Abstracts
Rearing leafhopper (Hemiptera: Cicadellidae) vectors free of Xylella fastidiosa is a requirement for studies of various aspects of vector-pathogen interactions. The selection of a plant that allows vector development but not bacterial multiplication is desirable to produce healthy vectors. In this study, two leafhopper hosts, Vernonia condensata ('boldo') and Aloysia virgata ('lixeira') were needle inoculated with citrus and coffee strains of X. fastidiosa to evaluate if these plants support pathogen colonization. The inoculated plants did not present symptoms and the pathogen was not detected by culture and PCR tests, neither soon after inoculation (7-14 days) nor later, at 1, 4, 6 and 12 months after inoculation. To obtain healthy adults of the leafhopper vectors Acrogonia citrina, Bucephalogonia xanthophis, Dilobopterus costalimai, Homalodisca ignorata and Oncometopia facialis, early-instar nymphs were reared on V. condensata. X. fastidiosa was not detected in any of 175 adults obtained. V. condensata and A. virgata are nonpropagative hosts of X. fastidiosa and enable the production of healthy leafhoppers for vector studies.
Vernonia condensata; citrus variegated chlorosis; coffee leaf scorch; sharpshooter vectors; rearing technique
A obtenção de cigarrinhas (Hemiptera: Cicadellidae) livres de Xylella fastidiosa é importante para estudos de interação entre essa bactéria e seus vetores, sendo desejável a seleção de uma planta que permita a criação desses insetos, mas não a multiplicação da bactéria. Neste estudo, duas plantas hospedeiras de cigarrinhas, Vernonia condensata (boldo) e Aloysia virgata (lixeira), foram inoculadas por agulha com as estirpes de citros e de cafeeiro de X. fastidiosa, para avaliar a possibilidade deste patógeno colonizá-las. Não foram observados sintomas, nem se detectou a bactéria por isolamento em meio de cultura e/ou PCR em períodos curtos (7 e 14 dias) ou longos (1, 4, 6 e 12 meses) após a inoculação. Para obtenção de adultos sadios das cigarrinhas vetoras, Acrogonia citrina, Bucephalogonia xanthophis, Dilobopterus costalimai, Homalodisca ignorata e Oncometopia facialis, ninfas de primeiros ínstares foram criadas em plantas de boldo. Não foi detectada X. fastidiosa em nenhum de 175 adultos obtidos da criação. V. condensata e A. virgata não permitem a colonização de X. fastidiosa, possibilitando assim a obtenção de cigarrinhas sadias para estudos com vetores.
Vernonia condensata; clorose variegada dos citros; requeima do cafeeiro; vetores; técnica de criação
ENTOMOLOGY
Identification of a non-host plant of Xylella fastidiosa to rear healthy sharpshooter vectors
Identificação de uma planta não-hospedeira de Xylella fastidiosa para criação de insetos vetores sadios
Rosangela Cristina MarucciI; Teresinha Augusta GiustolinI; Marcelo Pedreira de MirandaI; Helen MiqueloteI; Rodrigo Piacentini Paes de AlmeidaI, II; João Roberto Spotti LopesI * * Correspondig author < jrslopes@esalq.usp.br>
IUSP/ESALQ - Depto. de Entomologia, Fitopatologia e Zoologia Agrícola, C.P. 9 - 13418-900 - Piracicaba, SP - Brasil
IIUniversity of Hawaii, Dept. of Plant and Environmental Protection Sciences, 3050 Maile Way, Honolulu, HI 96822 USA
ABSTRACT
Rearing leafhopper (Hemiptera: Cicadellidae) vectors free of Xylella fastidiosa is a requirement for studies of various aspects of vector-pathogen interactions. The selection of a plant that allows vector development but not bacterial multiplication is desirable to produce healthy vectors. In this study, two leafhopper hosts, Vernonia condensata ('boldo') and Aloysia virgata ('lixeira') were needle inoculated with citrus and coffee strains of X. fastidiosa to evaluate if these plants support pathogen colonization. The inoculated plants did not present symptoms and the pathogen was not detected by culture and PCR tests, neither soon after inoculation (7-14 days) nor later, at 1, 4, 6 and 12 months after inoculation. To obtain healthy adults of the leafhopper vectors Acrogonia citrina, Bucephalogonia xanthophis, Dilobopterus costalimai, Homalodisca ignorata and Oncometopia facialis, early-instar nymphs were reared on V. condensata. X. fastidiosa was not detected in any of 175 adults obtained. V. condensata and A. virgata are nonpropagative hosts of X. fastidiosa and enable the production of healthy leafhoppers for vector studies.
