Abstracts
Pre-harvest sprouting (PHS) damage leads to occasional massive losses in all wheat producing areas, causing downgrading of grain quality, that severely limits end-use applications and results in substantial financial losses to farmers and food processors. Red grain color is a traditional marker for resistance to sprouting in wheat breeding programs, however red-grained genotype alone does not always guarantee effective resistance. The objective of this work was to find genes for resistance to PHS and investigate its inheritance in Brazilian wheat cultivars. Genetic variation for dormancy was investigated in the parents, F1 and 300 F2 lines derived from the cross Frontana × OR1 and its reciprocal. The germination/dormancy sprouted grains was evaluated on fifty seeds per replication, germinated in paper towel rolls at 20ºC for 5 days. A bimodal distribution for dormancy occurred in the Frontana/OR1 and OR1/Frontana derived F2 populations. The mean ratio of dormant and non-dormant seeds of the cross and its reciprocal was 85:1115, fitting a digenic model of 1:15 (P < 0.05). In fact, all non after-ripened F1 seeds germinated. The F2 distribution indicates that two major genes, here called A,a and B,b, control seed dormancy, which it appears to be recessive to dormancy. Only the homozygous aabb is dormant. As expected, there was no effect of maternal tissues.
Triticum aestivum; QTL; alpha-amylase; digenic model; phenotype
Germinação precoce na espiga (PHS) ocasiona perdas significativas nas regiões produtoras de trigo, causando depreciação na qualidade do produto final, o que resulta em prejuízos para os produtores e os moageiros. A cor vermelha do grão é um marcador tradicional para resistência à germinação nos programas de melhoramento de trigo, todavia, o genótipo de grão vermelho sózinho não garante resistência efetiva. O objetivo desse trabalho foi encontrar gene(s) para resistência à PHS e estudar a herança do caráter dormência de semente nos cultivares de trigo brasileiro. A variação genética para dormência foi investigada nos parentais, no F1 e em 300 linhas F2 derivadas do cruzamento de Frontana × OR1, e o cruzamento recíproco. Os grãos brotados (sprouted) e os dormentes foram avaliados pelo teste de germinação, em rolo de papel, a 20º C por 5 dias. Observou-se uma distribuição bimodal na população F2 para Frontana × OR1 e OR1 × Frontana. A taxa segregante de sementes dormentes e não dormentes foi de 85:1115, o que revela um modelo digênico de 1:15 (P < 0,05). Na verdade, todas as sementes F1, logo após a colheita, germinaram. Esse resultado e a distribuição F2 indicam que dormência parece ser recessiva e que há o envolvimento de dois genes, denominados de A,a e B,b controlando a dormência da semente de trigo. Somente o genótipo homozigoto aabb é dormente. Como esperado, não houve efeito dos tecidos maternais.
Triticum aestivum; QTL; alfa-amilase; modelo digênico; fenótipo
NOTE
Genetic control of seed dormancy and pre-harvest sprouting in wheat
Controle genético da dormência e da germinação precoce em trigo
Claudinei Andreoli* * Correspondencing author < andreoli@cnpso.embrapa.br> ; Manoel Carlos Bassoi; Dionisio Brunetta
Embrapa Soja, C.P. 231 - 86061-970 - Londrina, PR, Brasil
ABSTRACT
Pre-harvest sprouting (PHS) damage leads to occasional massive losses in all wheat producing areas, causing downgrading of grain quality, that severely limits end-use applications and results in substantial financial losses to farmers and food processors. Red grain color is a traditional marker for resistance to sprouting in wheat breeding programs, however red-grained genotype alone does not always guarantee effective resistance. The objective of this work was to find genes for resistance to PHS and investigate its inheritance in Brazilian wheat cultivars. Genetic variation for dormancy was investigated in the parents, F1 and 300 F2 lines derived from the cross Frontana × OR1 and its reciprocal. The germination/dormancy sprouted grains was evaluated on fifty seeds per replication, germinated in paper towel rolls at 20ºC for 5 days. A bimodal distribution for dormancy occurred in the Frontana/OR1 and OR1/Frontana derived F2 populations. The mean ratio of dormant and non-dormant seeds of the cross and its reciprocal was 85:1115, fitting a digenic model of 1:15 (P < 0.05). In fact, all non after-ripened F1 seeds germinated. The F2 distribution indicates that two major genes, here called A,a and B,b, control seed dormancy, which it appears to be recessive to dormancy. Only the homozygous aabb is dormant. As expected, there was no effect of maternal tissues.
