Abstracts
Phytostabilisation of a contaminated soil with heavy metals is considered a very appropriate technology to reduce erosion and dispersion of contaminants. A greenhouse study was conducted to evaluate the effects of both chemical amendments (calcium silicate and brewery sludge), and phytoremediation using the grass Brachiaria decumbens, on an industrial residue contaminated with Zn and Cd (industrial residue). Industrial residue samples placed into 30 L containers were amended with 20% brewery sludge, calcium silicate (2%, 3%), and 20% of brewery sludge + calcium silicate (2.5%, 4%), and were compared to the control treatment (non-amended residue). After pH stabilization, B. decumbens plants were grown on all treatments in order to evaluate the ability of the species to tolerate high Zn and Cd concentrations from the residue. Samples were collected twice, at planting and harvesting, for pH determination and simple extractions with water, sodium nitrate, acetic acid and DTPA. Differences in Zn and Cd concentrations in extracts allowed to estimate the concentrations of these elements in the most likely chemical forms they are found in the residue. Alkaline and organic industrial amendments reduced Zn and Cd percentages, both in the soluble and exchangeable fractions, as well as caused the predominance of Zn and Cd in the most stable chemical fractions, such as complexed and precipitated compounds. B. decumbens was tolerant to Zn and Cd from the industrial residue after addition of the amendments.
phytoremediation; inertization; contamination; heavy metals
A fitoestabilização de solos contaminados com metais pesados é considerada uma boa alternativa para reduzir a erosão e dispersão de contaminantes no ambiente. Foi conduzido um experimento em casa-de-vegetação com o objetivo de avaliar a contenção química (silicato de cálcio e lodo do biodigestor de uma cervejaria) e a fitorremediação pela Brachiaria decumbens, de um resíduo industrial contaminado com Zn e Cd, utilizando vasos de 30 L. Os tratamentos foram: resíduo industrial (testemunha); resíduo industrial + 20% lodo; resíduo industrial + silicato de cálcio (2%; 3%); (resíduo industrial + 20% lodo) + silicato de cálcio (2,5%; 4%). Após estabilização do pH, foram cultivados nos tratamentos plantas de B. decumbens, visando avaliar o potencial de tolerância a elevadas concentrações de Zn e Cd presentes no resíduo industrial. No plantio e colheita das plantas foram retiradas amostras dos diferentes tratamentos para determinação do pH e extrações simples com água, nitrato de sódio, ácido acético e DTPA. A partir das concentrações de Zn e Cd obtidas nas extrações, foram estimadas, através das diferenças nas quantidades extraíveis, as concentrações de Zn e Cd nas prováveis formas químicas. A adição de resíduos industriais, alcalino e orgânico, provocou redução nas percentagens de Zn e Cd nas frações solúvel e trocável, e predominância dos mesmos em frações químicas mais estáveis como complexados e precipitados. A B. decumbens apresentou tolerância ao Zn e Cd presentes no resíduo industrial após tratamento de contenção química.
fitorremediação; inertização; contaminação; metais pesados
SOILS AND PLANT NUTRITION
Chemical amendment and phytostabilization of an industrial residue contaminated with Zn and Cd
Correção química e fitoestabilização de um resíduo industrial contaminado com Zn e Cd
Fabiana Soares dos SantosI; Márcio Osvaldo Lima MagalhãesII; Nelson MazurII; Nelson Moura Brasil do Amaral SobrinhoII,* * Corresponding author < nelmoura@ufrrj.br>
IUFF/EEIMVR - Depto. de Agronegócios, Av. dos Trabalhadores, 420 - 27255-125 - Volta Redonda, RJ - Brasil
IIUFRRJ - Depto. de Solos, BR 465, km 7 - 26890-000 - Seropédica, RJ - Brasil
ABSTRACT
Phytostabilisation of a contaminated soil with heavy metals is considered a very appropriate technology to reduce erosion and dispersion of contaminants. A greenhouse study was conducted to evaluate the effects of both chemical amendments (calcium silicate and brewery sludge), and phytoremediation using the grass Brachiaria decumbens, on an industrial residue contaminated with Zn and Cd (industrial residue). Industrial residue samples placed into 30 L containers were amended with 20% brewery sludge, calcium silicate (2%, 3%), and 20% of brewery sludge + calcium silicate (2.5%, 4%), and were compared to the control treatment (non-amended residue). After pH stabilization, B. decumbens plants were grown on all treatments in order to evaluate the ability of the species to tolerate high Zn and Cd concentrations from the residue. Samples were collected twice, at planting and harvesting, for pH determination and simple extractions with water, sodium nitrate, acetic acid and DTPA. Differences in Zn and Cd concentrations in extracts allowed to estimate the concentrations of these elements in the most likely chemical forms they are found in the residue. Alkaline and organic industrial amendments reduced Zn and Cd percentages, both in the soluble and exchangeable fractions, as well as caused the predominance of Zn and Cd in the most stable chemical fractions, such as complexed and precipitated compounds. B. decumbens was tolerant to Zn and Cd from the industrial residue after addition of the amendments.