Key words:Vernonia condensata, citrus variegated chlorosis, coffee leaf scorch, sharpshooter vectors, rearing technique
RESUMO
A obtenção de cigarrinhas (Hemiptera: Cicadellidae) livres de Xylella fastidiosa é importante para estudos de interação entre essa bactéria e seus vetores, sendo desejável a seleção de uma planta que permita a criação desses insetos, mas não a multiplicação da bactéria. Neste estudo, duas plantas hospedeiras de cigarrinhas, Vernonia condensata (boldo) e Aloysia virgata (lixeira), foram inoculadas por agulha com as estirpes de citros e de cafeeiro de X. fastidiosa, para avaliar a possibilidade deste patógeno colonizá-las. Não foram observados sintomas, nem se detectou a bactéria por isolamento em meio de cultura e/ou PCR em períodos curtos (7 e 14 dias) ou longos (1, 4, 6 e 12 meses) após a inoculação. Para obtenção de adultos sadios das cigarrinhas vetoras, Acrogonia citrina, Bucephalogonia xanthophis, Dilobopterus costalimai, Homalodisca ignorata e Oncometopia facialis, ninfas de primeiros ínstares foram criadas em plantas de boldo. Não foi detectada X. fastidiosa em nenhum de 175 adultos obtidos da criação. V. condensata e A. virgata não permitem a colonização de X. fastidiosa, possibilitando assim a obtenção de cigarrinhas sadias para estudos com vetores.
Palavras-chave:Vernonia condensata, clorose variegada dos citros, requeima do cafeeiro, vetores, técnica de criação
INTRODUCTION
One of the limiting factors to the study of many vector-pathogen interactions is the development of an efficient rearing system to obtain pathogen-free insects. In certain cases, vectors are difficult to be reared in large number in the laboratory, and most candidate host-plants are also hosts of the pathogen of interest. Diseases caused by the xylem-limited bacterium Xylella fastidiosa Wells et al. fall within this category: vectors are difficult to be reared and the bacterium colonizes most of the hosts in which it is inoculated (Hill & Purcell, 1995; Freitag, 1951).
X. fastidiosa is transmitted to plants by the leafhopper (Hemiptera: Cicadellidae) vectors of the subfamily Cicadellinae, known as sharpshooters, and causes diseases in many crops of economic importance in North America, such as grape, almond, peach and others (Hopkins & Purcell, 2002). In South America, the bacterium causes diseases of citrus, coffee and plum (French & Kitajima, 1978; Rossetti et al., 1990; Paradela Filho et al., 1995). Citrus and coffee X. fastidiosa strains are phylogenetically very similar to each other and distant from all other X. fastidiosa groups (Mehta & Rosato, 2001), and citrus strains cause disease in coffee (Li et al., 2001). It is not known if coffee strains cause disease in citrus. In the nature, X. fastidiosa also infects a wide range of plants without symptoms (Freitag, 1951; Hopkins & Adlerz, 1988). These plants could be alternative hosts for the multiplication of vectors, and/or serve as sources of X. fastidiosa inoculum.
Studies on the Pierce's disease of the grapevine (PD) strain of X. fastidiosa have demonstrated that the bacterium is foregut-borne (non-circulative) in the vectors (Purcell & Finlay, 1979). Bacterial cells acquired from infected plants were observed to be polarly attached to the foregut, particularly in the cibarium (suction pump), precibarium, and anterior portion of the esophagous (Purcell et al., 1979; Brlansky et al., 1983). The pathogen multiplied in the vector (Hill & Purcell, 1995), and sharpshooter adults retained infectivity for life (Severin, 1949; Hill & Purcell, 1995). However, infective nymphs lose infectivity after molting, suggesting that transmissible bacterial cells are limited to the vector's foregut (Purcell & Finlay, 1979).
Protocols to rear sharpshooters for multiple generations in the laboratory have not been developed and a good strategy to obtain healthy insects for transmission experiments would be to select a host plant that supports nymphal development, but not the multiplication of X. fastidiosa. Vernonia condensata Baker (Asteraceae) and Aloysia virgata (Ruiz & Pavan) Juss (Verbenaceae) were found to frequently harbor large numbers of leafhopper sharpshooters and other xylem feeding insects (Almeida, 1999; Giustolin et al., 2002), including species identified as X. fastidiosa vectors to citrus (Roberto et al., 1996; Krügner et al., 2000). Previous work has shown that V. condensata ('boldo' or 'alumã') supported the development of sharpshooter nymphs (Almeida, 1999; Milanez et al., 2001). These two plant species were chosen as test plants and evaluated regarding the ability of two X. fastidiosa strains (citrus and coffee) to infect, multiply and survive in V. condensata and A. virgata. A report on the development of a sharpshooter rearing technique that uses these plants to produce non-infective vectors for transmission studies of X. fastidiosa is also presented.
