ABSTRACT:
The present study evaluated the growth of the species Mytella guyanensis and Mytella strigata on ropes suspended in Amazon Macrotidal Mangrove Coast. The mussels were farmed at a density of 840 ind. m-1 of rope, with the same shell height (mm) and live weight (g) for both species. The experiment was entirely randomized, with two treatments and 15 repetitions. Significant differences were reported regarding the growth (shell height and live weight) between the species (P>0.05). The daily growth rate was greater for M. guyanensis than for M. strigata. At the end of the cultivation cycle, only 451 ± 46 (59.9%) of M. guyanensis individuals reached commercial size per meter of rope compared to 670 ± 73 (89.3%) of M. strigata individuals. Survival rates were similar. Salinity and temperature increased progressively throughout the experiment with the reduction in rainfall and were within the range considered ideal for these species. In conclusion, the farming of both species of mussels was viable under estuarine conditions influenced by macrotides, with satisfactory survival rates and daily growth (shell height and weight).
Key words:
estuary; macrotides; Mytella guyanensis; Mytella strigata; mussel farming
RESUMO:
O presente trabalho avaliou o crescimento das espécies Mytella guyanensis e Mytella strigata em cordas suspensas na Costa de Manguezais de Macromarés da Amazônia. Os mexilhões foram cultivados na densidade de 840 ind. m-1 de corda, com a mesma altura de valva (mm) e peso vivo (g) para ambas as espécies. O experimento ocorreu inteiramente casualizado, com dois tratamentos e 15 repetições. Foram encontradas diferenças significativas quanto ao crescimento (altura da concha e peso vivo) entre as espécies (P>0,05). A taxa de crescimento diário foi maior para a M. guyanensis do que para M. strigata. Ao final do ciclo de cultivo, apenas 451 ± 46 (59,9%) indivíduos de M. guyanensis atingiram o tamanho comercial por metro de corda comparado a 670 ± 73 (89,3%) indivíduos de M. strigata. As taxas de sobrevivência foram semelhantes. A salinidade e a temperatura aumentaram progressivamente ao longo do experimento com a redução das chuvas e ficaram dentro da faixa considerada ideal para essas espécies. Em conclusão, o cultivo de ambas as espécies de mexilhões foi viável em condições estuarinas influenciadas pelas macromarés, com taxas de sobrevivência e crescimento diário satisfatórios (altura de valva e peso).
Palavras-chave:
estuário; macromarés; Mytella guyanensis; Mytella strigata; cultivo de mexilhões
INTRODUCTION:
Bivalve mollusks are farmed to produce healthy food to meet the demands of a growing world population. Several species of these organisms are targets of extractivism and farming around the world (COLOMBO et al., 2016COLOMBO J. et al. Proximate chemical composition and fatty acid profile of the blue musselMytilus edulis from rocky shores and long line cultures in San Jorge Gulf, Argentina. Rev Biol Mar Oceanogr, v.51, n.2, p.293-299, 2016. Available from: <Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0718-19572016000200007&lng=es&nrm=iso >. Accessed: Feb. 2, 2022. doi: 10.4067/S0718-19572016000200007.
http://www.scielo.cl/scielo.php?script=s...
; GUARALDE et al., 2020GUARALDE, A. R. et al. An Artemia franciscana bioassay for the monitoring of lipophilic phycotoxins in marine bivalve mollusc cultures: An alternative to screening testing? Revista Ambiente & Água, v.15, n.5, 2020. Available from: <Available from: https://doi.org/10.4136/ambi-agua.2549 >. Accessed: Feb. 15, 2022. doi: 10.4136/ambi-agua.2549.
https://doi.org/10.4136/ambi-agua.2549...
). The farming of these bivalves is the most productive form of aquaculture in salt water (IGARASHI, 2019IGARASHI, M. A. Aspects of technological development cycle of mussel farming in Brazil (A Review). Rev Unimar Ciências, v.28, n.1-2, p.1-19, 2019. Available from: <Available from: http://ojs.unimar.br/index.php/ciencias/article/view/1514/865 >. Accessed: May, 10, 2022.
http://ojs.unimar.br/index.php/ciencias/...
) and is practiced in Europe, the Americas, China, Japan, India, and countries of Southeast Asia that have coastal waters (CEBU, 2016CEBU, E. H. Bamboo Tray Module Mussel Farming. J Acad Res, v.1, n.4, p.22-39, 2016. Available from: <Available from: https://aquadocs.org/handle/1834/40344 >. Accessed: Jan. 5, 2022.
https://aquadocs.org/handle/1834/40344...
).
Mussels occupy the 6th position among the most farmed aquatic organisms in Brazilian aquaculture and are restricted to coastal states (IBGE, 2020IBGE - Instituto Brasileiro de Geografa e Estatística. Pesquisa da Pecuária Municipal 2017, 2018 e 2019, 2020. Available from: <Available from: https://sidra.ibge.gov.br/tabela/3940 >. Accessed: May, 15, 2022.
https://sidra.ibge.gov.br/tabela/3940...
). Mussel farming is mainly performed using low-cost artisanal structures, especially individual ropes, on which the mussels are placed in 0.8 to 1.5 m-long tubular nets suspended on simple longlines. Production is destined mainly for local consumers and the subsistence of the families involved in the activity (SUPLICY, 2017SUPLICY, F. M. Cultivo de mexilhões: sistema contínuo e mecanizado. Florianópolis: Epagri, 2017, 124p. Available from: <Available from: https://docweb.epagri.sc.gov.br/website_epagri/Cedap/Livro/4-%20Livro-maricultura-mexilhao-gestao-sistema-de-cultivo-semente-engorda-risco.pdf >. Accessed: Apr. 22, 2022.
https://docweb.epagri.sc.gov.br/website_...
; SILVESTRI et al., 2018SILVESTRI, F. et al. Characterization of vagile fauna associated with suspended mussel farms. Arq Cienc Mar, v.51, n.2, p.57-71, 2018. Available from: <Available from: http://www.periodicos.ufc.br/arquivosdecienciadomar/article/view/32531 >. Accessed: Apr. 22, 2022. doi: 10.32360/acmar.v51i2.32531.
http://www.periodicos.ufc.br/arquivosdec...
).
Brazilian mussel production is represented mainly by the farming of the species Perna perna (PIERRI et al., 2016PIERRI, B. S. et al. The brown mussel Perna perna in Brazil: native or exotic? Arq Bras Med Vet Zootec, v.68, n.2, p.404-414, 2016. Available from: <Available from: https://www.scielo.br/j/abmvz/a/G9pjBLNhnw6rF98JjjW9Zqb >. Accessed: Jan. 28, 2022. doi: 10.1590/1678-4162-8534.
https://www.scielo.br/j/abmvz/a/G9pjBLNh...
). However, other native mytilids are reported in the country, such as Mytilus edulis platensis, M. guyanensis, and M. strigata (PEREIRA et al., 2003). Although, traded by traditional shellfish gatherers, these species remain unviable from a productive standpoint owing to the lack of studies that support the production process in many regions of the country. However, some efforts have been developed toward understanding the biology and physiology of species such as M. guyanensis and M. strigata (ONODERA & HENRIQUES, 2017ONODERA, F. K.; HENRIQUES, M. B. Mortality of Mytella falcata and M. guyanensis exposed to different temperatures. Bol Inst Pesca, v.43, n.1, p.106-111, 2017. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1201 >. Accessed: Dec. 14, 2021. doi: 10.20950/1678-2305.2017v43n1p106.
https://www.pesca.sp.gov.br/boletim/inde...
).
Specifically, regarding M. guyanensis, the main studies are related to the reproductive biology of the species (CHRISTO et al., 2016CHRISTO S. W, et al. Reproductive aspects of mussels (Bivalvia, Mollusca) in the Paranaguá estuarine complex, Paraná, Brazil. Bol Inst Pesca, v.42, n.4, p.924-936, 2016. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1189 >. Accessed: Feb. 2, 2022. doi: 10.20950/1678-2305.2016v42n4p924.
https://www.pesca.sp.gov.br/boletim/inde...
