmes. O itraconazol apresentou altas concentrações inibitórias mínimas contra C. famata e C. tropicalis. A anfotericina B reduziu a biomassa e atividade metabólica dos biofilmes. C. famata induziu a mortalidade de C. elegans. Em conclusão, répteis e anfíbios são colonizados por leveduras capazes de produzir importantes fatores de virulência, especialmente por cepas de Candida spp. que apresentam baixa suscetibilidade a azólicos que podem resultar de desequilíbrio no ecossistema. Por fim, C. famata isolados desses animais apresentaram alta patogenicidade, mostrando a importância do estudo da microbiota fúngica de répteis e anfíbios.
Palavras-chave:
herpetofauna; leveduras; antifúngico; virulência; patogenicidade
INTRODUCTION:
Herpetology is the strand of zoology that studies amphibians and reptiles, by researches on their evolution, biology, behavior, husbandry, among others (BORGES-LEITE; RODRIGUES; BORGES-NOJOSA, 2014BORGES-LEITE, M. J.; RODRIGUES, J. F. M.; BORGES-NOJOSA, D. M. Herpetofauna of a coastal region of northeastern Brazil. Herpetology Notes, 2014. v.7, p.405-413.). As part of the studies focusing on the understanding of the biology and health of these animals, in free-ranging and captive conditions, researches aiming the characterization of their bacterial (NOWAKIEWICZ et al., 2015NOWAKIEWICZ, A. et al. Aerobic bacterial microbiota isolated from the cloaca of the European pond turtle (Emys orbicularis) in Poland. Journal of wildlife diseases, 2015. v.51, n.1, p.255-259. Available from: <Available from: https://meridian.allenpress.com/jwd/article/51/1/255/122437/Aerobic-Bacterial-Microbiota-Isolated-from-the >. Accessed: Feb. 20, 2020. doi: 10.7589/2013-07-157.
https://meridian.allenpress.com/jwd/arti...
; VEGA-MANRIQUEZ et al., 2018VEGA-MANRIQUEZ, D. X. et al. Identification of bacteria present in ulcerative stomatitis lesions of captive sea turtles Chelonia mydas. Veterinary research communications, 2018. v.42, n.3, p.251-254. Available from: <Available from: https://link.springer.com/article/10.1007%2Fs11259-018-9728-y >. Accessed: Jun. 02, 2020. doi: 10.1007/s11259-018-9728-y.
https://link.springer.com/article/10.100...
; CARDOSO-BRITO et al., 2019CARDOSO-BRITO, V. et al. Conjunctival bacterial flora and antimicrobial susceptibility of captive and free‐living sea turtles in Brazil. Veterinary ophthalmology, 2019. v.22, n.3, p.246-255. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/vop.12584 >. Accessed: Apr. 15, 2020. doi: 10.1111/vop.12584.
https://onlinelibrary.wiley.com/doi/abs/...
) and fungal (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
; BRILHANTE et al., 2015BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
https://link.springer.com/article/10.100...
a; SVEDESE et al., 2017SVEDESE, V. M. et al. Fungal microbiota from the oral mucosa of sympatric lizards from the Brazilian semiarid region. Herpetological Review , 2017. v.48, n.3, p.538-541. Available from: <Available from: https://www.researchgate.net/profile/Leonardo_Ribeiro3/publication/319950839_Fungal_microbiota_from_the_oral_mucosa_of_sympatric_lizards_from_the_Brazilian_semiarid_region/links/59c315ce458515af3060caf2/Fungal-microbiota-from-the-oral-mucosa-of-sympatric-lizards-from-the-Brazilian-semiarid-region.pdf >. Accessed: Jun. 20, 2020.
https://www.researchgate.net/profile/Leo...
) microbibota have been performed. These studies are essential to better understand the host-microbe interactions, considering that bacteria and fungi from the microbiota may become pathogenic, under specific circumstances, including the occurrence of natural and anthropogenic stressors in the environment (HERNÁNDEZ-GÓMEZ et al., 2020HERNÁNDEZ-GÓMEZ, O.; WUERTHNER, V.; HUA, J. Amphibian host and skin microbiota response to a common agricultural antimicrobial and internal parasite. Microbial ecology, 2020. v.79, n.1, p.175-191. Available from: <Available from: https://link.springer.com/article/10.1007%2Fs00248-019-01351-5 >. Accessed: May, 04, 2020. doi: 10.1007/s00248-019-01351-5.
https://link.springer.com/article/10.100...
).
In this context, several fungal diseases in reptiles and amphibians have been described, such as gastrointestinal and pulmonary candidiasis in turtles and Batrachochytrium dendrobatidis infections in amphibians, which are responsible for the population decline of several species (KOLBY et al., 2015KOLBY, J. E. et al. Presence of amphibian chytrid fungus (Batrachochytrium dendrobatidis) in rainwater suggests aerial dispersal is possible. Aerobiologia, 2015. v.31, n.3, p.411-419. Available from: <Available from: https://link.springer.com/article/10.1007/s10453-015-9374-6 >. Accessed: Mar. 04, 2020. doi: 10.1007/s10453-015-9374-6.
https://link.springer.com/article/10.100...
; BOSCH et al., 2017BOSCH, R. A. et al. First Reports of Tadpole Mouthpart Anomalies in a Cuban Toad (Anura: Bufonidae: Peltophryne). Herpetological Review, 2017. v.48, n.1, p.58-62.). However, studies on the fungal microbiota of these animals are limited (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
; BRILHANTE et al., 2015BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
https://link.springer.com/article/10.100...
a).
Fungal pathogenicity is associated with the production of virulence factors, such as phospholipases and proteases, as well as biofilm formation. Many of these factors have already been described in yeasts from other animal species (SIDRIM et al., 2016______ et al. Antifungal Resistance and Virulence Among Candida spp. from Captive Amazonian manatees and West Indian Manatees: Potential Impacts on Animal and Environmental Health. EcoHealth, 2016. v.13, n.2, p.328-338. Available from: <https://link.springer.com/article/10.1007%2Fs10393-015-1090-8>. Accessed: May, 05, 2020. doi: 10.1007/s10393-015-1090-8.
https://link.springer.com/article/10.100...
; ROCHA et al., 2017ROCHA, M. F. G. et al. Azole resistance in Candida albicans from animals: Highlights on efflux pump activity and gene overexpression. Mycoses , 2017. v.60, n.7, p.462-468. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12611 >. Accessed: 4 mar. 2020. doi: 10.1111/myc.12611.
https://onlinelibrary.wiley.com/doi/abs/...
). In addition to these virulence factors, decreased susceptibility to fluconazole, itraconazole and amphotericin B has also been described among yeasts from different animal species, highlighting the importance of evaluating the antifungal susceptibility of animal isolates (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
; BRILHANTE et al., 2015BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
https://link.springer.com/article/10.100...
a).