MATERIAL AND METHODS
Evaluation of V. condensata and A. virgata as hosts of X. fastidiosa
Two X. fastidiosa strains, CCT 6570 and CCT 6756 (both deposited at Coleção de Culturas Tropical, Fundação André Tosello, Campinas, SP), which cause citrus variegated chlorosis (CVC) and coffee leaf scorch (CLS), respectively, were used for inoculation of the two plant species. Cultures of each strain were obtained after two passages on solid periwinkle wilt gelrite (PWG) medium (Hill & Purcell, 1995) from purified inoculum preserved at -80°C. Bacterial colonies were scrapped from PWG with platinum loop and homogenized in phosphate buffer saline (PBS) until the resulting suspension became turbid. Concentrations of viable bacteria in the suspensions, as determined by dilution plating, were 1.1 x 107 colony forming units (CFU) mL-1 and 2.5 x 108 CFU mL-1 for the citrus and coffee strains, respectively. Twenty potted plants of V. condensata and five of A. virgata, obtained from healthy propagative material or seeds, were needle-inoculated with each strain. Ten seedlings of Citrus sinensis (L.) Osbeck (var. Caipira) and 15 of Coffea arabica L. (cv. Mundo Novo) were inoculated simultaneously with the citrus and coffee strains, respectively, and served as positive controls. Test plants were inoculated in three different points of the stem; each point received 5 mL of suspension, which was placed on the stem surface and pin-pricked five times with a No.0 entomological pin. Negative controls consisted of five to ten plants of each species inoculated with PBS. At 1, 4, 6 and 12 months after inoculation, leaves located immediately above the inoculation points were sampled and tested by culturing (primary isolation) and/or polymerase chain reaction (PCR) for the presence of X. fastidiosa.
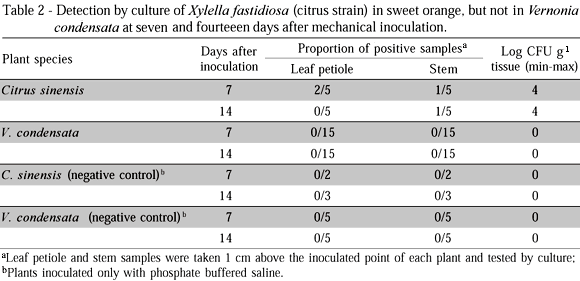
A second experiment was set to evaluate the short-term survival of X. fastidiosa cells in V. condensata. Thirty test plants of V. condensata and 10 plants of C. sinensis (positive control) were needle-inoculated (one point per plant) with 5 mL of a 8.8 x 108 CFU mL-1 suspension of the citrus strain. Ten plants of V. condensata and 5 of citrus were inoculated only with PBS (negative controls). At seven and 14 days after needle inoculation, samples of the leaf petiole and stem located 1 cm above the inoculated point were taken from each test plant, and submitted to primary isolation for detection of viable X. fastidiosa cells. Half of the plants were tested per period.
Primary isolation of X. fastidiosa
Bacteria were isolated from plants using the method of Hill & Purcell (1995) and adapted to citrus by Almeida et al. (2001). The petiole and part of the mid-vein of sampled leaves were cut from the leaf, weighed, surface sterilized, chopped, and transferred to glass tubes (16 mm in diameter) containing 2 mL of PBS. Samples were homogenized at 20,000 rpm in a tissue grinder and the resulting suspension was diluted 10-fold in a laminar flow chamber and plated on PWG. The plates were incubated at 28°C and the number of colony forming units (CFU) were counted after 14 days. Bacterial concentration in the plants (CFU g-1 of tissue) was estimated based on the initial weight of the samples, and the number of CFU counted in the dilution used. Colonies of both strains were routinely tested by PCR to certify that they were X. fastidiosa.
The primary isolation procedure described, which involves maceration of the plant tissue, may release plant inhibitors that limit the growth of X. fastidiosa on the culture media (Purcell & Saunders, 1999). To investigate a possible in vitro inhibition of X. fastidiosa, homogenized tissues of V. condensata and A. virgata were prepared in glass tubes with 2 mL of a suspension of »106 CFU mL-1 of the citrus X. fastidiosa strain (CCT 6570). Four treatments were prepared: (A) 0.05 g of surface-sterilized healthy leaf midvein and petiole of V. condensata; (B) 0.05 g of surface-sterilized healthy leaf midvein and petiole of A. virgata; (C) and (D) the cell suspension only. Treatments A, B and C were homogenized at 20,000 rpm as described, whereas D was a non-homogenized control. Suspensions were diluted to 10 and 100 fold after processing and plated on solid PWG medium and CFU were counted 14 d later. The treatments were replicated six times; each tube was a replicate. Data were submitted to ANOVA and Tukey Test (P < 0.05).