), population dynamics and habitat characterization (NISHIDA & LEONEL, 1995NISHIDA, A. K.; LEONEL, R. M. V. Occurrence, population dynamics and habitat characterization of Mytella guyanensis (Lamarck, 1819) (Mollusca, Bivalvia) in the Paraíba do Norte river estuary. Bolm Inst Oceanogr, v.43, p.49-57, 1995. Available from: <Available from: https://www.scielo.br/j/bioce/a/yQbtBn5zYWb7Sf9yzdhCpyN >. Accessed: Nov. 30, 2021. doi: 10.1590/S0373-55241995000100004.
https://www.scielo.br/j/bioce/a/yQbtBn5z...
), production estimates (PEREIRA et al., 2003), the analysis of parasites of the species (CEUTA & BOEHS, 2012CEUTA, L. O; BOEHS, G. Parasites of the mangrove mussel Mytella guyanensis (Bivalvia: Mytilidae) in Camamu Bay, Bahia, Brazil. Braz J Biol, v.72, n.3, p.421-427, 2012. Available from: <Available from: https://www.scielo.br/j/bjb/a/6BBh3qqr47vWVK35NDMKtwv >. Accessed: Jan. 5, 2022. doi: 10.1590/S1519-69842012000300002.
https://www.scielo.br/j/bjb/a/6BBh3qqr47...
), and growth under farming conditions (COSTA & NALESSO, 2002COSTA, K. G. E; NALESSO, R. C. Cultivo experimental de Mytella falcata (Orbigny, 1846) e M. guyanensis (Lamarck, 1819), no estuário do Rio Piraquê-açu (Aracruz-ES). Acta Limnol Bras, v.14, n.1, p.15-22, 2002. Available from: <Available from: http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d >. Accessed: Feb. 5, 2022.
http://www.alb.periodikos.com.br/article...
). Less information is available on M. strigata; the few studies conducted have addressed the relationship and adaptation to the environment (NARCHI & GALVÃO-BUENO, 1983NARCHI, W.; GALVÃO-BUENO, M. S. Anatomia funcional de Mytella charruana (D’Orbigny, 1846) (Bivalvia: Mytilidae). Bolm Zool Univ S Paulo, v.6, p.113-145, 1983. Available from: <Available from: https://www.revistas.usp.br/bolzoo/article/view/121956 >. Accessed: Mar. 26, 2022. doi: 10.11606/issn.2526-3358.bolzoo.1983.121956.
https://www.revistas.usp.br/bolzoo/artic...
), growth rates (DIARTE-PLATA et al., 2013DIARTE-PLATA, G. et al. Growth and survival of the musselMytella strigata(Bivalvia: Mytilidae) in suspension culture in Macapule lagoon, Sinaloa, Mexico. Hidrobiologica, v.23, n.3, p.374-385, 2013. Available from: <Available from: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972013000300010 >. Accessed: Mar. 22, 2022.
https://www.scielo.org.mx/scielo.php?scr...
), catch management (ARAÚJO et al., 2009ARAÚJO, A. R. R., et al. Management of the Mytella charruana (d’ Orbigny, 1846) fisheries along the coast of the Sergipe State: sustainability indicators. Rev Bras Eng Pesca, v.4, n.2, p.56-70, 2009. Available from: <Available from: https://ppg.revistas.uema.br/index.php/REPESCA/article/view/237 >. Accessed: Jan. 5, 2022. doi: 10.18817/repesca.v4i2.237.
https://ppg.revistas.uema.br/index.php/R...
), and genetic variability (SOUZA et al., 2015SOUZA, T. O. et al. Population structure and identification of two matrilinear and one patrilinear mitochondrial lineages in the mussel Mytella charruana. Estuar Coast Shelf Sci, v.156, p.165-174, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0272771414003308 >. Accessed: Apr. 22, 2022. doi: 10.1016/j.ecss.2014.11.009.
https://www.sciencedirect.com/science/ar...
).
Thus, there is a need for studies that evaluate the productive variables of these native species of mussels, especially along the Amazon Macrotidal Mangrove Coast (AMMC), which is a region characterized by the presence of fishing communities that use these organisms for the purposes of local consumption, craftwork, and mass sales as food.
This study evaluated the growth of the mussels M. guyanensis and M. strigata on suspended ropes under estuarine environmental conditions influenced by macrotides.
MATERIALS AND METHODS:
This study was conducted in the municipality of São José de Ribamar located on Maranhão Island (2°29’51.21”S e 44°03’14.61”W) in northeast Brazil (Figure 1). The region is part of the Amazon Macrotidal Mangrove Coast (AMMC), characterized by semidiurnal tides that reach 7.5 meters (SOUZA FILHO, 2005SOUZA FILHO, P. W. M. Costa de manguezais de macromaré da Amazônia: cenários morfológicos, mapeamento e quantificação de áreas usando dados de sensores remotos. Rev Bras Geofis, v.23, n.4, p.427-435, 2005. Available from: <Available from: https://www.scielo.br/j/rbg/a/548fTgMXRHTmSTYBXNhfxbc >. Accessed: Apr. 22, 2022. doi: 10.1590/S0102-261X2005000400006.
https://www.scielo.br/j/rbg/a/548fTgMXRH...
).
Geographic location of the study area: the municipality of São José de Ribamar, Maranhão, Brazil.
The climate is tropical and wet, with the influence of the equatorial air mass. Temperatures are high (26 - 29 ºC) throughout the year and the high precipitation results in two well-defined seasonal periods strongly marked by rains (ALVARES et al., 2013ALVARES, C. A. et al. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, v.22, n.6, p.711-728, 2013. Available from: <Available from: https://www.schweizerbart.de/papers/metz/detail/22/82078/Koppen_s_climate_classification_map_for_Brazil?af=crossref >. Accessed: Jan. 5, 2022. doi: 10.1127/0941-2948/2013/0507.
https://www.schweizerbart.de/papers/metz...
; NASCIMENTO et al., 2017NASCIMENTO, F. C. A. et al. Statistical Analysis of Dry and Rainy Event of Maranhão Rainfall. Rev Bras de Meteorol, v.32, n.3, p.375-386, 2017. Available from: <Available from: https://www.scielo.br/j/rbmet/a/Jt4QgwVYxYzphGKVGypvWwk >. Accessed: Mar. 26, 2022. doi: 10.1590/0102-77863230005.
https://www.scielo.br/j/rbmet/a/Jt4QgwVY...
). Data from the Instituto Nacional de Meteorologia (INMET [National Meteorological Institute]) reveal a total precipitation of 3046.3 mm for 2019, with an annual average of 2114.3 mm in the previous 30 years (1989 to 2019). The precipitation data show a typical seasonal cycle during the study period. The highest precipitation in the rainy season (January to June) occurred in March (818.2 mm). The dry season was from August to December, with an accumulated precipitation of less than 30 mm from August to October (Figure 2).
Average monthly precipitation based on the previous 30 years (1989 to 2019); monthly precipitation recorded in 2019 in the estuary of Santo Antônio River, Maranhão, Brazil. Gray dashed line - upper and lower bounds of 95% confidence interval.
The experiment was conducted for six months in the period of least rainfall in the region (dry season - July to December), as shown in figure 2. Seeds of the mussel M. strigata were obtained through artificial collectors (polyethylene terephthalate bottles) at the study site. The seeds of M. guyanensis do not attach to artificial substrates and were; therefore extracted directly from natural banks in the region. The seeds of M. strigata and M. guyanensis used in the experiment had shell heights of 20.1 ± 0.9 and 20.4 ± 0.7 mm, respectively.
The experimental design was entirely randomized with two treatments (ropes with M. strigata and M. guyanensis) and 15 repetitions, totaling 30 experimental units composed of ropes measuring one meter in length with a density of 840 ind. m-1 of rope (1200 g.m-1). The individuals were encased in a set of two tubular 60-mm nets forming two socks (one within the other). The inner sock was made of cotton and the outer sock was made of polyamide. In the center, a 5-mm nylon line was used to ensure the suspension of the ropes until the end of cultivation. The ropes were attached to a floating raft in the estuarine region of the city of São José de Ribamar (02°28’51.21”S and 44°03’14.61”W) (Figure 1).