Key words: phytoremediation, inertization, contamination, heavy metals
RESUMO
A fitoestabilização de solos contaminados com metais pesados é considerada uma boa alternativa para reduzir a erosão e dispersão de contaminantes no ambiente. Foi conduzido um experimento em casa-de-vegetação com o objetivo de avaliar a contenção química (silicato de cálcio e lodo do biodigestor de uma cervejaria) e a fitorremediação pela Brachiaria decumbens, de um resíduo industrial contaminado com Zn e Cd, utilizando vasos de 30 L. Os tratamentos foram: resíduo industrial (testemunha); resíduo industrial + 20% lodo; resíduo industrial + silicato de cálcio (2%; 3%); (resíduo industrial + 20% lodo) + silicato de cálcio (2,5%; 4%). Após estabilização do pH, foram cultivados nos tratamentos plantas de B. decumbens, visando avaliar o potencial de tolerância a elevadas concentrações de Zn e Cd presentes no resíduo industrial. No plantio e colheita das plantas foram retiradas amostras dos diferentes tratamentos para determinação do pH e extrações simples com água, nitrato de sódio, ácido acético e DTPA. A partir das concentrações de Zn e Cd obtidas nas extrações, foram estimadas, através das diferenças nas quantidades extraíveis, as concentrações de Zn e Cd nas prováveis formas químicas. A adição de resíduos industriais, alcalino e orgânico, provocou redução nas percentagens de Zn e Cd nas frações solúvel e trocável, e predominância dos mesmos em frações químicas mais estáveis como complexados e precipitados. A B. decumbens apresentou tolerância ao Zn e Cd presentes no resíduo industrial após tratamento de contenção química.
Palavras-chave: fitorremediação, inertização, contaminação, metais pesados
INTRODUCTION
Soil contamination with heavy metals is a major environmental problem. Their negative impacts affect not only the soil quality, but also several other environmental parameters. Effective and lasting in situ strategies have been developed for the remediation of soils contaminated with heavy metals. Phytoremediation has also been adopted and seems to be a promising technique for the remediation of contaminated areas (Gleba et al., 1999; Garbisu et al., 2002; Kamnev, 2003). It is a harmless and low cost technique involves the use of green plants in the management of contaminated soils, and can be widely used.
Phytostabilization and phytoextraction are the most usual phytoremediation techniques adopted for soils contaminated with heavy metals. Phytostabilization consists of using green plants to reduce the mobility of contaminant agents through revegetation strategies (Garbisu & Alkorta, 2001). In this case, the plant species tolerance, biological cycle, rusticity and ability to grow on unvegetated soils are characteristics that may contribute for the success of the stabilization of plants in soils contaminated with heavy metals. This study aimed to (i) evaluate the chemical behavior of Zn and Cd present in the industrial residue from a Zn industry, after remediation procedures using an alkaline corrective with brewery sludge, and (ii) to evaluate the effectiveness of the residue phytostabilization with the grass Brachiaria decumbens.