Key words:Triticum aestivum , QTL, a-amylase, digenic model, phenotype
RESUMO
Germinação precoce na espiga (PHS) ocasiona perdas significativas nas regiões produtoras de trigo, causando depreciação na qualidade do produto final, o que resulta em prejuízos para os produtores e os moageiros. A cor vermelha do grão é um marcador tradicional para resistência à germinação nos programas de melhoramento de trigo, todavia, o genótipo de grão vermelho sózinho não garante resistência efetiva. O objetivo desse trabalho foi encontrar gene(s) para resistência à PHS e estudar a herança do caráter dormência de semente nos cultivares de trigo brasileiro. A variação genética para dormência foi investigada nos parentais, no F1 e em 300 linhas F2 derivadas do cruzamento de Frontana × OR1, e o cruzamento recíproco. Os grãos brotados (sprouted) e os dormentes foram avaliados pelo teste de germinação, em rolo de papel, a 20º C por 5 dias. Observou-se uma distribuição bimodal na população F2 para Frontana × OR1 e OR1 × Frontana. A taxa segregante de sementes dormentes e não dormentes foi de 85:1115, o que revela um modelo digênico de 1:15 (P < 0,05). Na verdade, todas as sementes F1, logo após a colheita, germinaram. Esse resultado e a distribuição F2 indicam que dormência parece ser recessiva e que há o envolvimento de dois genes, denominados de A,a e B,b controlando a dormência da semente de trigo. Somente o genótipo homozigoto aabb é dormente. Como esperado, não houve efeito dos tecidos maternais.
Palavras-chave:Triticum aestivum, QTL, a-amilase, modelo digênico, fenótipo
INTRODUCTION
Pre-harvest sprouting (PHS) damage leads to occasional massive losses in all wheat (Triticum aestivum L.) producing areas, causing downgrading of grain quality that severely limits end-use applications and results in substantial financial losses to farmers and food processors. Lack of adequate dormancy results in pre-harvest sprouting in the field under wet weather conditions. Pre-harvest sprouting is the germination of the grains before harvest due to rainfalls and high humidity (Figure 1). During this process, it occurs an increase of a-amylase activity in the endosperm, resulting in the degradation of starch to reduced sugars, which causes great losses to the bakery industry. Tolerance to sprouting damage and embryo dormancy is therefore a highly desirable but complex trait that is regulated by a number of key resistance genes that strongly interact with environmental conditions. Several workers have noted that seed dormancy appears to be closely associated with red grain color. However, PHS occurs sporadically in all red commercial varieties, implicating that the red-grained genotypes alone do not always guarantee effective resistance (Flintham & Gale, 1988; Flintham, 2000).
Lawson et al. (1997), who had worked with Australian background, showed that in two recombinant populations PHS was under a simple genetic control involving two genes. A new major gene (PHS) was identified on the long arm of chromosome 4A as controlling the difference between two red-grained cultivars with widely different dormancy (Flintham et al., 2002). The PHS gene appeared to exert its effect in the embryo, in contrast to the R gene expression in the maternal testa tissue. In the same arm, Anderson et al. (1993), Kato et al. (2001), Mares et al. (2002), Bassoi (2002) have identified two Quantitative Trait Loci (QTL) controlling seed dormancy. Moreover, Li et al. (2003) have found two QTLs for seed dormancy on chromosomes 2H and 5HL in barley. The major QTL for dormancy coincided with the QTL for pre-harvest sprouting at chromosome 5HL and explained 61% of the phenotypic variation.
The objective of this work was to assess genes for resistance to pre-harvest sprouting (PHS) in Brazilian wheat cultivars and to study their inheritance in T. aestivum.
MATERIAL AND METHODS
A F2 population from the cross Frontana × OR1 and its reciprocal were used in this study. Frontana is an old, tall, red-grained cultivar with high level of seed dormancy and resistance to leaf rust and Fusarium Head Blight (FHB) and OR1 is a modern short, high-yielding red-grained cultivar with low dormancy, and susceptible to PHS. Genetic variation for dormancy was investigated in the parents, F1 and 300 F2 lines derived from F1 hybrids grown at Embrapa Experimental Station, in Londrina, Paraná. When grains reached about 13% to 15% moisture content, at the harvest point, on September 4, a single head per plant was collected and seeds were tested for germinability and dormancy. Grains were gently threshed and germination/dormancy was immediately evaluated on wet paper towel rolls at 20ºC for 5 days. For the test, two hundred seeds were used for F1 and parents, and 300 seeds for the F2 progenies of each cross. The germination was scored for number of sprouted grains with visible rupture of the pericarp. A c2 analysis was performed to test for 3:1 and 15:1 ratios in the F2 progenies at 5% level of probability (P < 0.05).