Sampling was performed at 45-day intervals. At each sampling, three ropes from each treatment were removed from the water. Subsamples of 50 individuals from each rope were measured for shell height (mm) with the aid of calipers (precision: 0.1 mm) and weighed using a digital scale; the total weight (g) was recorded to three decimal places. After 180 days, shell height was measured and the number of live mussels per rope was counted to determine the distribution frequency of M. guyanensis and M. strigata per size class (shell height) and survival at the end of the cultivation cycle. The standardization of the biometrics of the shells during the experiment was based on the method proposed by GALTSOFF (1964GALTSOFF, P. S. The American oyster Crassostrea virginica. Fish Bull, v.64, n.1, p.11-28, 1964. Available from: <Available from: https://link.springer.com/article/10.2307/1350854 >. Accessed: Jan. 22, 2022. doi: 10.2307/1350854.
https://link.springer.com/article/10.230...
). The environmental variables of temperature (°C) and salinity of the water (g.kg-1) at the cultivation site were measured using the YSI Multiprobe 556 MPS every 45 days.
Throughout the experiment, a qualitative analysis of fouling organisms and vagile fauna on the mussel ropes was performed every 45 days. The material collected in the field was fixed in 4% formalin for 24 hours and taken to the laboratory for freezing to preserve the organisms and maintain their natural characteristics.
The Student’s t-test (P < 0.05) was used to analyze the data, as this test enables the assessment of growth between two sets of data (two species, in the present case) as well as the comparison of survival rates.
RESULTS:
Growth
At the end of the 180-day experimental period, the species M. guyanensis and M. strigata reached a mean shell height of 50.1 ± 0.9 and 40.9 ± 0.9 mm and mean live weight of 7.4 ± 0.3 and 4.0 ± 0.2 g, respectively. The mean daily growth rate of M. guyanensis and M. strigata was 0.17 and 0.12 mm for shell height and 0.04 and 0.02 g for live weight gain, respectively.
Regarding the performance of the biometric variables (shell height and live weight) throughout the experimental period, no significant differences in growth were found between the two species in the first 45 days of cultivation (P > 0.05) (Figure 3). However, growth in terms of both shell height and weight gain was significantly higher for M. guyanensis than for M. strigata beginning at 90 days through to the end of cultivation (P < 0.05). Regarding the mean total weight of the mussels, a significant weight gain was recorded for M. strigata beginning at 135 days of cultivation (P < 0.05).
Productive variables. (A) Mean shell height (mm) and (B) mean total weight (g) of M. strigata and M. guyanensis farmed in the macrotidal estuary of the Amazon. Different letters indicate a significant difference between treatments (P<0.05, Student’s t-test).
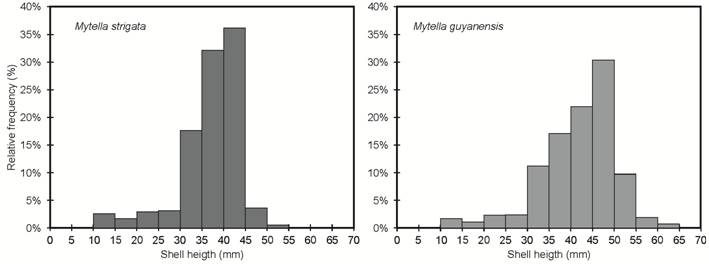
At the end of the experiment, the ropes with M. guyanensis had a significantly higher mean total weight (6.3 ± 0.42 kg.m-1) than those with M. strigata (4.9 ± 0.96 kg.m-1). A total of 451 ± 46 (59.9%) and 670 ± 73 (89.3%) individuals of M. guyanensis and M. strigata, respectively, reached commercial size (≥ 40 and ≥ 30 mm) per meter of rope (Figure 4). Further, 302 ± 23 (40.1%) M. guyanensis and 80 ± 10 (10.7%) M. strigata individuals did not achieve the commercial size per meter of rope.
Productive variables. (A) Mean shell height (mm) and (B) mean distribution frequency per size class (shell height) of M. strigata and M. guyanensis farmed in the macrotidal estuary of the Amazon.
No significant difference was reported in the survival rate of M. guyanensis (86.7 ± 5.6%) and M. strigata (85.3 ± 13.0%) at the end of the cultivation cycle (P > 0.05).
Environmental variables
As the study was conducted in the dry season (July to December), the rainfall was 328.3 mm at the beginning, and 2.1 mm at the end of the experiment. The behavior of the precipitation data in relation to the historical series for the site is displayed in figure 2, confirming the local pattern and variation throughout the experimental period (Figure 5). Salinity ranged from 29.0 ± 1.9 to 34.0 ± 1.4 g.kg-1 and temperature ranged from 28.5 ºC ± 0.7 to 31.0 ºC ± 2.1; both variables increased progressively throughout the experiment but with no abrupt variations (Figure 5). Turbidity ranged from 37 to 87 NTU and was higher in the rainy period and lower in the dry period.
Environmental variables measud during the experimental farming of M. guyanensis and M. strigata in the macrotidal estuary of the Amazon.
Fouling organisms and vagile fauna
The qualitative analysis of fouling organisms and vagile fauna during the experimental period revealed a high agglomeration of barnacles of the species Amphibalanus amphitrite and Amphibalanus improvisus, especially in November and December. To a lesser extent, the oyster Crassostrea rhizophorae, sponges Mycale sp., the gastropod Stramonita haemastoma, and other individuals of M. guyanensis and M. strigata were found externally on the cultivation rope.
DISCUSSION:
Mytella guyanensis has allometric growth or an adjustable differential at Y = aLb (SIBAJA & VILLALOBOS, 1986SIBAJA, W. G.; VILLALOBOS, C. Crecimiento del mejillón chora Mytella guayanensis (Bivalvía: Mytilidae) en el Golfo de Nicoya, Costa Rica. Rev Biol Trop, v.34, 2, p.231-236, 1986. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/view/24300 >. Accessed: Mar. 7, 2022.
https://revistas.ucr.ac.cr/index.php/rbt...
) and a larger shell compared to M. strigata, with adults reaching a height of 63 to 86 mm (REIS JÚNIOR et al., 2016REIS JÚNIOR, J. J. C. et al. Morphometric analysis and meat yieldof Mytilidae caught in the state of Sergipe. Scientia Plena, v.12, n.12, p.1-11, 2016. Available from: <Available from: https://www.scientiaplena.org.br/sp/article/view/3066 >. Accessed: May, 19, 2022. doi: 10.14808/sci.plena.2016.120201.
https://www.scientiaplena.org.br/sp/arti...
); sexual maturation occurs at around 18 mm (CRUZ & VILLALOBOS, 1993CRUZ, R. A.; VILLALOBOS, C. R. Shell length at sexual maturity and spawning cycle of Mytella guyanensis (Bivalvia: Mytilidae) from Costa Rica. Rev Biol Trop, v.41, n.1, p.89-92, 1993. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/download/23311/23613/ >. Accessed: Mar. 22, 2022. doi: 10.15517/rbt.v41i1.23311.
https://revistas.ucr.ac.cr/index.php/rbt...
). In contrast, M. strigata has negative allometric growth, with a maximum value height of 44 to 55 mm and sexual maturation at around 10 mm (PEREIRA et al., 2003; REIS JÚNIOR et al., 2016) Thus, the performance of the cultivated specimens was satisfactory, reaching valve heights of 50.1±0.9 and 40.9±0.9 mm and a live weight of 7.4±0.3 and 4.0±0.2 g, for M. guyanensis and M. strigata, respectively. Regarding the significantly higher growth of M. guyanensis compared to M. strigata, we point out the biology of the species itself, because; although, they coexist in the same environment and have very similar morphologies, they differ in terms of physiology and behavior (NARCHI & GALVÃO-BUENO, 1983NARCHI, W.; GALVÃO-BUENO, M. S. Anatomia funcional de Mytella charruana (D’Orbigny, 1846) (Bivalvia: Mytilidae). Bolm Zool Univ S Paulo, v.6, p.113-145, 1983. Available from: <Available from: https://www.revistas.usp.br/bolzoo/article/view/121956 >. Accessed: Mar. 26, 2022. doi: 10.11606/issn.2526-3358.bolzoo.1983.121956.
https://www.revistas.usp.br/bolzoo/artic...