This study identified yeasts from free-ranging reptiles and amphibians from the Caatinga biome. Afterwards, phospholipase and protease production and biofilm formation by these fungi were evaluated, as well as their antifungal susceptibility in planktonic and biofilm growth. Finally, the pathogenicity of C. famata in Caenorhabditis elegans infection model was also investigated.
MATERIALS AND METHODS:
Strains
The yeasts of this study are part of the fungal collection of the Specialized Medical Mycology Center (CEMM) of Federal University of Ceará (UFC). The genera Candida, Cryptococcus and Rhodotorula were included. They were recovered from the oral cavity and cloaca of free-ranging reptiles and amphibians, and the skin surface of amphibians. Samples were collected from seven reptile species (Lygophis dilepis, Boa constrictor, Micrurus ibiboboca, Psomorphis jeobetis, Iguana iguana and Tropidurus hispidus) and six amphibian species (Rhinella granulosa, Rhinella jimi, Leptodactylus latrans, Leptodactylus vastus, Leptodactylus sp. and Pithecopus nordetinus). This study was carried out under authorization of the Chico Mendes Institute of Biodiversity (ICMBio) under protocol number SISBIO-51820-1.
Phenotypic and molecular identification
Yeasts were grown on Sabouraud agar supplemented with chloramphenicol, and, after 48 hours, colonies suggestive of Candida were seeded on CHROMagar Candida ® to verify their purity. The yeasts were submitted to the following biochemical tests: urease production on Christensen’s urea agar; carbon and nitrogen assimilation. The yeast micromorphology was then evaluated by Dalmau slide culture on cornmeal-Tween-80 agar (DE HOOG et al., 2000HOOG, G. S. DE et al. Atlas of clinical fungi. 2. ed. Baarn: The Nederlands: Centraalbureau voor Schimmslcultures, 2000.; KURTZMAN et al., 2010KURTZMAN, C. P.; FELL, J. W.; BOEKHOUT, T. The yeasts: a taxonomic study. [S.l.]: Elsevier, 2010.).
C. famata and C. parapsilosis colonies were purified on CHROMagar Candida® and grown on YEPD agar (1% yeast extract, 2% dextrose, 2% peptone) and C. neoformans was grown on potato dextrose agar. After 48 hour, an inoculum was prepared from each sample in sterile milli-Q water and submitted to DNA extraction. The genetic material extraction was done with the High Pure PCR Template Preparation kit (Roche Applied Science kit, Germany) (CORDEIRO et al., 2018______ et al. Phenotype-driven strategies for screening Candida parapsilosis complex for molecular identification. Brazilian Journal of Microbiology, 2018. v.49, p.193-198. Available from: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328843/>. Accessed: Mar. 04, 2020. doi: 10.1016/j.bjm.2017.11.004.
https://www.ncbi.nlm.nih.gov/pmc/article...
).
The method of TAVANTI et al. (2005TAVANTI, A. et al. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. Journal of Clinical Microbiology , jan. 2005. v.43, n.1, p.284-292. Available from: <Available from: https://jcm.asm.org/content/43/1/284.long >. Accessed: Mar. 04, 2020. doi: 10.1128/JCM.43.1.284-292.2005.
https://jcm.asm.org/content/43/1/284.lon...
) was used for molecular identification of the cryptic species of the C. parapsilosis complex. The amplification of the SADH gene (5’-GTTGATGCTTTGGATTGT-3’) was carried out by polymerase chain reaction (PCR). The products of this PCR were digested by the enzyme BanI (TermoLab) for up to 16 h at 37 °C. Afterwards, DNA products were analyzed by electrophoresis in 2% agarose gel to visualize the obtained digestion patterns and identify the strains according to the number and size of the DNA bands (CORDEIRO et al., 2018______ et al. Phenotype-driven strategies for screening Candida parapsilosis complex for molecular identification. Brazilian Journal of Microbiology, 2018. v.49, p.193-198. Available from: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328843/>. Accessed: Mar. 04, 2020. doi: 10.1016/j.bjm.2017.11.004.
https://www.ncbi.nlm.nih.gov/pmc/article...
).
C. famata complex was identified according to FENG et al. (2014FENG, X. et al. Development of two molecular approaches for differentiation of clinically relevant yeast species closely related to Candida guilliermondii and Candida famata. Journal of Clinical Microbiology, 2014. v.52, n.9, p.3190-3195. Available from: <Available from: https://jcm.asm.org/content/52/9/3190.long >. Accessed: Mar. 04, 2020. doi: 10.1128/JCM.01297-14.
https://jcm.asm.org/content/52/9/3190.lo...
), by PCR amplification using the primers ITS2F (5’-GATGTATTAGGTAGGTTTATCCAACTCGT-3’) and 26SR (5’-TCATTTCAACCCCAATACCTC-3’). Afterwards, the amplicon was digested with BsaHI and XbaI, at 37 ºC, for up to 16 hours. Finally, the digestion products were submitted to agarose gel electrophoresis. C. famata presents three DNA bands (447 bp, 375 bp and 290 bp), Debaryomyces nepalensis four bands (447 bp, 375 bp, 214 bp and 76 bp) and C. palmioleopbhila two bands (660 bp and 446 bp) (BRILHANTE et al., 2016a).
C. neoformans was identified according to the methodology described by VELEGRAKI et al. (2001VELEGRAKI, A. et al. Prospective use of RFLP analysis on amplified Cryptococcus neoformans URA5 gene sequences for rapid identification of varieties and serotypes in clinical samples. Medical Mycology , 2001. v.39, n.5, p.409-417. Available from: <Available from: https://academic.oup.com/mmy/article/39/5/409/960965 >. Accessed: Mar. 04, 2020. doi: 10.1080/mmy.39.5.409.417.
https://academic.oup.com/mmy/article/39/...
) by the restriction fragment length polymorphism (RFLP) of the amplicon of the primers for the gene URA5 (forward: 5’-ACGGTGAGGGCGGTACTATG-3’; reverse: 5’-AAGACCTCTGAACACCGTAC-3’).C. neoformans complex genotypes were identified based on the obtained digestion pattern with the AluI restriction enzyme at 37 ºC, for up to 16 hours. Digestion products were submitted to agarose gel electrophoresis and restriction patterns were analyzed.
Phospholipase and protease production assay
The test was performed based on the method described by SIDRIM et al. (2010SIDRIM, J. J. C. et al. Candida species isolated from the gastrointestinal tract of cockatiels (Nymphicus hollandicus): in vitro antifungal susceptibility profile and phospholipase activity. Veterinary microbiology, 2010. v.145, n.3, p.324-328. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378113510001872?via%3Dihub >. Accessed: Feb. 01, 2020. doi: 10.1016/j.vetmic.2010.04.006.
https://www.sciencedirect.com/science/ar...