PCR detection of X. fastidiosa in plants
DNA extraction from plant samples was carried out as described by Minsavage et al. (1994), except that higher dilution of the extract (1:100) and higher concentration of ascorbic acid (0.1 mol L-1) (Pinto & Leite Jr., 1999). PCR was carried out by using the primers RST31 and RST33 that amplify a 733-bp DNA fragment of several strains of X. fastidiosa (Minsavage et al., 1994), and CVC1/272-2-int for specific amplification of a 500-bp product of the citrus (Pooler & Hartung, 1995) and coffee (Coletta Filho & Machado, 2001) strains of the bacterium. The two primer pairs were used in the same reaction (multiplex PCR). Amplification was carried out under the conditions reported by Minsavage et al. (1994). After amplification, 2 mL of dye (0.25% bromophenol blue; 40% sucrose) were added to each sample and the PCR products were separated by 1.5% agarose gel eletrophoresis in buffer TBE (89 mM Tris; 89 mM boric acid; 2 mM EDTA, pH 8.0) with ethidium bromide (0.5 mg mL-1). DNA fragments were visualized under UV light and documented in the Eagle Eye II system (Stratagene, La Jolla, CA 92037, EUA).
Rearing technique
To obtain sharpshooter eggs, field-collected adults were confined for oviposition (7-14 days) on healthy V. condensata or C. sinensis (X. fastidiosa-free, certified nursery trees). V. condensata was used as oviposition and development host for the sharpshooter Bucephalogonia xanthophis (Berg), whereas C. sinensis was the oviposition host for Acrogonia citrina Marucci & Cavichioli, Dilobopterus costalimai Young, Homalodisca ignorata Melichar, and Oncometopia facialis (Signoret). During oviposition, the insects were confined on the plants inside rearing cages (32 ´ 32 ´ 50 cm) or cloth (dacron-organdy) bags in the greenhouse. Plants of V. condensata containing eggs of B. xanthophis were transferred directly to larger rearing cages (50 ´ 60 ´ 70 cm) for nymphal development.
When citrus was used as oviposition host, egg-bearing leaves were detached from the plants and placed inside Petri dishes. Citrus petioles were covered with moistened cotton to keep leaves turgid. The Petri dishes were kept in an incubator at 25 ± 2oC and checked every morning (9h00-12h00) for eclosion of nymphs. Soon after eclosion, first-instar nymphs were transferred by plastic vials to healthy plants of V. condensata, inside the larger rearing cage (50 ´ 60 ´ 70 cm). Nymphs of B. xanthophis were allowed to develop up to the adult stage on V. condensata, which proved to be a good feeding and developmental host for this particular species. For the other sharpshooter species, healthy nursery trees of C. sinensis grafted on Citrus limonia Osbeck (rangpur lime) were latter added to the cage for development of 3rd- to 5th-instar nymphs and adult emergence.
PCR detection of X. fastidiosa in the insects
X. fastidiosa transmission by vectors from citrus to citrus is inefficient (1.0 to 12.0% per O. facialis and B. xanthophis, respectively) (Krügner et al., 2000), and transmission experiments take a long time to complete (incubation period of approximately 6 months) (Lopes et al., 1996). To determine if any of the insects had X. fastidiosa, independently of their ability to transmit the bacterium, they were tested using a PCR-based diagnostic method.
Samples of sharpshooter adults of the various species obtained from the rearing system were tested. Because of the relatively low population of X. fastidiosa cells in vector heads (Hill & Purcell, 1995) and the possible presence of PCR inhibitors in the insect vectors, a special technique that includes chelating agents (Ciapina & Lemos, 2001) was used to extract DNA from sharpshooters. A nested-PCR assay was used to detect X. fastidiosa in the samples. External primers 272-1 and 272-2 were used in a first reaction to amplify a DNA fragment of 700 bp, which was then used as a template for a second reaction (nested-PCR) with the internal and specific primers CVC-1 and 272-2-int (Pooler & Hartung, 1995). The PCR mix for the first reaction was prepared as follows: 1X buffer (Tris 20 mmol L-1; KCl 50 mmol L-1; pH 8.4 - Life Technologies); 2 mmol L-1 MgCl2; 200 mmol L-1 of each dNTP; 1U Taq DNA polymerase; 1 mL (0.5 mmol L-1) of each external primer and 8 mL of sample DNA for a final volume of 20 mL. PCR conditions were: 1 cycle at 94oC for 2 min, followed by 35 cycles at 94oC for 1 min, 62oC for 1 min, 72oC for 1,5 min, and then by one final cycle at 72oC for 5 min. The same mix and conditions were used for the nested-PCR, except that 3 mL of the first reaction product were used as template for the internal primers. Nested-PCR products were visualized by agarose gel electrophoresis as described for plant samples.