; SIBAJA & VILLALOBOS, 1986; COSTA & NALESSO, 2002COSTA, K. G. E; NALESSO, R. C. Cultivo experimental de Mytella falcata (Orbigny, 1846) e M. guyanensis (Lamarck, 1819), no estuário do Rio Piraquê-açu (Aracruz-ES). Acta Limnol Bras, v.14, n.1, p.15-22, 2002. Available from: <Available from: http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d >. Accessed: Feb. 5, 2022.
http://www.alb.periodikos.com.br/article...
; CHRISTO et al., 2016CHRISTO S. W, et al. Reproductive aspects of mussels (Bivalvia, Mollusca) in the Paranaguá estuarine complex, Paraná, Brazil. Bol Inst Pesca, v.42, n.4, p.924-936, 2016. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1189 >. Accessed: Feb. 2, 2022. doi: 10.20950/1678-2305.2016v42n4p924.
https://www.pesca.sp.gov.br/boletim/inde...
).
The daily growth rate of M. guyanensis was lower than that reported in some studies (SIBAJA, 1988SIBAJA, W. G. Fijación larval y crecimiento del mejillón Mytella guayanensis L. (Bivalvia: Mytilidae) en Isla Chira, Costa Rica. Rev Biol Trop, 36: 453-456, 1988. Available from: <https://revistas.ucr.ac.cr/index.php/rbt/article/download/23854/24037>. Accessed: Jan. 8, 2022.; JUÁREZ & PERALTA, 2020) but not the lowest ever recorded; SIBAJA & VILLALOBOS (1986) and MORA & ALPIZAR (1998MORA, D. A.; ALPÍZAR, B. M. Crecimiento de Mytella guyanensis (Bivalvia: Mytilidae) en balsas flotantes. Rev Biol Trop, v.46, n.6, p.21-26, 1998. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/view/29630 >. Accessed: Jan. 15, 2022.
https://revistas.ucr.ac.cr/index.php/rbt...
) reported lower growth rates. The daily growth rate of M. strigata was also lower than that reported in some experiments (PEREIRA & GRAÇA LOPES, 1995PEREIRA, O. M.; GRAÇA LOPES, R. Fixação de sementes de Mytella falcata (sururu) em coletores artificiais no Canal de Bertioga, Estuário de Santos, Estado de São Paulo, Brasil. Bol Inst Pesca, v.22, n.1, p.165-173, 1995. Available from: <Available from: https://www.bvs-vet.org.br/vetindex/periodicos/boletim-do-instituto-de-pesca/22-(1995)-1/fixacao-de-sementes-de-mytella-falcata-sururu-em-coletores-artificiais >. Accessed: Nov. 27, 2021.
https://www.bvs-vet.org.br/vetindex/peri...
) but higher than that reported by COSTA & NALESSO (2002COSTA, K. G. E; NALESSO, R. C. Cultivo experimental de Mytella falcata (Orbigny, 1846) e M. guyanensis (Lamarck, 1819), no estuário do Rio Piraquê-açu (Aracruz-ES). Acta Limnol Bras, v.14, n.1, p.15-22, 2002. Available from: <Available from: http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d >. Accessed: Feb. 5, 2022.
http://www.alb.periodikos.com.br/article...
).
However, the results were similar to those obtained in experiments conducted in the same region (Macrotidal Mangrove Coast of the Amazon) (MONTELES et al., 2019MONTELES, J. S. et al. Productive parameters of Mytella charruana farmed in macrotidal mangroves on the amazonian coast, Brazil. In: SILVA F. F (Org.). Aquicultura e pesca: adversidades e resultados 2. Ponta Grossa: Atena Editora, p.151-165, 2019. Available from: <Available from: https://www.atenaeditora.com.br/arquivos/ebooks/aquicultura-e-pesca-adversidades-e-resultados-2 >. Accessed: May, 22, 2022. doi: 10.22533/at.ed.16119151016.
https://www.atenaeditora.com.br/arquivos...
). The environmental conditions of the region may explain why the daily growth rate was lower than that reported in some studies. The Macrotidal Mangrove Coast of the Amazon has peculiar meteorological and oceanographic conditions, such as a high annual temperature, little annual thermal variation, and the considerable contribution of particulate matter from the numerous mangroves in the region.
Hence, particulate matter is a limiting factor to the cultivation of mussels such as M. guyanensis and M. strigata by directly affecting primary production, diminishing food availability, and affecting the metabolism of the species, leading to greater energy expenditure (MORENO et al., 2010MORENO, D. et al. Métodos para identificar, diagnosticar y evaluar el grado de eutrofia. Contatos, 78: 25-33, 2010. Available from: <Available from: http://www2.izt.uam.mx/newpage/contactos/anterior/n78ne/eutrofia2.pdf >. Accessed: Mar. 26, 2022.
http://www2.izt.uam.mx/newpage/contactos...
). MORENO et al. (2010) reported that locations at which turbidity (measured using a Secchi disc) is lower than 3 m offer low quality for marine farming. However, DÍAZ et al. (2019DÍAZ, C. et al. Comparative growth of Mytilus chilensis (Hupé 1854) and Mytilus galloprovincialis (Lamarck 1819) in aquaculture longline system in Chile. Aquaculture, v.507, p.21-27, 2019. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0044848617312280 >. Accessed: Mar. 16, 2022. doi: 10.1016/j.aquaculture.2019.03.057.
https://www.sciencedirect.com/science/ar...
) did not find this generalization to hold for the mussels cultivated in Chile (Mytilus chilensis and Mytilus galloprovincialis), demonstrating that each species had its peculiarities in terms of environmental variables. Thus, there is no setback to the development of M. guyanensis and M. strigata in the region, given that these species are native and, therefore, adapted to the high circulation of particulate matter in the water column.
The mean daily growth rate for M. strigata was higher than that reported by COSTA & NALESSO (2002COSTA, K. G. E; NALESSO, R. C. Cultivo experimental de Mytella falcata (Orbigny, 1846) e M. guyanensis (Lamarck, 1819), no estuário do Rio Piraquê-açu (Aracruz-ES). Acta Limnol Bras, v.14, n.1, p.15-22, 2002. Available from: <Available from: http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d >. Accessed: Feb. 5, 2022.
http://www.alb.periodikos.com.br/article...
) but lower than that reported by PEREIRA & GRAÇA LOPES (1995PEREIRA, O. M.; GRAÇA LOPES, R. Fixação de sementes de Mytella falcata (sururu) em coletores artificiais no Canal de Bertioga, Estuário de Santos, Estado de São Paulo, Brasil. Bol Inst Pesca, v.22, n.1, p.165-173, 1995. Available from: <Available from: https://www.bvs-vet.org.br/vetindex/periodicos/boletim-do-instituto-de-pesca/22-(1995)-1/fixacao-de-sementes-de-mytella-falcata-sururu-em-coletores-artificiais >. Accessed: Nov. 27, 2021.
https://www.bvs-vet.org.br/vetindex/peri...
). These variations in growth rate are directly related to environmental variations in the different regions, as demonstrated by the similar results reported in other studies conducted on the Macrotidal Mangrove Coast of the Amazon (MONTELES et al., 2019MONTELES, J. S. et al. Productive parameters of Mytella charruana farmed in macrotidal mangroves on the amazonian coast, Brazil. In: SILVA F. F (Org.). Aquicultura e pesca: adversidades e resultados 2. Ponta Grossa: Atena Editora, p.151-165, 2019. Available from: <Available from: https://www.atenaeditora.com.br/arquivos/ebooks/aquicultura-e-pesca-adversidades-e-resultados-2 >. Accessed: May, 22, 2022. doi: 10.22533/at.ed.16119151016.
https://www.atenaeditora.com.br/arquivos...
). However, this variation does not seem to be a setback, as both species reached the commercial size (40 mm and 30 mm) (PEREIRA et al., 2003; PEREIRA et al., 2018;) within 90 days of farming. Regarding the mean daily weight gain of 0.04 and 0.02 g (live weight) for M. guyanensis and M. strigata, respectively, the results are similar to those reported in previous studies (MORA & ALPIZAR, 1998MORA, D. A.; ALPÍZAR, B. M. Crecimiento de Mytella guyanensis (Bivalvia: Mytilidae) en balsas flotantes. Rev Biol Trop, v.46, n.6, p.21-26, 1998. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/view/29630 >. Accessed: Jan. 15, 2022.
https://revistas.ucr.ac.cr/index.php/rbt...