RESULTS AND DISCUSSION
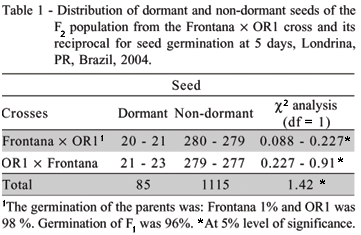
Germination of F1 and F2 seeds from crosses between dormant Frontana and non-dormant OR1 and their reciprocals were compared. A bimodal distribution for dormancy was observed in the F2 populations derived from Frontana × OR1 and OR1 × Frontana crosses (Figure 2). The mean ratio of dormant to non-dormant seeds over both types of crosses was 85:1115, fitting a digenic model of 1:15 (P < 0.05, Table 1). The c2 for the pooled data, 1115 non-dormant: 85 dormant, was 1,42 (df = 1, P < 0.05). These results indicate that two major genes, hereinafter called A,a and B,b, control seed dormancy, which appears to be recessive to dormancy. Only the homozygous aabb is dormant. In fact, all F1 non after-ripened seeds, derived from Frontana × OR1 and its reciprocal, germinated, indicating that there is no effect of the maternal tissues (Table 1). Interestingly, the genes for dormancy in the dormant line from Canada, RL4137 (Frontana/3/McMurachy/ Exchange//2*Redman), one of the cultivars most used to introduce PHS resistance in breeding programs in the world (Bassoi & Flintham, 2005; Flintham, 1993) and the modern dormant Brazilian cultivar, BRS177 (PF83899/PF813//PF2714) came from FRONTANA. The line PF2714 is derived from Frontana. This allows us to suggest that most likely the genes for seed dormancy and PHS in wheat germplasm in the world come from Frontana's derived lines.
Only two cases of major gene, controlling PHS and dormancy, have been reported in wheat: the triplicate R homoeoloci associated with red grain colour (Flintham & Gale, 1996) and the locus Phs (Flintham, 2000). It is evident that red grain colour and dormancy are not phenotypes of the same pleiotropic gene as it has been reported by Flintham et al. (2002), since R alleles do not fully guarantee dormancy in wet conditions.
Thus, the plant breeding strategy for introducing resistance to pre-harvest sprouting in wheat is to incorporate major genes from Frontana or derived lines, such as RL 4137 or BRS 177, into modern breeding lines.
CONCLUSIONS
The F2 distribution indicates that dormancy in this cross appeared to be recessive and two major genes, A,a e B,b, are involved in the control of seed dormancy. Only the homozygous aabb is dormant. As expected, there was no effect of maternal tissues.
Received November 11, 2005
Accepted October 02, 2006
- ANDERSON, J.A.; SORREL, M.E.; TANKSLEY, S.D. RFLP analysis of genomic regions associated with resistance to pre harvest sprouting in wheat. Crop Science, v.33, p.453-459, 1993.
- BASSOI, M.C. Quantitative trait analysis of grain dormancy in wheat (Triticum aestivum L. Thell). Norwich: John Innes Centre & University of East Anglia, 2002. 240p. (Ph.D. - Thesis).
- BASSOI, M.C.; FLINTHAM, J. Relationship between grain colour and preharvest sprouting in wheat. Pesquisa Agropecuária Brasileira, v.40, p.981-988, 2005.
- FLINTHAM, J.E. Grain colour and sprout-resistance in wheat. In: WALKER-SIMMONS, M.K.; RIED, J.L. (Ed.). Pre-harvest sprouting in cereals. 6.ed. Saint Paul: American Association of Cereal Chemists, 1993. p.30-36.
- FLINTHAM, J.E. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Science Research, v.10, p.43-50, 2000.
- FLINTHAM, J.E.; GALE, M.D. Genetics of pre harvest sprouting and associated traits in wheat. Plant Varieties & Seeds, v.1, p.87-97, 1988.
- FLINTHAM, J.E.; GALE, M.D. Dormancy gene maps in homoeologous cereal genomes. In: NODA, K.; MARES, D.J. (Ed.). Pre-harvest sprouting in cereals. Tokyo: Center for Academic Societies, 1996. p.143-149.
- FLINTHAM, J.E.; ADLAM, R.E.; BASSOI, M.C.; HOLSWORTH, M.; GALE, M.D. Mapping genes for resistance to sprouting damage in wheat. Euphytica, v.126, p.39-45, 2002.
- KATO, K.; NAKAMURA, W.; TABIKI, T.; MIURA, H.; SAWADA, S. Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theoretical & Applied Genetic, v.102, p.980-985, 2001.
- LAWSON, W.R.; GOWIN, I.D.; COOPER, M.; BRENNAN, P.S. Genetic analysis of pre harvest sprouting tolerance in three wheat crosses. Australian Journal of Agricultural Research, v.48, p.215-221, 1997.
- LI, C.D.; TARR, A.; LANCE, R.C.M.; HARASYMOW, S.; UHLMANN, J.; WESTOR, S.; YOUNG, K.J.; GRIME, C.R.; CAKIR, M.; BROUGHTON, S.; APPELS, R. A major QTL controlling seed dormancy and pre-harvest sprouting/grain a-amylase in two-rowed barley (Hordeum vulgare L.). Australian Journal of Agricultural Research, v.54, p.1303-1313, 2003.
- MARES, D.J.; MRVA, K.; TAM, M.; SHARP, P. Dormancy in white-grained wheat: Progress towards identification of genes and molecular markers. Euphytica, v.126, p.47-53, 2002.
Publication Dates
-
Publication in this collection
22 Dec 2006 -
Date of issue
Dec 2006
History
-
Accepted
02 Oct 2006 -
Received
11 Nov 2005