). Egg yolk agar medium, prepared with 2% Sabouraud dextrose agar, supplemented with 1 mol/L of sodium chloride, 0.05 mol/L of calcium chloride and 8% sterile egg yolk emulsion, was used. The egg yolk emulsion was preheated to 40 °C in a water bath and then incorporated into the sterile medium. After preparation, the medium was distributed into Petri dishes (90 mm), forming a 4 mm layer. Thus, a fungal inocula at a turbidity of 5 on McFarland scale were prepared in 0.9% sterile saline solution, from yeast colonies with 24 to 72 hours of growth. Then, 10 μL of the inoculum were added to sterile filter paper disks (5 mm) and placed on the surface of the agar. Finally, the plates were incubated for seven days, with daily observation, and reading was based on the measurement of the precipitation zone (opaque halo) around the yeast colony. Phospholipase activity was expressed as PHz, which represents the ratio between colony diameter and precipitation zone diameter (PRICE at al., 1982PRICE, M. F.; WILKINSON, I. D.; GENTRY, L. O. Plate method for detection of phospholipase activity in Candida albicans. Medical Mycology, 1982. v.20, n.1, p.7-14. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/7038928/ >. Accessed: Sep. 01, 2019. doi: 10.1080/00362178285380031.
https://pubmed.ncbi.nlm.nih.gov/7038928/...
). When PHz = 1, the isolate was negative for the production of extracellular phospholipases, but if PHz <1, the isolate was positive for phospholipase production, where PHz >0.64 indicates weak enzymatic activity, while PHz <0.64 indicates strong enzymatic production. C. albicans ATCC 10231 was used as positive control for phospholipase production.
Protease production was evaluated according to CORDEIRO et al. (2015CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
https://academic.oup.com/mmy/article/53/...
). For this purpose, bovine serum albumin agar (BSA; Sigma, USA) at pH 3.5 was used. For each tested yeast, fungal inoculum in saline (0.9%) was adjusted to 5 on McFarland scale, after which 10 μL of the inoculum were added to sterile paper discs and transferred to the surface of the BSA agar. Plates were incubated at 35 °C, for 5 days. The proteolytic activity was given by PRz, defined as the ratio between the diameter of the yeast colony and total diameter (colony plus proteolysis zone). Thus, PRz <1 indicates the presence of protease activity, while PRz = 1 indicates the absence of enzymatic activity. C. albicans strain ATCC 10231 was used as positive control for protease production.
Biofilm formation
The method described by CORDEIRO et al. (2015CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
https://academic.oup.com/mmy/article/53/...
) was used for biofilm production. A total of 29 isolates (Candida spp., C. neoformans and Rhodotorula rubra) were used in this test. Yeast cells were resuspended in RPMI 1640 broth (Sigma, USA) and the suspension was adjusted to the concentration of 1 × 106 cells/mL. Subsequently, 200 μL of the inoculum were transferred to 96-well flat-bottomed polystyrene plates, which were incubated at 35 °C for 48 hours. Wells containing only culture medium without inoculum were used as negative control. The whole experiment was carried out in triplicate. After the incubation, the supernatant was carefully aspirated, and the wells were washed twice with PBS-Tween 20 (0.05%). Subsequently, the wells were washed with 100 μL of 100% methanol and the supernatant was aspirated. After drying, 100 μL of 0.3% crystal violet was added to each well. After 20 minutes, the dye solution was aspirated, and the wells were washed twice with sterile distilled water. Then, 150 μL of a 33% acetic acid solution was added to the wells and left for 30 seconds. The volume was then transferred to a new 96-well plate, which was read immediately using a spectrophotometer at 590 nm to obtain optical density (OD) values. The cutoff values (ODc) for the biofilm formation assay were defined as three standard deviations above the mean OD of the negative control. Strains were classified as non-biofilm producers (OD≤ODc), weak producers (ODc<OD≤2xODc), moderate producers (2xODc<OD≤4xODc) or strong producers (OD>4xODc).
Planktonic antifungal susceptibility assay
To evaluate the antifungal susceptibility of yeasts (Candida spp., C. neoformans and R. rubra; n=29) in planktonic growth, the broth microdilution assay standardized by the Clinical Laboratory Standards Institute, document M27-A3 was applied (CLSI, 2008CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard - third edition. M27-A3. ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2008.). For this purpose, RPMI 1640 broth was used. The antifungals used were fluconazole (FLC, 0.125-64 μg/mL; Sigma Chemical Corporation, USA) itraconazole (ITC, 0.03215-16 μg/mL; Sigma Chemical Corporation, USA), voriconazole (VRC, 0.03215-16 μg/mL Sigma Chemical Corporation, USA) and amphotericin B (AMB, 0.03125-16 μg/mL; Sigma Chemical Corporation, USA). Tests were performed in 96-well polypropylene plates. Cells were suspended in saline solution (0.9%) until reaching 0.5 turbidity on the McFarland scale. Then the inoculum was diluted 1:50 and 1:20 in RPMI 1640 broth to obtain the final inoculum of 0.5-2.5 × 103 cells/mL. The tests were performed in duplicate. The minimum inhibitory concentration (MIC) was determined as the lowest concentration capable of inhibiting fungal growth by 50%, when compared to the drug-free growth control, and 100% inhibition for AMB (CLSI, 2008CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard - third edition. M27-A3. ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2008.). For quality control of the test, the strains of Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used.
Antifungal susceptibility assay of the biofilm
The antifungal susceptibility assay of the biofilm was performed as described by BRILHANTE et al. (2016b), with modifications. For this test, 29 isolates (Candida spp., C. neoformans and R. rubra) were analyzed. Biofilm was formed as described above. After biofilm maturation, the drugs diluted in RPMI were added. Antifungal drugs were tested at concentrations of 2-32 μg/mL for AMB and 8-128 μg/mL for ITC. For each tested strain, drug-free wells (growth control) and yeast-free wells (sterility control) were included. After the incubation period (48 h), the culture medium was removed and the wells were washed twice with PBS-Tween 20. Finally, biofilm metabolic activity was quantified using the tetrazolium salt reduction test 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT; Sigma Chemical Co., USA). Aliquots of 100 μL of XTT-menadione solution [0.5 mg/L XTT and 1 μM of menadione (Sigma Chemical Co., USA)], prepared in PBS and filtered through a 0.22 μM membrane were incubated in the dark, at 37 °C, for 3 hours. After the incubation period, the XTT solution was transferred to the wells of a new plate, followed by spectrophotometric reading at 492 nm. The sessile minimum inhibitory concentration (SMIC) against mature biofilms was determined as the minimum concentration capable of reducing 50% (SMIC50) and 90% (SMIC90) of the biofilm metabolic activity, when compared to the drug-free growth control of the respective strain). Subsequently, biofilm biomass was spectrophotometrically quantified by the crystal violet staining technique, as described above. The assay was performed in triplicate.