; COSTA & NALESSO, 2002; MONTELES et al., 2019; UREÑA-JUÁREZ & PERALTA, 2020UREÑA-JUÁREZ, P.; PERALTA, C. D. Cultivo en suspensión de Mytella guyanensis (Bivalvia: Mytilidae) en Isla Chira, Costa Rica: implicaciones ambientales y biológicas. Repertório Científico, v.23, n.2, p.76-88. Available from: <Available from: https://revistas.uned.ac.cr/index.php/repertorio/article/view/3029/4285 >.2020 Accessed: Apr. 20, 2022. doi: 10.22458/rc.v23i2.3029.
https://revistas.uned.ac.cr/index.php/re...
). Although, the productive variables for M. guyanensis in the region of the Macrotidal Mangrove Coast of the Amazon have not been well defined, MONTELES et al. (2019) obtained success in the farming of M. strigata in a 240-day cycle, considering 60 days of seed recruitment and 180 days of grow-out on suspended ropes. The shell height and weight gain results for both species in the present study indicated the productive viability of the species in a sock farming system.
With regard to the productive performance of M. guyanensis and M. strigata, the two species began to differ significantly in terms of shell growth and weight beginning at 90 days, revealing greater growth of M. guyanensis. However, REIS JÚNIOR et al. (2016REIS JÚNIOR, J. J. C. et al. Morphometric analysis and meat yieldof Mytilidae caught in the state of Sergipe. Scientia Plena, v.12, n.12, p.1-11, 2016. Available from: <Available from: https://www.scientiaplena.org.br/sp/article/view/3066 >. Accessed: May, 19, 2022. doi: 10.14808/sci.plena.2016.120201.
https://www.scientiaplena.org.br/sp/arti...
) reported that; although, M. guyanensis achieved greater shell height and weight than M. strigata, the mean yield of meat in natura was lower (33.2% vs. 50.3%, respectively). Therefore, the mean yield of meat is a point that favors M. strigata over M. guyanensis.
At 180 days of cultivation on ropes, M. guyanensis and M. strigata had achieved commercial size (≥ 40 and ≥ 30 mm, respectively). However, M. strigata had a higher percentage of individuals (89%) ready for market (670 ± 73 ind.) and a lower percentage of individuals not attaining the commercial size (80 ± 10 ind.; 10.7%) compared to M. guyanensis com [59.9% (451 ± 46 ind.) and 40.1% (302 ± 23 ind.), respectively].
In Costa Rica, UREÑA-JUÁREZ & PERALTA (2020)UREÑA-JUÁREZ, P.; PERALTA, C. D. Cultivo en suspensión de Mytella guyanensis (Bivalvia: Mytilidae) en Isla Chira, Costa Rica: implicaciones ambientales y biológicas. Repertório Científico, v.23, n.2, p.76-88. Available from: <Available from: https://revistas.uned.ac.cr/index.php/repertorio/article/view/3029/4285 >.2020 Accessed: Apr. 20, 2022. doi: 10.22458/rc.v23i2.3029.
https://revistas.uned.ac.cr/index.php/re...
reported that M. guyanensis reached commercial size (≥ 40 mm) after 120 days of farming on floating boxes. DIARTE-PLATA et al. (2013DIARTE-PLATA, G. et al. Growth and survival of the musselMytella strigata(Bivalvia: Mytilidae) in suspension culture in Macapule lagoon, Sinaloa, Mexico. Hidrobiologica, v.23, n.3, p.374-385, 2013. Available from: <Available from: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972013000300010 >. Accessed: Mar. 22, 2022.
https://www.scielo.org.mx/scielo.php?scr...
) obtained a growth of 46.99 ± 0.26 mm for M. strigata after 238 days of suspended cultivation using “oyster boxes” under the environmental conditions of Sinaloa, Mexico. The disadvantage in cultivation time for M. guyanensis and the advantage of M. strigata in the cited studies may be directly related to environmental conditions and the farming structures used, and do not imply the unviability of production, rather only indicate the adjustment to local conditions.
In the present study, the number of individuals of M. guyanensis that did not achieve commercial size was higher than that of M. strigata, suggesting the possibility of a cultivation time longer than 180 days for the species on the Macrotidal Mangrove Coast of the Amazon. However, the cultivation time would be less than that required by Perna perna, which belongs to the same family (Mytilidae) and has a larger commercial size (80 mm) that takes about 12 months to reach (recruitment to harvest) in the same farming system of suspended ropes (SUPLICY, 2018SUPLICY, F. M. Evaluation of initial density effect on the productivity of Perna perna mussels in Santa Catarina State. Agropecuária Catarinense, v.31, n.1, p.77-81, 2018. Available from: <Available from: https://publicacoes.epagri.sc.gov.br/RAC/article/view/253 >. Accessed: Apr. 29, 2022. doi: 10.22491/RAC.2018.v31n1.11.
https://publicacoes.epagri.sc.gov.br/RAC...
). For the individuals of M. guyanensis and M. strigata that did not reach the commercial size and belonged to very small size classes, the management and use of a new farming cycle is a possibility, as it occurs with P. perna (SUPLICY, 2018).
The mean survival rates for M. guyanensis and M. strigata (86.7 ± 5.6% and 85.3 ± 13.0%, respectively) after six months of farming were close to those reported by other authors. UREÑA-JUÁREZ & PERALTA (2020)UREÑA-JUÁREZ, P.; PERALTA, C. D. Cultivo en suspensión de Mytella guyanensis (Bivalvia: Mytilidae) en Isla Chira, Costa Rica: implicaciones ambientales y biológicas. Repertório Científico, v.23, n.2, p.76-88. Available from: <Available from: https://revistas.uned.ac.cr/index.php/repertorio/article/view/3029/4285 >.2020 Accessed: Apr. 20, 2022. doi: 10.22458/rc.v23i2.3029.
https://revistas.uned.ac.cr/index.php/re...
reported a survival rate of 87 ± 4.5% of M. guyanensis individuals and DIARTE-PLATA et al. (2013DIARTE-PLATA, G. et al. Growth and survival of the musselMytella strigata(Bivalvia: Mytilidae) in suspension culture in Macapule lagoon, Sinaloa, Mexico. Hidrobiologica, v.23, n.3, p.374-385, 2013. Available from: <Available from: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972013000300010 >. Accessed: Mar. 22, 2022.
https://www.scielo.org.mx/scielo.php?scr...
) reported a rate of 81.25% for M. strigata. Therefore, the results of the present study demonstrated that both species are adapted to the farming conditions in the peculiar environment of the Macrotidal Mangrove Coast of the Amazon.
The occurrence of vagile and fouling fauna on the cultivation structures was not a negative factor for the farming of the two species, as only a few organisms were associated. The region of the experiment demonstrated low fitness for the natural uptake of mussel seeds, which contributed to the low fixation of these seeds on the ropes during the cultivation period without altering the initial density or compromising the growth of the individuals. However, the environmental conditions, especially the high salinity, contributed to the occurrence of interspecific competition, which requires care and management throughout the production process. For instance, oysters and barnacles compete for space and food, and this can lead to the loss of production by alerting the visual aspect of the shells and affecting the growth of the individuals (ALVARENGA & NALESSO, 2006ALVARENGA, L., NALESSO, R. C. Preliminary assessment of the potential for mangrove oyster cultivation in Piraquê-açu river estuary (Aracruz, ES). Braz Arch Biol Technol, v.49, n.1, p.163-169, 2006. Available from: <Available from: https://www.scielo.br/j/babt/a/3YwFvdnCJzkCFpqMK43B9fp >. Accessed: Jan. 1, 2022. doi: 10.1590/S1516-89132006000100019.
https://www.scielo.br/j/babt/a/3YwFvdnCJ...
). Moreover, the gastropod Stramonita haemastoma is a known predator of bivalves, and studies have linked its presence in farming activities to the death of oysters and mussels (PEZY et al., 2019PEZY, J. P. et al. First record of the gastropod Stramonita haemastoma (Linnaeus, 1767) in the English Channel. BioInvasions Rec, v.8, n.2, p.266-272, 2019. Available from: <Available from: https://hal-normandie-univ.archives-ouvertes.fr/hal-02153586 >. Accessed: Jun. 25, 2022. doi: 10.3391/bir.2019.8.2.08.
https://hal-normandie-univ.archives-ouve...