Three positive controls were included in all PCR assays: (1) cultured cells of X. fastidiosa, and DNA extracted from (2) infected plants and (3) insects that had previously fed on infected plants. MiliQ water and DNA extracted from healthy insects were used as negative controls.
RESULTS AND DISCUSSION
Evaluation of V. condensata and A. virgata as hosts of X. fastidiosa
In the first experiment, X. fastidiosa was not detected by culture or by PCR in any of the plants of V. condensata and A. virgata either at one, four, six or 12 months after needle inoculation of the citrus (CVC) or coffee (CLS) strains of the pathogen (Table 1). In addition, no symptoms were observed in these plants when compared to the negative controls. As expected, viable X. fastidiosa cells were recovered in cultures from C. sinensis and C. arabica plants (positive controls) inoculated with the citrus and coffee strains, respectively, at all evaluation dates. At 12 months after inoculation, the pathogen was detected by PCR and culture in most citrus (70%) and coffee (93.3%) plants. In general, the proportions of infected plants and the concentrations of cultivable cells of X. fastidiosa were higher in coffee than in citrus, suggesting that the former plant is a better propagative host for the pathogen. None of the non-inoculated (negative control) plants of the four species were positive for X. fastidiosa (data not shown).
In the second experiment, X. fastidiosa was not detected by culture from stems and leaves of V. condensata sampled one centimeter above the inoculated point at seven or 14 days after inoculation of the citrus strain. In contrast, the bacterium was successfully cultured from citrus stems (10%) and leaves (40%) at seven days, and from stems (20%) at 14 days after inoculation (Table 2).
Tissue homogenates of V. condensata and A. virgata produced no inhibitory effect on the growth of X. fastidiosa on solid medium. Bacterial suspensions homogenized with either plant species actually had higher populations of surviving X. fastidiosa after plating than did the homogenized and non-homogenized suspensions in PBS alone (P < 0.05) (Table 3). The lower bacterial numbers recovered in the non-homogenized treatment might have occurred because of cell clumping rather than cell death. Similar results were obtained by Almeida et al. (2001), who observed higher viability of X. fastidiosa suspensions homogenized with citrus tissue than with PBS alone. The higher survival of X. fastidiosa in the presence of plant homogenates cannot be explained. The failure to culture X. fastidiosa in V. condensata and A. virgata (Tables 1 and 2) probably did not resulted from inhibitory effect of the plant homogenates on bacterial growth.
X. fastidiosa has a broad host plant range, although its infection may not always result in visible disease symptoms (Hopkins & Purcell, 2002). After studying colonization and survival of the PD strain of X. fastidiosa in different trees and shrubs, Purcell & Saunders (1999) classified the host plants in different categories in relation to multiplication (propagative and non-propagative), movement (systemic and non-systemic) and persistence (pathological and non-pathological) of the pathogen. Highly susceptible species such as California blackberry (Rubus ursinus Cham. & Schltdl.) and large periwinkle (Vinca major L.) were classified as propagative, systemic and overwintering hosts. For other hosts, such as willows (Salix lasiolepis Benth.), X. fastidiosa established primary infections that died after a few weeks without symptom development; these plants were classified as nonpathological hosts. Based on these criteria, both V. condensata and A. virgata would fit in the non-propagative, non-systemic and non-pathological category. No early (primary) or late infection of X. fastidiosa could be detected in these plants by culture or PCR, indicating that they might be immune to this pathogen. However, only two strains of X. fastidiosa (CVC and CLS) were tested in these inoculation experiments. The host range varies among strains of the bacterium, and therefore the possibility that other strains might colonize V. condensata or A. virgata cannot be excluded. Despite these considerations, V. condensata and A. virgata can be designated as adequate hosts to ensure production of healthy sharpshooters for transmission studies related to CVC and CLS, because there is no evidence of infection and colonization of these plants by the citrus and coffee strains of X. fastidiosa.