C. famata pathogenesis against Caenorhabditis elegans
The test was performed according to the protocol established by BRILHANTE et al. (2016b), with modifications. C. famata was the only species included in this assay, as it presented a more representative number of isolates (n=13). Thus, strains of C. famata and L4 stage nematodes were used in this experiment. The nematodes were previously cultured in nematode growth medium (NGM) containing Escherichia coli OP50 as a food source. Yeast strains were grown on BHI agar (Brain Heart Infusion - Himedia®) medium supplemented with 100 μg/mL of ciprofloxacin, at 35 °C, for up to 72 hours. C. albicans ATCC 10231 was used as a positive pathogenicity control. E. coli OP50 strain was also previously cultured on BHI agar without ciprofloxacin at 30 °C, for 24 hours, and used as a negative pathogenicity control. Subsequently, the nematodes were washed from the plates containing NGM with M9 buffer and transferred to the plates containing the microorganisms. The plates were kept in an incubator at 25 ºC for 2 hours, for the nematodes to feed on the microorganisms. After incubation, three consecutive nematode washes were performed to remove the microorganisms adhered to the worm cuticle. Approximately 50 nematodes were transferred to 6-well cell culture plates containing 1.5 mL of liquid medium composed of M9 (79%), BHI broth (20%), 100 μg/mL cholesterol in ethanol and 100 μg/mL of ciprofloxacin. Plates were then incubated at 25 °C and nematode viability was analyzed after 0, 24, 48, 72 and 96 hours. Thus, animals that had fungal structures growing outwards from the worm pseudocoelom and/or those that did not respond to mechanical stimuli were considered dead. Each nematode considered dead was removed from the well throughout the experiment. At the end of the period, a survival curve was established.
Statistical analysis
Friedman’s test, followed by Dunn’s post hoc test, was used to compare biomass and metabolic activity of Candida spp. mature biofilms, after exposure to AMB and ITC, to those of the drug-free growth control. To analyze C. famata induced mortality rate of C. elegans, Kaplan-Meyer survival curves were constructed and the log-rank and Breslow tests were used. P-values lower that 5% indicated significant conclusions.
RESULTS:
The microorganisms were identified by morphological and molecular methods, as follows: C. famata (13/29), Candida tropicalis (7/29), C. parapsilosis sensu stricto (3/29), Candida metapsilosis (1/29), Candida guilliermondii (1/29), C. neoformans (2/29) and R. rubra (2/29) (Table 1).
Regarding enzymatic production, 16/29 identified yeasts were phospholipases positive, of which 5/16 had strong activity (PHz <0.64). As for protease activity, 10/29 strains were positive, with PRz ranging from 0.59-0.86 (Table 1).
Concerning planktonic antifungal susceptibility, MIC ranges against C. famata complex were 0.03125-2 μg/mL for AMB, 0.5-4 μg/mL for FLC, <0.03125-4 μg/mL for ITC and <0.03125-0.5 μg/mL for VRC (Table 1). MICs against C. tropicalis ranged from 0.0625-0.25 μg/mL for AMB, 0.5-4 μg/mL for FLC, 0.0625-16 μg/mL for ITC and 0.03125-1 μg/mL for VRC. As for the C. parapsilosis complex, the MIC ranges were 0.03125-0.25 μg/mL for AMB, 0.5-4 μg/mL for FLC, <0.03125-0.0625 μg/mL for ITC and <0.03125-0.0625 μg/mL for VRC. The obtained MICs against C. guilliermondii were 0.125 μg/mL for AMB, 1 μg/mL for FLC, 0.0625 μg/mL for ITC and 0.03125 μg/mL for VRC.
As for the non-Candida isolates, MICs against C. neoformans were 4 μg/mL for FLC and ranged from 0.5-2 μg/mL for AMB, 0.03125-0.0625 μg/mL for ITC and 0.03125-0.125 μg/mL for VRC. Finally, the obtained MICs against R. rubra were <0.03125-0.5 μg/mL for AMB, <0.25-2 μg/mL for FLC, 0.5-1 μg/mL for ITC and 1-8 μg/mL for VRC (Table 1).
All tested strains (n=29) produced biofilms, of which 10/29 were moderate producers and 19/29 were strong producers. Overall, for Candida spp. AMB caused biomass reduction (P<0.05) starting at 8 µg/mL and decreased (P<0.05) metabolic activity at all tested concentrations (2-32 µg/mL), while ITC only reduced (P<0.05) biofilm biomass and metabolic activity at the two highest concentrations (64 and 128 µg/mL).
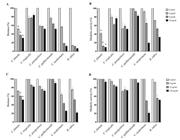
Concerning the antifungal susceptibility of mature biofilms of C. famata, AMB caused a biomass reduction of 61-71% and a metabolic activity decrease of 57- 90%, and the highest tested concentration (32 µg/mL) was defined as the SMIC90. ITC reduced biofilm biomass by 28-47% and metabolic activity by 7-12% (Figure 1). Regarding C. tropicalis biofilms, AMB reduced their biomass by 20-28% and metabolic activity by 17-36%. In addition, ITC caused little biomass reduction (2-16%) and a decrease of 5-18% in metabolic activity (Figure 1). As for C. parapsilosis complex biofilms, AMB caused 46-48% and 37-49% reduction in biomass and metabolic activity, respectively, while ITC induced 16-25% biofilm biomass reduction and a decrease of 25-31% in biofilm metabolic activity (Figure 1). At last, C. guilliermondii biofilms presented a maximum decrease in biomass and metabolic activity of 47% and 16%, respectively, after exposure to AMB. ITC reduced the biomass of these biofilms by 8% and their metabolic activity by 2-9% (Figure 1).
Antifungal susceptibility of mature biofilms of yeasts (n=29) from free-ranging reptiles and amphibians, with emphasis on Candida spp. (13 C. famata; 7 C. tropicalis, 4 C. parapsilosis species complex and 1 C. guilliermondii). Amphotericin B (A and B) caused biomass reduction starting at 8 µg/mL (A) and decreased metabolic activity at all tested concentrations (2-32 µg/mL; B). Itraconazole (C and D) only reduced biofilm biomass (C) and metabolic activity (D) at the highest concentration (128 µg/mL). Data are expressed as mean and standard deviation of crystal violet (biomass) and XTT (metabolic activity) absorbance values normalized with those of the drug-free growth control. *indicates statistically significant (P<0.05) differences between the crystal violet and XTT absorbance values obtained for Candida spp. biofilms after exposure to antifungal drugs and those obtained for their respective drug-free growth control. Species other than C. famata were not included in the statistical analyses because of the reduced number of isolates.
As for biofilms of C. neoformans, AMB caused a biomass decrease of 89-90% and a reduction in metabolic activity of 6-82%. ITC reduced the biomass by 33-73% and the biofilm metabolic activity by 31-75% (SMIC50 = 32 µg/mL). Moreover, R. rubra biofilms suffered 86-94% decrease in biomass and 28-67% reduction in metabolic activity, after exposure to AMB. ITC reduced the biomass of these biofilms by 25-70% and their metabolic activity by 8-49%.