). To a lesser extent, the alga Acanthophora spicifera and bryozoan Bowerbankia sp. also compete for space.
The satisfactory growth of the two species in the region may be associated with the local environmental conditions. Located near the equator, the Macrotidal Mangrove Coast of the Amazon has high temperatures and salinity, which benefit the development of the two species. According to the literature, both M. guyanensis and M. strigata are euryhaline species (PEREIRA-BARROS, 1987PEREIRA-BARROS, J. B. Ecologia do sururu Mytella falcata (Mytilidae) da Lagoa Mundaú (Maceió-AL). Boletim de Estudos de Ciências do Mar, v.6, p.84-86, 1987.; LEONEL & SILVA, 1988LEONEL, R. M. V.; SILVA, I. N. Estudo da sobrevivência e da capacidade de isolamento de Mytella guyanensis (Mollusca - Bivalvia) em diferentes salinidades. Rev Nordest Biol, v.6, n.1, p.35-41, 1988. Available from: <Available from: https://periodicos.ufpb.br/index.php/revnebio/article/view/17982 >. Accessed: Nov. 10, 2021.
https://periodicos.ufpb.br/index.php/rev...
; RICE et al., 2016RICE, M. A. et al. Identification and salinity tolerance of the western hemisphere mussel Mytella charruana (D’Orbigny, 1842) in the Philippines. J Shellfish Res, v.35, n.4, p.865-873, 2016. Available from: <Available from: https://doi.org/10.2983/035.035.0415 >. Accessed: Jan. 22, 2022. doi: 10.2983/035.035.0415.
https://doi.org/10.2983/035.035.0415...
;). For instance, M. guyanensis can tolerate a salinity ranging from 5 to 35 g.Kg-1 (LEONEL & SILVA, 1988) and M. strigata can tolerate salinity ranging from 2 to 40 g.Kg-1 (YUAN et al., 2010YUAN, W. et al. Exploring the Survival Threshold: a study of salinity tolerance of the nonnative mussel Mytella charruana. J Shellfish Res, v.29, n.2, p.415-422, 2010. Available from: <Available from: https://bioone.org/journals/journal-of-shellfish-research/volume-29/issue-2/035.029.0218/Exploring-the-Survival-Threshold--A-Study-of-Salinity-Tolerance/10.2983/035.029.0218.short >. Accessed: Apr. 17, 2022. doi: 10.2983/035.029.0218.
https://bioone.org/journals/journal-of-s...
), demonstrating the viability of farming. As native species to the region, both are adapted to high temperatures and salinity. Temperature, which plays an important role in the growth, feeding rate, survival, and reproduction of aquatic organisms, was within the tolerable range for the two species, as reported by ONODERA & HENRIQUES (2017ONODERA, F. K.; HENRIQUES, M. B. Mortality of Mytella falcata and M. guyanensis exposed to different temperatures. Bol Inst Pesca, v.43, n.1, p.106-111, 2017. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1201 >. Accessed: Dec. 14, 2021. doi: 10.20950/1678-2305.2017v43n1p106.
https://www.pesca.sp.gov.br/boletim/inde...
). Thus, the local temperature and salinity are favorable to the development of mussel farming in the region.
Studies that assess the farming of mussels in these areas can contribute data to assist in sustainable production and the reduction of anthropogenic pressure on the natural banks of these mussels, enabling the continuity of the stock and ensuring food for future generations. Although, all the parameters tested were favorable for cultivation, they were restricted to the assayed density of 840 ind. m-1 of rope; therefore, further research is warranted to investigate the effect that different stocking densities have on the productive parameters (growth and survival) of these species.
CONCLUSION:
The mussels Mytella guyanensis and Mytella strigata can be farmed on suspended ropes under estuarine conditions influenced by macrotides, demonstrating satisfactory daily growth rates (shell height and weight). Although, the survival rates at the end of the experiment were similar, commercial size was attained faster in M. strigata cultivation ropes, indicating the need for a cultivation time greater than 180 days for M. guyanensis. The environmental conditions of the Macrotidal Mangrove Coast of the Amazon did not constitute a problem for the natural development of the mussels studied herein, as both species reached their commercial size, indicating the farming viability of the two species in the dry season.
ACKNOWLEDGMENTS
The authors are grateful to the members of the Mariculture Center (NUMAR) for contributing to the execution of the different steps of this research; the Instituto Federal de Educação, Ciência e Tecnologia do Maranhão (IFMA [Federal Institute of Education, Science and Technology of the State of Maranhão]), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq [National Council of Scientific and Technological Development]), and the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA [State of Maranhão Foundation for Research and Scientific and Technological Development]) for fostering this project.
REFERENCES
- ALVARENGA, L., NALESSO, R. C. Preliminary assessment of the potential for mangrove oyster cultivation in Piraquê-açu river estuary (Aracruz, ES). Braz Arch Biol Technol, v.49, n.1, p.163-169, 2006. Available from: <Available from: https://www.scielo.br/j/babt/a/3YwFvdnCJzkCFpqMK43B9fp >. Accessed: Jan. 1, 2022. doi: 10.1590/S1516-89132006000100019.
» https://doi.org/10.1590/S1516-89132006000100019.» https://www.scielo.br/j/babt/a/3YwFvdnCJzkCFpqMK43B9fp - ALVARES, C. A. et al. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, v.22, n.6, p.711-728, 2013. Available from: <Available from: https://www.schweizerbart.de/papers/metz/detail/22/82078/Koppen_s_climate_classification_map_for_Brazil?af=crossref >. Accessed: Jan. 5, 2022. doi: 10.1127/0941-2948/2013/0507.
» https://doi.org/10.1127/0941-2948/2013/0507.» https://www.schweizerbart.de/papers/metz/detail/22/82078/Koppen_s_climate_classification_map_for_Brazil?af=crossref - ARAÚJO, A. R. R., et al. Management of the Mytella charruana (d’ Orbigny, 1846) fisheries along the coast of the Sergipe State: sustainability indicators. Rev Bras Eng Pesca, v.4, n.2, p.56-70, 2009. Available from: <Available from: https://ppg.revistas.uema.br/index.php/REPESCA/article/view/237 >. Accessed: Jan. 5, 2022. doi: 10.18817/repesca.v4i2.237.
» https://doi.org/10.18817/repesca.v4i2.237» https://ppg.revistas.uema.br/index.php/REPESCA/article/view/237 - CEBU, E. H. Bamboo Tray Module Mussel Farming. J Acad Res, v.1, n.4, p.22-39, 2016. Available from: <Available from: https://aquadocs.org/handle/1834/40344 >. Accessed: Jan. 5, 2022.
» https://aquadocs.org/handle/1834/40344 - CEUTA, L. O; BOEHS, G. Parasites of the mangrove mussel Mytella guyanensis (Bivalvia: Mytilidae) in Camamu Bay, Bahia, Brazil. Braz J Biol, v.72, n.3, p.421-427, 2012. Available from: <Available from: https://www.scielo.br/j/bjb/a/6BBh3qqr47vWVK35NDMKtwv >. Accessed: Jan. 5, 2022. doi: 10.1590/S1519-69842012000300002.
» https://doi.org/10.1590/S1519-69842012000300002.» https://www.scielo.br/j/bjb/a/6BBh3qqr47vWVK35NDMKtwv - CHRISTO S. W, et al. Reproductive aspects of mussels (Bivalvia, Mollusca) in the Paranaguá estuarine complex, Paraná, Brazil. Bol Inst Pesca, v.42, n.4, p.924-936, 2016. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1189 >. Accessed: Feb. 2, 2022. doi: 10.20950/1678-2305.2016v42n4p924.
» https://doi.org/10.20950/1678-2305.2016v42n4p924.» https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1189 - COLOMBO J. et al. Proximate chemical composition and fatty acid profile of the blue musselMytilus edulis from rocky shores and long line cultures in San Jorge Gulf, Argentina. Rev Biol Mar Oceanogr, v.51, n.2, p.293-299, 2016. Available from: <Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0718-19572016000200007&lng=es&nrm=iso >. Accessed: Feb. 2, 2022. doi: 10.4067/S0718-19572016000200007.