Rearing system for production of healthy vectors
The rearing system developed allowed the production of healthy adult sharpshooters of various species known to be vectors of X. fastidiosa to citrus. Several lab-reared individuals of each species [A. citrina (10), B. xanthophis (24), D. costalimai (69), H. ignorata (46) and O. facialis (26)] were tested, but none of them was positive for X. fastidiosa using nested-PCR. Bacterial DNA was detected only in samples of individuals that had been previously exposed to infected plants (positive controls). These results were expected based on the information available on transmission mechanisms of the pathogen. Because transovarial transmission of X. fastidiosa is known not to occur (Freitag, 1951), newly-emerged nymphs will be free of this bacterium, unless they feed on infected tissue. In the case accidental feeding on infected plant occurs, any infective nymph will lose ability to transmit the pathogen during development on plants without X. fastidiosa, because the foregut-borne inoculum is lost with the old cuticle after an ecdysis (Purcell & Finlay, 1979; Purcell et al., 1979).
Based on the results of the inoculation study, both V. condensata and A. virgata can be used as early instar hosts which will induce loss of infectivity. However, V. condensata was used only because previous studies showed that it allows satisfactory survival of nymphs of D. costalimai (58%) and O. facialis (78%) (Milanez et al., 2001). It also permits rapid development of nymphs of Acrogonia sp. (37.5 days), D. costalimai (34.2 days) and O. facialis (63 days) at 25oC (Milanez et al., 2002). These nymphal development periods were shorter than those reported for these sharpshooters on C. sinensis (sweet orange) by Paiva et al. (2001) and Almeida & Lopes (1999) at similar temperatures. In addition, V. condensata can be easily propagated by stem cuttings and grows fast (30-60 days) under greenhouse conditions, with very few pest problems. Aphids are often observed, but can be eliminated with a selective insecticide (e.g. pirimicarb)
Measurement of the developmental (egg-adult) period was not made but general observations indicated approximate durations of 40 days for B. xanthophis, and 90 days for O. facialis in this system. Because of the fluctuating temperatures in the greenhouse, development was slower during fall and winter (April-August), when nocturnal temperatures were lower. According to Milanez et al. (2002), nymphal development is favored by temperatures in the range of 20-25oC.
Among the sharpshooters, B. xanthophis was reared with most success. This species readily oviposits in V. condensata and most eclosed nymphs survive on this host until the adult stage. Because the reared adults mated and laid eggs on this host, a continuous supply of healthy adults was obtained for 20 generations so far. When needed, field supplies of B. xanthophis adults and nymphs could be easily collected on young shoots of the ornamental shrub Duranta repens L. ("pingo-de-ouro"), which is commonly planted along sidewalks and gardens. Possibly because of its attractive green-yellow coloration, this plant is often visited by B. xanthophis. For the other sharpshooters studied, the final number of adults produced per rearing cage was low, despite the fairly large number of first-instar nymphs that were obtained in the laboratory and transferred to V. condensata. Milanez et al. (2002) reported maximum viabilities of 58, 80 and 30% for nymphs of Acrogonia sp., D. costalimai and O. facialis reared on V. condensata. By using nursery trees of sweet orange (C. sinensis) grafted on rangpur lime as rearing hosts, Paiva et al. (2001) obtained higher survival rates (50-96%) for nymphs of the same sharpshooter species. Interestingly, rangpur lime alone was a preferred host for oviposition of D. costalimai and O. facialis compared to V. condensata, but did not allow a reasonable survival (>25%) of the nymphs (Milanez et al., 2001). Therefore, in the rearing system used herein, it was decided to use healthy nursery trees of C. sinensis to improve survival of 3rd-5th instar nymphs and oviposition by the resulting adults of A. citrina, D. costalimai, H. ignorata, and O. facialis.
Insects which feed on xylem sap are in general polyphagous (Press & Whittacker, 1993). Some species studied also have an interesting behavior of changing preferred host plants in the field as seasons change, probably because of modifications in the physiology of the host (Purcell, 1976). Because xylem sap is an extremely diluted diet, sharpshooters have interesting energetic dilemmas (Novotny & Wilson, 1997), which make them feed and excrete many times their body weight everyday (Mittler, 1967). In addition, these insects are extremely efficient absorbers of amino acids and other compounds in their diet, using in most cases up to 99% of the amino acid content of the xylem sap (Andersen et al., 1989). Because of these interesting behavioral features, the combination of V. condensata and citrus in the same cage may complement each other nutritionally as hosts for the sharpshooters, and maybe promote mating and egg-laying.
ACKNOWLEDGMENTS
To Paulo C. Ferraz for technical assistance; Eliana G. Lemos and Luciana P. Ciapina (Dept. of Technology UNESP/FCAV/Jaboticabal) for sharing the sharpshooter DNA extraction method and the nested-PCR protocol. To CAPES, FAPESP, CBP&D/Café and FUNDECITRUS for scholarships and financial support.