Finally, C. famata strains induced C. elegans death, reducing worm viability by up to 60%, after 48 hours of exposure. The mortality rate induced by this yeast species was greater (P<0.0001) than that obtained for both negative (E. coli OP50) and positive (C. albicans ATCC 102301) controls of pathogenicity (Figure 2).
Pathogenicity of Candida famata (n=13) recovered from free-ranging reptiles and amphibians, using the nematode model Caenorhabditis elegans. ATCC 10231 (Candida albicans) used as positive control for pathogenicity; E. coli OP50 is the negative control for pathogenicity. Different survival rates were observed among the three survival curves evaluated throughout the observation period. Higher mortality was associated with C. famata strains when compared to ATCC 10231 (P<0.0001) and when compared to E. coli OP50 (P<0.0001).
DISCUSSION:
The data on the microbiota of reptiles and amphibians are scarce. The present study identified yeasts from reptile and amphibian microbiota. Candida was the most frequently isolated genus, with C. famata and C. tropicalis as the most prevalent species. C. parapsilosis complex and C. guilliermondii also were identified. Studies on animal microbiota have shown the genus Candida as the most prevalent yeasts (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
; BRILHANTE et al., 2015BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
https://link.springer.com/article/10.100...
a; SIDRIM et al., 2016______ et al. Antifungal Resistance and Virulence Among Candida spp. from Captive Amazonian manatees and West Indian Manatees: Potential Impacts on Animal and Environmental Health. EcoHealth, 2016. v.13, n.2, p.328-338. Available from: <https://link.springer.com/article/10.1007%2Fs10393-015-1090-8>. Accessed: May, 05, 2020. doi: 10.1007/s10393-015-1090-8.
https://link.springer.com/article/10.100...
). Similar to our findings, other authors have also described C. famata and C. tropicalis in reptiles, such as turtles (BRILHANTE et al., 2015a) and tortoises (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
).
Besides the genus Candida, the species C. neoformans and R. rubra were also identified. It is important to mention that the genus Cryptococcus has not been commonly reported in reptiles and amphibians. However, MCNAMARA et al. (1994MCNAMARA, T. S. et al. Cryptococcosis in a Common Anaconda (Eunectes murinus). Journal of Zoo and Wildlife Medicine, 1994. v.25, n.1, p.128-132. Available from: <Available from: https://www.jstor.org/stable/20095345 >. Accessed: May, 20, 2020. doi: 10.2307/20095345.
https://www.jstor.org/stable/20095345...
) isolated C. neoformans causing infection in a captive snake (Eunectes murinus).
Despite being present in the microbiota, these yeasts produce several virulence factors and are commonly associated with infections, thus, knowledge on animal microbiota is relevant for the understanding of several biological characteristics. In this study, C. famata, C. tropicalis and C. parapsilosis sensu stricto produced both phospholipases and proteases, but only the two former species presented strong phospholipase-producing isolates (Pz<0.64). Production of virulence factors such as proteases and phospholipases contribute to Candida pathogenicity and they are well elucidated for C. albicans (BERMAN & SUDBERY, 2002BERMAN, J.; SUDBERY, P. E. Candida albicans: A molecular revolution built on lessons from budding yeast. Nature Reviews Genetics, 1 dez. 2002. v.3, n.12, p.918-930. Available from: <Available from: https://www.nature.com/articles/nrg948 >. Accessed: Mar. 04, 2020. doi: 10.1038/nrg948.
https://www.nature.com/articles/nrg948...
; FANNING & MITCHELL, 2012FANNING, S.; MITCHELL, A. P. Fungal biofilms. PLoS pathogens, 2012. v.8, n.4, p.e1002585. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320593/ >. Accessed: May, 03, 2020. doi: 10.1371/journal.ppat.1002585.
https://www.ncbi.nlm.nih.gov/pmc/article...
).
Concerning the antifungal susceptibility assay, high ITC MICs (≥2 µg/mL) were observed against C. famata (2/13) and C. tropicalis (3/7). This finding is relevant because the tested animals were free-ranging wild animals that had never been treated with antifungal drugs, suggesting the presence of antifungal selective pressure in the environment, possibly due to the use of azoles in agricultural practices. It is important to emphasize that high azole MICs have been reported against yeasts from the microbiota of several animal species (BRILHANTE et al., 2015BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
https://link.springer.com/article/10.100...
b; 2016a; CORDEIRO et al., 2015CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
https://academic.oup.com/mmy/article/53/...
; SIDRIM et al., 2016______ et al. Antifungal Resistance and Virulence Among Candida spp. from Captive Amazonian manatees and West Indian Manatees: Potential Impacts on Animal and Environmental Health. EcoHealth, 2016. v.13, n.2, p.328-338. Available from: <https://link.springer.com/article/10.1007%2Fs10393-015-1090-8>. Accessed: May, 05, 2020. doi: 10.1007/s10393-015-1090-8.
https://link.springer.com/article/10.100...
). Moreover, it is important to emphasize that high azole MICs against C. tropicalis are commonly reported (KOTHAVADE et al., 2010KOTHAVADE, R. J. et al. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. Journal of Medical Microbiology , 2010. v.59, n.8, p.873-880. Available from: <Available from: http://jmm.microbiologyresearch.org/pubmed/content/journal/jmm/10.1099/jmm.0.013227-0 >. Accessed: Feb. 02, 2020. doi: 10.1099/jmm.0.013227-0.
http://jmm.microbiologyresearch.org/pubm...
; CORDEIRO et al., 2015CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
https://academic.oup.com/mmy/article/53/...
). As for C. parapsilosis species complex, the obtained isolates were susceptible to the tested antifungals, corroborating previous reports (BRILHANTE et al., 2018______ et al. Antifungal susceptibility and virulence of Candida parapsilosis species complex: An overview of their pathogenic potential. Journal of Medical Microbiology , 1 jul. 2018. v.67, n.7, p.903-914. Available from: <https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000756#tab2>. Accessed: Mar. 4, 2020. doi: 10.1099/jmm.0.000756.
https://www.microbiologyresearch.org/con...
).
AMB presented high MIC against one isolate of C. neoformans (2 μg/mL), while azole antifungals (FLC, VRC and ITC) showed low MIC ranges. High AMB MICs are not commonly reported in the literature, but some authors have reported MIC ranges of 0.25-1 μg/mL (PERFECT et al., 1996PERFECT, J. R. et al. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrobial agents and chemotherapy, 1996. v.40, n.8, p.1910-3. Available from: <Available from: https://aac.asm.org/content/40/8/1910.long >. Accessed: Feb. 25, 2020. doi: 10.1128/AAC.40.8.1910.
https://aac.asm.org/content/40/8/1910.lo...
) and 0.06-0.12 μg/mL (GAGO et al., 2017GAGO, S. et al. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses, 2017. v.60, n.1, p.40-50. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12543 >. Accessed: Mar. 04, 2020. doi: 10.1111/myc.12543.
https://onlinelibrary.wiley.com/doi/abs/...