» https://doi.org/10.4067/S0718-19572016000200007» http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0718-19572016000200007&lng=es&nrm=iso - COSTA, K. G. E; NALESSO, R. C. Cultivo experimental de Mytella falcata (Orbigny, 1846) e M. guyanensis (Lamarck, 1819), no estuário do Rio Piraquê-açu (Aracruz-ES). Acta Limnol Bras, v.14, n.1, p.15-22, 2002. Available from: <Available from: http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d >. Accessed: Feb. 5, 2022.
» http://www.alb.periodikos.com.br/article/627b10d6782aad05cb235d6d - CRUZ, R. A.; VILLALOBOS, C. R. Shell length at sexual maturity and spawning cycle of Mytella guyanensis (Bivalvia: Mytilidae) from Costa Rica. Rev Biol Trop, v.41, n.1, p.89-92, 1993. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/download/23311/23613/ >. Accessed: Mar. 22, 2022. doi: 10.15517/rbt.v41i1.23311.
» https://doi.org/10.15517/rbt.v41i1.23311.» https://revistas.ucr.ac.cr/index.php/rbt/article/download/23311/23613/ - DIARTE-PLATA, G. et al. Growth and survival of the musselMytella strigata(Bivalvia: Mytilidae) in suspension culture in Macapule lagoon, Sinaloa, Mexico. Hidrobiologica, v.23, n.3, p.374-385, 2013. Available from: <Available from: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972013000300010 >. Accessed: Mar. 22, 2022.
» https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-88972013000300010 - DÍAZ, C. et al. Comparative growth of Mytilus chilensis (Hupé 1854) and Mytilus galloprovincialis (Lamarck 1819) in aquaculture longline system in Chile. Aquaculture, v.507, p.21-27, 2019. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0044848617312280 >. Accessed: Mar. 16, 2022. doi: 10.1016/j.aquaculture.2019.03.057.
» https://doi.org/10.1016/j.aquaculture.2019.03.057.» https://www.sciencedirect.com/science/article/pii/S0044848617312280 - GALTSOFF, P. S. The American oyster Crassostrea virginica Fish Bull, v.64, n.1, p.11-28, 1964. Available from: <Available from: https://link.springer.com/article/10.2307/1350854 >. Accessed: Jan. 22, 2022. doi: 10.2307/1350854.
» https://doi.org/10.2307/1350854.» https://link.springer.com/article/10.2307/1350854 - GUARALDE, A. R. et al. An Artemia franciscana bioassay for the monitoring of lipophilic phycotoxins in marine bivalve mollusc cultures: An alternative to screening testing? Revista Ambiente & Água, v.15, n.5, 2020. Available from: <Available from: https://doi.org/10.4136/ambi-agua.2549 >. Accessed: Feb. 15, 2022. doi: 10.4136/ambi-agua.2549.
» https://doi.org/10.4136/ambi-agua.2549.» https://doi.org/10.4136/ambi-agua.2549 - IBGE - Instituto Brasileiro de Geografa e Estatística. Pesquisa da Pecuária Municipal 2017, 2018 e 2019, 2020. Available from: <Available from: https://sidra.ibge.gov.br/tabela/3940 >. Accessed: May, 15, 2022.
» https://sidra.ibge.gov.br/tabela/3940 - IGARASHI, M. A. Aspects of technological development cycle of mussel farming in Brazil (A Review). Rev Unimar Ciências, v.28, n.1-2, p.1-19, 2019. Available from: <Available from: http://ojs.unimar.br/index.php/ciencias/article/view/1514/865 >. Accessed: May, 10, 2022.
» http://ojs.unimar.br/index.php/ciencias/article/view/1514/865 - LEONEL, R. M. V.; SILVA, I. N. Estudo da sobrevivência e da capacidade de isolamento de Mytella guyanensis (Mollusca - Bivalvia) em diferentes salinidades. Rev Nordest Biol, v.6, n.1, p.35-41, 1988. Available from: <Available from: https://periodicos.ufpb.br/index.php/revnebio/article/view/17982 >. Accessed: Nov. 10, 2021.
» https://periodicos.ufpb.br/index.php/revnebio/article/view/17982 - MONTELES, J. S. et al. Productive parameters of Mytella charruana farmed in macrotidal mangroves on the amazonian coast, Brazil. In: SILVA F. F (Org.). Aquicultura e pesca: adversidades e resultados 2. Ponta Grossa: Atena Editora, p.151-165, 2019. Available from: <Available from: https://www.atenaeditora.com.br/arquivos/ebooks/aquicultura-e-pesca-adversidades-e-resultados-2 >. Accessed: May, 22, 2022. doi: 10.22533/at.ed.16119151016.
» https://doi.org/10.22533/at.ed.16119151016.» https://www.atenaeditora.com.br/arquivos/ebooks/aquicultura-e-pesca-adversidades-e-resultados-2 - MORA, D. A.; ALPÍZAR, B. M. Crecimiento de Mytella guyanensis (Bivalvia: Mytilidae) en balsas flotantes. Rev Biol Trop, v.46, n.6, p.21-26, 1998. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/view/29630 >. Accessed: Jan. 15, 2022.
» https://revistas.ucr.ac.cr/index.php/rbt/article/view/29630 - MORENO, D. et al. Métodos para identificar, diagnosticar y evaluar el grado de eutrofia. Contatos, 78: 25-33, 2010. Available from: <Available from: http://www2.izt.uam.mx/newpage/contactos/anterior/n78ne/eutrofia2.pdf >. Accessed: Mar. 26, 2022.
» http://www2.izt.uam.mx/newpage/contactos/anterior/n78ne/eutrofia2.pdf - NARCHI, W.; GALVÃO-BUENO, M. S. Anatomia funcional de Mytella charruana (D’Orbigny, 1846) (Bivalvia: Mytilidae). Bolm Zool Univ S Paulo, v.6, p.113-145, 1983. Available from: <Available from: https://www.revistas.usp.br/bolzoo/article/view/121956 >. Accessed: Mar. 26, 2022. doi: 10.11606/issn.2526-3358.bolzoo.1983.121956.
» https://doi.org/10.11606/issn.2526-3358.bolzoo.1983.121956.» https://www.revistas.usp.br/bolzoo/article/view/121956 - NASCIMENTO, F. C. A. et al. Statistical Analysis of Dry and Rainy Event of Maranhão Rainfall. Rev Bras de Meteorol, v.32, n.3, p.375-386, 2017. Available from: <Available from: https://www.scielo.br/j/rbmet/a/Jt4QgwVYxYzphGKVGypvWwk >. Accessed: Mar. 26, 2022. doi: 10.1590/0102-77863230005.
» https://doi.org/10.1590/0102-77863230005.» https://www.scielo.br/j/rbmet/a/Jt4QgwVYxYzphGKVGypvWwk - NISHIDA, A. K.; LEONEL, R. M. V. Occurrence, population dynamics and habitat characterization of Mytella guyanensis (Lamarck, 1819) (Mollusca, Bivalvia) in the Paraíba do Norte river estuary. Bolm Inst Oceanogr, v.43, p.49-57, 1995. Available from: <Available from: https://www.scielo.br/j/bioce/a/yQbtBn5zYWb7Sf9yzdhCpyN >. Accessed: Nov. 30, 2021. doi: 10.1590/S0373-55241995000100004.
» https://doi.org/10.1590/S0373-55241995000100004.» https://www.scielo.br/j/bioce/a/yQbtBn5zYWb7Sf9yzdhCpyN - ONODERA, F. K.; HENRIQUES, M. B. Mortality of Mytella falcata and M. guyanensis exposed to different temperatures. Bol Inst Pesca, v.43, n.1, p.106-111, 2017. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1201 >. Accessed: Dec. 14, 2021. doi: 10.20950/1678-2305.2017v43n1p106.
» https://doi.org/10.20950/1678-2305.2017v43n1p106.» https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/1201 - PEREIRA-BARROS, J. B. Ecologia do sururu Mytella falcata (Mytilidae) da Lagoa Mundaú (Maceió-AL). Boletim de Estudos de Ciências do Mar, v.6, p.84-86, 1987.
- PEREIRA, O. M. et al. Production estimate of Mytella falcata and M. guyanensis in natural beds of Ilha Comprida estuary (São Paulo, Brasil). Bol Inst Pesca, v.29, n.2, p.139-149, 2018. Available from: <Available from: https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/Pereira >. Accessed: Dec. 28, 2021.