Received January 24, 2003
Accepted August 26, 2003
- ALMEIDA, R.P.P. Multiplicação e movimentação de Xylella fastidiosa em mudas de Citrus sinensis e sua eficiência de aquisição e inoculação por vetores. Piracicaba: USP/ESALQ, 1999. 57p. (Dissertação - Mestrado)
- ALMEIDA, R.P.P.; LOPES, J.S. Desenvolvimento de Imaturos de Dilobopterus costalimai Young e Oncometopia facilais (Signoret) e Homalodisca ignorata Melichar (Hemiptera: Cicadellidae) em citros. Anais da Sociedade Entomológica do Brasil, v.28, p.179-182, 1999.
- ALMEIDA, R.P.P.; PEREIRA, E.F.; PURCELL, A.P.; LOPES, J.R.S. Multiplication and movement of a citrus strain of Xylella fastidiosa within sweet orange. Plant Disease, v.85, p.382-386, 2001.
- ANDERSEN, P.C.; BRODBECK, B.V.; MIZELL, R.F. III. Metabolism of amino acids and organic acids and sugars extracted from the xylem fluid of 4 host plants by Homalodisca coagulata Entomologia Experimentalis et Applicata, v.50, p.149-159, 1989.
- BRLANSKY, R.H.; TIMMER, L.W.; FRENCH, W.J.; MCCOY, R.E. Colonization of the sharpshooter vectors, Oncometopia nigricans and Homalodisca coagulata, by xylem-limited bacteria. Phytopathology, v.73, p.530-535, 1983.
- CIAPINA, L.P.; LEMOS, E.G.M. Rapid DNA extraction methodology to detect Xylella fastidiosa in sharpshooter leafhoppers by nested-PCR (compact disc). In: SIMPÓSIO GENOMA FUNCIONAL DA XYLELLA FASTIDIOSA, 1., Serra Negra, 2001. Posters São Paulo: FAPESP, 2001.
- COLETTA FILHO, H.D.; MACHADO, M.A. Hospedeiros, transmissão e técnicas de diagnóstico da bactéria Xylella fastidiosa Laranja, v.22, p.121-132, 2001.
- FREITAG, J.H. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopathology, v.41, p.920-934, 1951.
- FRENCH, W.J.; KITAJIMA, E.W. Occurrence of Plum Leaf scald in Brazil and Paraguay. Plant Disease Reporter, v.62, p.1035-1038, 1978.
- GIUSTOLIN, T.A.; LOPES, J.R.S.; MENDES, M.A.; MORAES, R.C.B.; RODRIGUES. Levantamento de hospedeiros alternativos das cigarrinhas vetoras de Xylella fastidiosa In: CONGRESSO BRASILEIRO DE ENTOMOLOGIA, 19., Manaus, 2002. Resumos Manaus: INPA, 2002. p.215.
- HILL, B.L.; PURCELL, A.H. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata Phytopathology, v.85, p.209-212, 1995.
- HOPKINS, D.L.; ADLERZ, W.C. Natural hosts of Xylella fastidiosa in Florida. Plant Disease, v.72, p.429-431, 1988.
- HOPKINS D.L.; PURCELL, A.H. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent disease. Plant Disease, v.86, p.1056-1066, 2002.
- KRÜGNER, R.; LOPES, M.T.V. de C.; SANTOS, J.S.; BERETTA, M.J.G.; LOPES, J.R.S. Transmission efficiency of Xylella fastidiosa to citrus by sharpshooters and identification of two new vector species. In: CONFERENCE OF INTERNATIONAL ORGANIZATION OF CITRUS VIROLOGISTS, 14., Campinas, 2000. Proceedings. Riverside: IOCV, 2000. p.423.
- LI, W.B.; PRIA JR., W.D.; TEIXEIRA, D.C.; MIRANDA, V.S.; AYRES, A.J.; FRANCO, C.F. Coffee leaf scorch caused by a strain of Xylella fastidiosa from citrus. Plant Disease, v.85, p.501-505, 2001.
- LOPES, J.R.S.; BERETTA, M.J.G.; HARAKAVA, R.; ALMEIDA, R.P.P.; KRÜGNER. R.; GARCIA JR., A. Confirmação da transmissão por cigarrinhas do agente causal da clorose variegada dos citros, Xylella fastidiosa Fitopatologia Brasileira, v.21, p.343, 1996. Suplemento/Apresentado ao 29. Congresso Brasileiro de Fitopatologia, Campo Grande, 1996 Resumo/
- MEHTA, A.; ROSATO, Y.B. Phylogenetic relationships of Xylella fastidiosa strains from different host, based on 16S rDNA and 16S-23S intergenic spacer sequences. International Journal of Systematic and Evolutionary microbiology, v.51, p.311-318, 2001.