) against C. neoformans. Moreover, VRC MICs against R. rubra were high, as they were comparable to MICs obtained against clinical Rhodotorula isolates (PRENEY et al., 2003PRENEY, L. et al. Experimental evaluation of antifungal and antiseptic agents against Rhodotorula spp. Mycoses , 2003. v.46, n.11-12, p.492-495. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.0933-7407.2003.00930.x?sid=nlm%3Apubmed >. Accessed: Mar. 04, 2020. doi: 10.1046/j.0933-7407.2003.00930.x.
https://onlinelibrary.wiley.com/doi/abs/...
; SERENA et al., 2004SERENA, C. et al. In vitro antifungal susceptibilities of uncommon basidiomycetous yeasts. Antimicrobial agents and chemotherapy , 2004. v.48, n.7, p.2724-2726. Available from: <Available from: https://aac.asm.org/content/48/7/2724.long >. Accessed: Mar. 04, 2020. doi: 10.1128/AAC.48.7.2724-2726.2004.
https://aac.asm.org/content/48/7/2724.lo...
).
As for mature biofilm antifungal susceptibility, C. famata biofilms were more susceptible to AMB (SMIC90 = 32 µg/mL) than ITC, whose SMIC50 was not reported. C. famata biofilm susceptibility is poorly investigated. C. famata complex species are commonly found in natural substrates and as part of the microbiota of some animal species, including humans, but it is considered an atypical human pathogen (BENITES et al., 2013BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
https://www.researchgate.net/publication...
; BRILHANTE et al., 2017______ et al. Yeasts From Scarlet Ibises (Eudocimus ruber): A Focus on Monitoring the Antifungal Susceptibility of Candida famata and Closely Related Species. Medical mycology, 2017. v.55, n.7, p.725-732. Available from: <https://academic.oup.com/mmy/article/55/7/725/2999704>. Accessed: Mar. 4, 2020. doi: 10.1093/MMY/MYW144.
https://academic.oup.com/mmy/article/55/...
).
C. tropicalis mature biofilms showed low antifungal susceptibility, against which SMIC90 or SMIC50 values were not found. C. tropicalis resistance in animal isolates has been reported (CORDEIRO et al., 2015CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
https://academic.oup.com/mmy/article/53/...
; BRILHANTE et al., 2016a), which is associated with overexpression of efflux pump-related genes (BIZERRA et al., 2008BIZERRA, F. C. et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS yeast research, 2008. v.8, n.3, p.442-50. Available from: <Available from: https://academic.oup.com/femsyr/article/8/3/442/599186 >. Accessed: Mar. 04, 2020. doi: 10.1111/j.1567-1364.2007.00347.x.
https://academic.oup.com/femsyr/article/...
), which are usually overexpressed when fungi are grown in their biofilm forms. AMB was also more effective than ITC against C. parapsilosis species complex and C. guilliermondii biofilms, but SMIC90 values were not reported. Concerning C. neoformans and R. rubra biofilms, both AMB and ITC presented inhibitory activity, reducing their biomass and metabolic activity.
As for the pathogenicity of C. famata using C. elegans infection model, high mortality rates were observed 48 and 96 hours after exposing the worms to this yeast species. Similar results have been observed for other Candida species (BRILHANTE et al., 2016b), which highlights the pathogenic potential of this yeast genus. These values were significantly higher than those for the negative pathogenicity control, demonstrating the pathogenic potential of C. famata, which is possibly associated with the expression and production of the virulence factors, observed in this study.
In summary, the microbiota of reptiles and amphibians contains yeasts able to produce important virulence factors, such as hydrolytic enzymes and biofilms. In addition, itraconazole resistance was observed among C. famata and C. tropicalis. Finally, C. famata strains increased the mortality rates of C. elegans.
ACKNOWLEDGEMENTS
This research was financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasil - Finance code 428737/2018-8.
REFERENCES
- BENITES, N. R. et al. Bacterial And Fungal Microflora Present in The Cloacae of Domestically Kept Red-Footed Tortoises (Geochelone carbonaria). Veterinária e Zootecnia, 2013. v.20, n.1, p.102-110. Available from: <Available from: https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed >. Accessed: Mar. 04, 2020.
» https://www.researchgate.net/publication/262637891_Microbiota_Bacteriana_e_Fungica_Presentes_na_Cloaca_de_Jabutis-Piranga_Geochelone_carbonaria_Criados_em_Domicilio_Bacterial_And_Fungal_Microflora_Present_in_The_Cloacae_of_Domestically_Kept_Red-Footed - BERMAN, J.; SUDBERY, P. E. Candida albicans: A molecular revolution built on lessons from budding yeast. Nature Reviews Genetics, 1 dez. 2002. v.3, n.12, p.918-930. Available from: <Available from: https://www.nature.com/articles/nrg948 >. Accessed: Mar. 04, 2020. doi: 10.1038/nrg948.
» https://doi.org/10.1038/nrg948.» https://www.nature.com/articles/nrg948 - BIZERRA, F. C. et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS yeast research, 2008. v.8, n.3, p.442-50. Available from: <Available from: https://academic.oup.com/femsyr/article/8/3/442/599186 >. Accessed: Mar. 04, 2020. doi: 10.1111/j.1567-1364.2007.00347.x.
» https://doi.org/10.1111/j.1567-1364.2007.00347.x.» https://academic.oup.com/femsyr/article/8/3/442/599186 - BORGES-LEITE, M. J.; RODRIGUES, J. F. M.; BORGES-NOJOSA, D. M. Herpetofauna of a coastal region of northeastern Brazil. Herpetology Notes, 2014. v.7, p.405-413.
- BOSCH, R. A. et al. First Reports of Tadpole Mouthpart Anomalies in a Cuban Toad (Anura: Bufonidae: Peltophryne). Herpetological Review, 2017. v.48, n.1, p.58-62.
- BRILHANTE, R. S. N.; RODRIGUES, P. H. De A.; et al. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia, 2015. v.180, n.5-6, p.421-426. Available from: <Available from: https://link.springer.com/article/10.1007/s11046-015-9923-0 >. Accessed: Apr. 25, 2020. doi: 10.1007/s11046-015-9923-0.
» https://doi.org/10.1007/s11046-015-9923-0» https://link.springer.com/article/10.1007/s11046-015-9923-0 - ______; SILVA, S. T. C.; et al. Emergence of azole-resistant Candida albicans in small ruminants. Mycopathologia , 2015. v.180, n.3-4, p.277-280. Available from: <https://link.springer.com/article/10.1007%2Fs11046-015-9888-z>. Accessed: May, 20, 2020. doi: 10.1007/s11046-015-9888-z.