» https://www.pesca.sp.gov.br/boletim/index.php/bip/article/view/Pereira - PEREIRA, O. M.; GRAÇA LOPES, R. Fixação de sementes de Mytella falcata (sururu) em coletores artificiais no Canal de Bertioga, Estuário de Santos, Estado de São Paulo, Brasil. Bol Inst Pesca, v.22, n.1, p.165-173, 1995. Available from: <Available from: https://www.bvs-vet.org.br/vetindex/periodicos/boletim-do-instituto-de-pesca/22-(1995)-1/fixacao-de-sementes-de-mytella-falcata-sururu-em-coletores-artificiais >. Accessed: Nov. 27, 2021.
» https://www.bvs-vet.org.br/vetindex/periodicos/boletim-do-instituto-de-pesca/22-(1995)-1/fixacao-de-sementes-de-mytella-falcata-sururu-em-coletores-artificiais - PEZY, J. P. et al. First record of the gastropod Stramonita haemastoma (Linnaeus, 1767) in the English Channel. BioInvasions Rec, v.8, n.2, p.266-272, 2019. Available from: <Available from: https://hal-normandie-univ.archives-ouvertes.fr/hal-02153586 >. Accessed: Jun. 25, 2022. doi: 10.3391/bir.2019.8.2.08.
» https://doi.org/10.3391/bir.2019.8.2.08.» https://hal-normandie-univ.archives-ouvertes.fr/hal-02153586 - PIERRI, B. S. et al. The brown mussel Perna perna in Brazil: native or exotic? Arq Bras Med Vet Zootec, v.68, n.2, p.404-414, 2016. Available from: <Available from: https://www.scielo.br/j/abmvz/a/G9pjBLNhnw6rF98JjjW9Zqb >. Accessed: Jan. 28, 2022. doi: 10.1590/1678-4162-8534.
» https://doi.org/10.1590/1678-4162-8534.» https://www.scielo.br/j/abmvz/a/G9pjBLNhnw6rF98JjjW9Zqb - REIS JÚNIOR, J. J. C. et al. Morphometric analysis and meat yieldof Mytilidae caught in the state of Sergipe. Scientia Plena, v.12, n.12, p.1-11, 2016. Available from: <Available from: https://www.scientiaplena.org.br/sp/article/view/3066 >. Accessed: May, 19, 2022. doi: 10.14808/sci.plena.2016.120201.
» https://doi.org/10.14808/sci.plena.2016.120201.» https://www.scientiaplena.org.br/sp/article/view/3066 - RICE, M. A. et al. Identification and salinity tolerance of the western hemisphere mussel Mytella charruana (D’Orbigny, 1842) in the Philippines. J Shellfish Res, v.35, n.4, p.865-873, 2016. Available from: <Available from: https://doi.org/10.2983/035.035.0415 >. Accessed: Jan. 22, 2022. doi: 10.2983/035.035.0415.
» https://doi.org/10.2983/035.035.0415.» https://doi.org/10.2983/035.035.0415 - SIBAJA, W. G. Fijación larval y crecimiento del mejillón Mytella guayanensis L. (Bivalvia: Mytilidae) en Isla Chira, Costa Rica. Rev Biol Trop, 36: 453-456, 1988. Available from: <https://revistas.ucr.ac.cr/index.php/rbt/article/download/23854/24037>. Accessed: Jan. 8, 2022.
- SIBAJA, W. G.; VILLALOBOS, C. Crecimiento del mejillón chora Mytella guayanensis (Bivalvía: Mytilidae) en el Golfo de Nicoya, Costa Rica. Rev Biol Trop, v.34, 2, p.231-236, 1986. Available from: <Available from: https://revistas.ucr.ac.cr/index.php/rbt/article/view/24300 >. Accessed: Mar. 7, 2022.
» https://revistas.ucr.ac.cr/index.php/rbt/article/view/24300 - SILVESTRI, F. et al. Characterization of vagile fauna associated with suspended mussel farms. Arq Cienc Mar, v.51, n.2, p.57-71, 2018. Available from: <Available from: http://www.periodicos.ufc.br/arquivosdecienciadomar/article/view/32531 >. Accessed: Apr. 22, 2022. doi: 10.32360/acmar.v51i2.32531.
» https://doi.org/10.32360/acmar.v51i2.32531.» http://www.periodicos.ufc.br/arquivosdecienciadomar/article/view/32531 - SOUZA FILHO, P. W. M. Costa de manguezais de macromaré da Amazônia: cenários morfológicos, mapeamento e quantificação de áreas usando dados de sensores remotos. Rev Bras Geofis, v.23, n.4, p.427-435, 2005. Available from: <Available from: https://www.scielo.br/j/rbg/a/548fTgMXRHTmSTYBXNhfxbc >. Accessed: Apr. 22, 2022. doi: 10.1590/S0102-261X2005000400006.
» https://doi.org/10.1590/S0102-261X2005000400006.» https://www.scielo.br/j/rbg/a/548fTgMXRHTmSTYBXNhfxbc - SOUZA, T. O. et al. Population structure and identification of two matrilinear and one patrilinear mitochondrial lineages in the mussel Mytella charruana Estuar Coast Shelf Sci, v.156, p.165-174, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0272771414003308 >. Accessed: Apr. 22, 2022. doi: 10.1016/j.ecss.2014.11.009.
» https://doi.org/10.1016/j.ecss.2014.11.009.» https://www.sciencedirect.com/science/article/pii/S0272771414003308 - SUPLICY, F. M. Cultivo de mexilhões: sistema contínuo e mecanizado. Florianópolis: Epagri, 2017, 124p. Available from: <Available from: https://docweb.epagri.sc.gov.br/website_epagri/Cedap/Livro/4-%20Livro-maricultura-mexilhao-gestao-sistema-de-cultivo-semente-engorda-risco.pdf >. Accessed: Apr. 22, 2022.
» https://docweb.epagri.sc.gov.br/website_epagri/Cedap/Livro/4-%20Livro-maricultura-mexilhao-gestao-sistema-de-cultivo-semente-engorda-risco.pdf - SUPLICY, F. M. Evaluation of initial density effect on the productivity of Perna perna mussels in Santa Catarina State. Agropecuária Catarinense, v.31, n.1, p.77-81, 2018. Available from: <Available from: https://publicacoes.epagri.sc.gov.br/RAC/article/view/253 >. Accessed: Apr. 29, 2022. doi: 10.22491/RAC.2018.v31n1.11.
» https://doi.org/10.22491/RAC.2018.v31n1.11.» https://publicacoes.epagri.sc.gov.br/RAC/article/view/253 - UREÑA-JUÁREZ, P.; PERALTA, C. D. Cultivo en suspensión de Mytella guyanensis (Bivalvia: Mytilidae) en Isla Chira, Costa Rica: implicaciones ambientales y biológicas. Repertório Científico, v.23, n.2, p.76-88. Available from: <Available from: https://revistas.uned.ac.cr/index.php/repertorio/article/view/3029/4285 >.2020 Accessed: Apr. 20, 2022. doi: 10.22458/rc.v23i2.3029.
» https://doi.org/10.22458/rc.v23i2.3029.» https://revistas.uned.ac.cr/index.php/repertorio/article/view/3029/4285 - YUAN, W. et al. Exploring the Survival Threshold: a study of salinity tolerance of the nonnative mussel Mytella charruana J Shellfish Res, v.29, n.2, p.415-422, 2010. Available from: <Available from: https://bioone.org/journals/journal-of-shellfish-research/volume-29/issue-2/035.029.0218/Exploring-the-Survival-Threshold--A-Study-of-Salinity-Tolerance/10.2983/035.029.0218.short >. Accessed: Apr. 17, 2022. doi: 10.2983/035.029.0218.
» https://doi.org/10.2983/035.029.0218.» https://bioone.org/journals/journal-of-shellfish-research/volume-29/issue-2/035.029.0218/Exploring-the-Survival-Threshold--A-Study-of-Salinity-Tolerance/10.2983/035.029.0218.short
-
CR-2021-0777.R1
Edited by
Publication Dates
-
Publication in this collection
16 Sept 2022 -
Date of issue
2023
History
-
Received
28 Oct 2021 -
Accepted
22 July 2022 -
Reviewed
27 Aug 2022