- MILANEZ, J.M.; PARRA, J.R.P.; MAGRI, D.C. Alternation of host plants as a survival mechanism of leafhoppers Dilobopterus costalimai and Oncometopia facialis (Hemiptera: Cicadellidae), vectors of the citrus variegated chlorosis (CVC). Scientia Agricola, v.58, p.699-702, 2001.
- MILANEZ, J.M.; PARRA, J.R.P.; CUSTÓDIO, I.A.; MAGRI, D.C.; CERA, C. Biologia e exigências térmicas de três espécies de cigarrinhas vetoras da bactéria Xylella fastidiosa Laranja, v.23, p.127-140, 2002.
- MINSAVAGE, G.V.; THOMPSON, C.M.; HOPKINS, D.L.; LEITE, R.M.V.B.C.; STALL, R.E. Development of a polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology, v.84, p.446-461, 1994.
- MITTLER, T.E. Water tensions in plants an entomological approach. Annals of the Entomological Society of America, v.60, p.1074-1076, 1967.
- NOVOTNY, V.; WILSON, M.R. Why are there no small species among xylem-sucking insects? Evolutionary Ecology, v.11, p.419-437, 1997.
- PAIVA, P.E.B.; BENVENGA, S.R.; GRAVENA, S. Aspectos biológicos das cigarrinhas Acrogonia gracilis (Osborn), Dilobopterus costalimai Young e Oncometopia facialis (Signoret) (Hemiptera: Cicadellidae) em Citrus sinensis L. Osbeck. Neotropical Entomology, v.30, p.25-28, 2001.
- PARADELA FILHO, O.; SUGIMORI, M.H.; RIBEIRO, I.J.A.; MACHADO, M.A.; LARANJEIRA, F.F.; GARCIA JR., A.; BERETTA, M.J.G.; HARAKAWA, R.; RODRIGUES NETO, J.; BERIAN, L.O.S. Primeira constatação em cafeeiro no Brasil da Xylella fastidiosa causadora da clorose variegada dos citros. Laranja, v.16, p.135-136, 1995.
- PINTO, F.G.S.; LEITE JR., R.P. Detecção de Xylella fastidiosa em Coffea spp. através da técnica de PCR. Fitopatologia Brasileira, v.24, p.254, 1999. Suplemento.
- POOLER, M.R.; HARTUNG, J.S. Specific PCR detection and identification of Xylella fastidiosa strains causing citrus variegated chlorosis. Current Microbiology, v.31, p.377-381, 1995.
- PRESS, M.C.; WHITTAKER, J.B. Exploitation of the xylem stream by parasitic organisms. Philosophical Transactions of the Royal Society London, v.341, p.101-111, 1993.
- PURCELL, A.H. Seasonal changes in host plant preference of the blue-green sharpshooter, Hordnia circellata (Homoptera: Cicadellidae). The Pan-Pacific Entomologist, v.52, p.33-37, 1976.
- PURCELL, A.H.; FINLAY, A.H. Evidence for noncirculative transmission of Pierce's disease bacterium by sharpshooter leafhoppers. Phytopathology, v.69, p.393-395, 1979.
- PURCELL, A.H.; SAUNDERS, S.R. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Disease, v.83, p.825-830, 1999.
- PURCELL, A.H.; FINLAY, A.H.; McLEAN, D.L. Pierce's disease bacterium: mechanism of transmission by leafhopper vectors. Science, v.206, p.839-841, 1979.
- ROBERTO, S.R.; COUTINHO, A.; LIMA, J.E.O.; MIRANDA, V.S.; CARLOS, E.F. Transmissão de Xylella fastidiosa pelas cigarrinhas Dilobopterus costalimai, Acrogonia terminalis e Oncometopia facialis em citros. Fitopatologia Brasileira, v.21, p.517-518, 1996.
- ROSSETTI, V.; GARNIER, M.B.; BOVÉ, J.M.; BERETTA, M.J.G.; TEIXEIRA, A.R.R.; QUAGGIO, J.A.; DE NEGRI, J.D. Présence de bactéries dans le xyléme d'orangers atteints de chlorose variégée une nouvelle maladie des agrumes au Brésil. Comptes Rendus de l'Académie des Sciences Paris, v.310, p.345-349, 1990.
- SEVERIN, H.H.P. Transmission of the virus of Pierce's disease of grapevines by leafhoppers. Hilgardia, v.19, p.190-206, 1949.
Publication Dates
-
Publication in this collection
05 Nov 2003 -
Date of issue
Dec 2003
History
-
Accepted
26 Aug 2003 -
Received
24 Jan 2003