» https://doi.org/10.1007/s11046-015-9888-z.» https://link.springer.com/article/10.1007%2Fs11046-015-9888-z - ______ ; MAIA-JÚNIOR, J. E.; et al. Yeasts from the microbiota of bats: A focus on the identification and antimicrobial susceptibility of cryptic species of Candida Journal of Medical Microbiology, 2016. v.65, n.10, p.1225-1228. Available from: <https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000340#tab2>. Accessed: Mar. 04, 2020. doi: 10.1099/jmm.0.000340.
» https://doi.org/doi: 10.1099/jmm.0.000340» https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000340#tab2 - ______; OLIVEIRA, J. S.; et al. Candida tropicalis from veterinary and human sources shows similar in vitro hemolytic activity, antifungal biofilm susceptibility and pathogenesis against Caenorhabditis elegans Veterinary Microbiology, 2016. v.192, p.213-219. Available from: <https://www.sciencedirect.com/science/article/pii/S0378113516301997?via%3Dihub>. Accessed: May, 20, 2020. doi: 10.1016/j.vetmic.2016.07.022.
» https://doi.org/10.1016/j.vetmic.2016.07.022.» https://www.sciencedirect.com/science/article/pii/S0378113516301997?via%3Dihub - ______ et al. Yeasts From Scarlet Ibises (Eudocimus ruber): A Focus on Monitoring the Antifungal Susceptibility of Candida famata and Closely Related Species. Medical mycology, 2017. v.55, n.7, p.725-732. Available from: <https://academic.oup.com/mmy/article/55/7/725/2999704>. Accessed: Mar. 4, 2020. doi: 10.1093/MMY/MYW144.
» https://academic.oup.com/mmy/article/55/7/725/2999704 - ______ et al. Antifungal susceptibility and virulence of Candida parapsilosis species complex: An overview of their pathogenic potential. Journal of Medical Microbiology , 1 jul. 2018. v.67, n.7, p.903-914. Available from: <https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000756#tab2>. Accessed: Mar. 4, 2020. doi: 10.1099/jmm.0.000756.
» https://doi.org/10.1099/jmm.0.000756.» https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000756#tab2 - CARDOSO-BRITO, V. et al. Conjunctival bacterial flora and antimicrobial susceptibility of captive and free‐living sea turtles in Brazil. Veterinary ophthalmology, 2019. v.22, n.3, p.246-255. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/vop.12584 >. Accessed: Apr. 15, 2020. doi: 10.1111/vop.12584.
» https://doi.org/10.1111/vop.12584.» https://onlinelibrary.wiley.com/doi/abs/10.1111/vop.12584 - CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard - third edition. M27-A3. ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2008.
- CORDEIRO, R. De A. et al. Candida tropicalis isolates obtained from veterinary sources show resistance to azoles and produce virulence factors. Medical mycology , 2015. v.53, n.2, p.145-152. Available from: <Available from: https://academic.oup.com/mmy/article/53/2/145/2812401 >. Accessed: Feb. 15, 2020. doi: 10.1093/mmy/myu081.
» https://doi.org/10.1093/mmy/myu081.» https://academic.oup.com/mmy/article/53/2/145/2812401 - ______ et al. Phenotype-driven strategies for screening Candida parapsilosis complex for molecular identification. Brazilian Journal of Microbiology, 2018. v.49, p.193-198. Available from: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328843/>. Accessed: Mar. 04, 2020. doi: 10.1016/j.bjm.2017.11.004.
» https://doi.org/10.1016/j.bjm.2017.11.004.» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328843/ - FANNING, S.; MITCHELL, A. P. Fungal biofilms. PLoS pathogens, 2012. v.8, n.4, p.e1002585. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320593/ >. Accessed: May, 03, 2020. doi: 10.1371/journal.ppat.1002585.
» https://doi.org/10.1371/journal.ppat.1002585.» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320593/ - FENG, X. et al. Development of two molecular approaches for differentiation of clinically relevant yeast species closely related to Candida guilliermondii and Candida famata Journal of Clinical Microbiology, 2014. v.52, n.9, p.3190-3195. Available from: <Available from: https://jcm.asm.org/content/52/9/3190.long >. Accessed: Mar. 04, 2020. doi: 10.1128/JCM.01297-14.
» https://doi.org/10.1128/JCM.01297-14.» https://jcm.asm.org/content/52/9/3190.long - GAGO, S. et al. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses, 2017. v.60, n.1, p.40-50. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12543 >. Accessed: Mar. 04, 2020. doi: 10.1111/myc.12543.
» https://doi.org/10.1111/myc.12543.» https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12543 - HERNÁNDEZ-GÓMEZ, O.; WUERTHNER, V.; HUA, J. Amphibian host and skin microbiota response to a common agricultural antimicrobial and internal parasite. Microbial ecology, 2020. v.79, n.1, p.175-191. Available from: <Available from: https://link.springer.com/article/10.1007%2Fs00248-019-01351-5 >. Accessed: May, 04, 2020. doi: 10.1007/s00248-019-01351-5.
» https://doi.org/10.1007/s00248-019-01351-5.» https://link.springer.com/article/10.1007%2Fs00248-019-01351-5 - HOOG, G. S. DE et al. Atlas of clinical fungi. 2. ed. Baarn: The Nederlands: Centraalbureau voor Schimmslcultures, 2000.
- KOLBY, J. E. et al. Presence of amphibian chytrid fungus (Batrachochytrium dendrobatidis) in rainwater suggests aerial dispersal is possible. Aerobiologia, 2015. v.31, n.3, p.411-419. Available from: <Available from: https://link.springer.com/article/10.1007/s10453-015-9374-6 >. Accessed: Mar. 04, 2020. doi: 10.1007/s10453-015-9374-6.
» https://doi.org/10.1007/s10453-015-9374-6.» https://link.springer.com/article/10.1007/s10453-015-9374-6 - KOTHAVADE, R. J. et al. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. Journal of Medical Microbiology , 2010. v.59, n.8, p.873-880. Available from: <Available from: http://jmm.microbiologyresearch.org/pubmed/content/journal/jmm/10.1099/jmm.0.013227-0 >. Accessed: Feb. 02, 2020. doi: 10.1099/jmm.0.013227-0.
» https://doi.org/10.1099/jmm.0.013227-0.» http://jmm.microbiologyresearch.org/pubmed/content/journal/jmm/10.1099/jmm.0.013227-0 - KURTZMAN, C. P.; FELL, J. W.; BOEKHOUT, T. The yeasts: a taxonomic study. [S.l.]: Elsevier, 2010.
- MCNAMARA, T. S. et al. Cryptococcosis in a Common Anaconda (Eunectes murinus). Journal of Zoo and Wildlife Medicine, 1994. v.25, n.1, p.128-132. Available from: <Available from: https://www.jstor.org/stable/20095345 >. Accessed: May, 20, 2020. doi: 10.2307/20095345.
» https://doi.org/10.2307/20095345.» https://www.jstor.org/stable/20095345 - NOWAKIEWICZ, A. et al. Aerobic bacterial microbiota isolated from the cloaca of the European pond turtle (Emys orbicularis) in Poland. Journal of wildlife diseases, 2015. v.51, n.1, p.255-259. Available from: <Available from: https://meridian.allenpress.com/jwd/article/51/1/255/122437/Aerobic-Bacterial-Microbiota-Isolated-from-the >. Accessed: Feb. 20, 2020. doi: 10.7589/2013-07-157.
» https://doi.org/10.7589/2013-07-157.» https://meridian.allenpress.com/jwd/article/51/1/255/122437/Aerobic-Bacterial-Microbiota-Isolated-from-the - PERFECT, J. R. et al. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans Antimicrobial agents and chemotherapy, 1996. v.40, n.8, p.1910-3. Available from: <Available from: https://aac.asm.org/content/40/8/1910.long >. Accessed: Feb. 25, 2020. doi: 10.1128/AAC.40.8.1910.
» https://doi.org/10.1128/AAC.40.8.1910.» https://aac.asm.org/content/40/8/1910.long - PRENEY, L. et al. Experimental evaluation of antifungal and antiseptic agents against Rhodotorula spp. Mycoses , 2003. v.46, n.11-12, p.492-495. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.0933-7407.2003.00930.x?sid=nlm%3Apubmed >. Accessed: Mar. 04, 2020. doi: 10.1046/j.0933-7407.2003.00930.x.
» https://doi.org/10.1046/j.0933-7407.2003.00930.x.» https://onlinelibrary.wiley.com/doi/abs/10.1046/j.0933-7407.2003.00930.x?sid=nlm%3Apubmed - PRICE, M. F.; WILKINSON, I. D.; GENTRY, L. O. Plate method for detection of phospholipase activity in Candida albicans Medical Mycology, 1982. v.20, n.1, p.7-14. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/7038928/ >. Accessed: Sep. 01, 2019. doi: 10.1080/00362178285380031.
» https://doi.org/10.1080/00362178285380031.» https://pubmed.ncbi.nlm.nih.gov/7038928/ - ROCHA, M. F. G. et al. Azole resistance in Candida albicans from animals: Highlights on efflux pump activity and gene overexpression. Mycoses , 2017. v.60, n.7, p.462-468. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12611 >. Accessed: 4 mar. 2020. doi: 10.1111/myc.12611.
» https://doi.org/10.1111/myc.12611.» https://onlinelibrary.wiley.com/doi/abs/10.1111/myc.12611 - SERENA, C. et al. In vitro antifungal susceptibilities of uncommon basidiomycetous yeasts. Antimicrobial agents and chemotherapy , 2004. v.48, n.7, p.2724-2726. Available from: <Available from: https://aac.asm.org/content/48/7/2724.long >. Accessed: Mar. 04, 2020. doi: 10.1128/AAC.48.7.2724-2726.2004.
» https://doi.org/10.1128/AAC.48.7.2724-2726.2004.» https://aac.asm.org/content/48/7/2724.long - SIDRIM, J. J. C. et al. Candida species isolated from the gastrointestinal tract of cockatiels (Nymphicus hollandicus): in vitro antifungal susceptibility profile and phospholipase activity. Veterinary microbiology, 2010. v.145, n.3, p.324-328. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378113510001872?via%3Dihub >. Accessed: Feb. 01, 2020. doi: 10.1016/j.vetmic.2010.04.006.
» https://doi.org/10.1016/j.vetmic.2010.04.006.» https://www.sciencedirect.com/science/article/pii/S0378113510001872?via%3Dihub - ______ et al. Antifungal Resistance and Virulence Among Candida spp. from Captive Amazonian manatees and West Indian Manatees: Potential Impacts on Animal and Environmental Health. EcoHealth, 2016. v.13, n.2, p.328-338. Available from: <https://link.springer.com/article/10.1007%2Fs10393-015-1090-8>. Accessed: May, 05, 2020. doi: 10.1007/s10393-015-1090-8.
» https://doi.org/10.1007/s10393-015-1090-8.» https://link.springer.com/article/10.1007%2Fs10393-015-1090-8 - SVEDESE, V. M. et al. Fungal microbiota from the oral mucosa of sympatric lizards from the Brazilian semiarid region. Herpetological Review , 2017. v.48, n.3, p.538-541. Available from: <Available from: https://www.researchgate.net/profile/Leonardo_Ribeiro3/publication/319950839_Fungal_microbiota_from_the_oral_mucosa_of_sympatric_lizards_from_the_Brazilian_semiarid_region/links/59c315ce458515af3060caf2/Fungal-microbiota-from-the-oral-mucosa-of-sympatric-lizards-from-the-Brazilian-semiarid-region.pdf >. Accessed: Jun. 20, 2020.
» https://www.researchgate.net/profile/Leonardo_Ribeiro3/publication/319950839_Fungal_microbiota_from_the_oral_mucosa_of_sympatric_lizards_from_the_Brazilian_semiarid_region/links/59c315ce458515af3060caf2/Fungal-microbiota-from-the-oral-mucosa-of-sympatric-lizards-from-the-Brazilian-semiarid-region.pdf - TAVANTI, A. et al. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. Journal of Clinical Microbiology , jan. 2005. v.43, n.1, p.284-292. Available from: <Available from: https://jcm.asm.org/content/43/1/284.long >. Accessed: Mar. 04, 2020. doi: 10.1128/JCM.43.1.284-292.2005.
» https://doi.org/10.1128/JCM.43.1.284-292.2005.» https://jcm.asm.org/content/43/1/284.long - VEGA-MANRIQUEZ, D. X. et al. Identification of bacteria present in ulcerative stomatitis lesions of captive sea turtles Chelonia mydas Veterinary research communications, 2018. v.42, n.3, p.251-254. Available from: <Available from: https://link.springer.com/article/10.1007%2Fs11259-018-9728-y >. Accessed: Jun. 02, 2020. doi: 10.1007/s11259-018-9728-y.
» https://doi.org/10.1007/s11259-018-9728-y.» https://link.springer.com/article/10.1007%2Fs11259-018-9728-y - VELEGRAKI, A. et al. Prospective use of RFLP analysis on amplified Cryptococcus neoformans URA5 gene sequences for rapid identification of varieties and serotypes in clinical samples. Medical Mycology , 2001. v.39, n.5, p.409-417. Available from: <Available from: https://academic.oup.com/mmy/article/39/5/409/960965 >. Accessed: Mar. 04, 2020. doi: 10.1080/mmy.39.5.409.417.
» https://doi.org/10.1080/mmy.39.5.409.417.» https://academic.oup.com/mmy/article/39/5/409/960965
-
CR-2020-0742.R3
Publication Dates
-
Publication in this collection
05 Apr 2021 -
Date of issue
2021
History
-
Received
10 Aug 2020 -
Accepted
12 Dec 2020 -
Reviewed
05 Feb 2021