MATERIAL AND METHODS
This study analyzed an industrial residue from the extraction process of Zn from calamine ore, situated at the Sepetiba Bay, in Rio de Janeiro, Brazil (43º50'W and 22º56'S). Residues accumulated over about 30 years may reach up to 2 million m3, and may contaminate soil, plants, marine sediments and millions of fishes and mollusks with heavy metals, even banishing fishing activities. Residue samples were collected in December, 2002. A 100kg-sample was formed joining sub-samples collected from the top, middle and bottom of a residue pile (ABNT, 1987a), which were later air-dried, ground and sieved through a 2mm mesh. Organic carbon was determined as proposed by Yeomans & Bremner (1988). Macronutrients, sodium, aluminum and pHH2O (soil:water ratio 1:2.5) were determined according to EMBRAPA (1997): Ca, Mg and Al were extracted by KCl 1M; H + Al by calcium acetate 0.5M; Na, P and K by Mehlich-1 (HCl 0,05M + H2SO4 0,0125M). The residue had the following properties: soil pH (soil:water ratio 1:2.5) 5.6, 1 g kg-1 organic carbon, 2500 mmolc kg-1 Ca, 410 mmolc kg-1 Mg, 46 mmolc kg-1 H + Al, 0 mmolc kg-1 Al, 9.80 mmolc kg-1 Na, 0.174 g kg-1 P, 0.094 g kg-1 K. The high Ca, Mg, P and K concentrations in the residue may be related to the high content of these nutrients in the calamine ore.
Samples were digested with nitric-perchloric acid 2:1 (Scott, 1978), in order to obtain the pseudototal contents of some heavy metals in the residue. Solubilization and leaching tests were made to determine soluble and leaching contents, according to ABNT (1987b, 1987c). The Zn, Cd, Cu, Fe, Mn and Pb contents were determined in extracts obtained by digestion. In the solubility test, analysis were made with an atomic absorption spectrophotometer. Only Cd and Pb contents were determined from extracts obtained in the leaching test, since these elements are hazardous to the environment.
To evaluate the inertization potential of the industrial residue, an alkaline industrial corrective composed of calcium silicate and brewery sludge was used as amendment to reduce Zn and Cd solubility. The calcium silicate corrective was obtained from the replacement of the pipeline covering of a Brazilian Oil Company. The organic carbon content in the sludge was found to be 20%, determined through the method of Yeomans & Bremner (1988). Both products were air dried, ground and sieved through 2mm mesh, in order to estimate heavy metals total contents and pH in water (1:2.5). To evaluate the potential of organic matter in immobilizing heavy metals, the dose of brewery sludge was estimated to have an amendment mixture with organic matter representing 2% of the weight of the pots. Calcium silicate was prepared with the support of a neutralization curve, to obtain a balance pH value ranging from 6.5 to 7.0 in the final mixture.
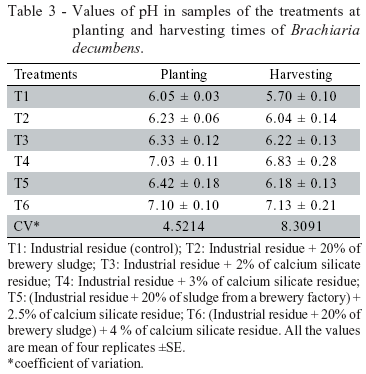
The experiment was carried out in a greenhouse, and consisted in filling up 30L containers with the residues and amendments according to the following treatments: T1: Industrial residue (control); T2: Industrial residue + 20% of brewery sludge; T3: Industrial residue + 2% of calcium silicate residue; T4: Industrial residue + 3% of calcium silicate residue; T5: (Industrial residue + 20% of brewery sludge) + 2.5% of calcium silicate residue; T6: (Industrial residue + 20% of brewery sludge) + 4 % of calcium silicate residue. These mixtures were incubated until pH stabilization, indicated by the neutralization curve over about 30 days.
Brachiaria decumbens seeds were sown on sterilized sand. Thirty-day-old seedlings were transferred of the pots and grown for other 45 days. Pots were irrigated manually up to 80% of the field capacity. Residues were sampled twice, at planting and at harvesting of B. decumbens, for the determination of the pH in water (1:2.5) and the performance of simple extractions, using the following extractors: (i) water, (ii) sodium nitrate 0.1 mol L-1 (Keller & Védy, 1994); (iii) DTPA (Lindsay & Norvell, 1978); and (iv) acetic acid 0.043 mol L-1 (Ure et al., 1993). The Cd and Zn contents in the extracts were determined through atomic absorption spectrophotometry.
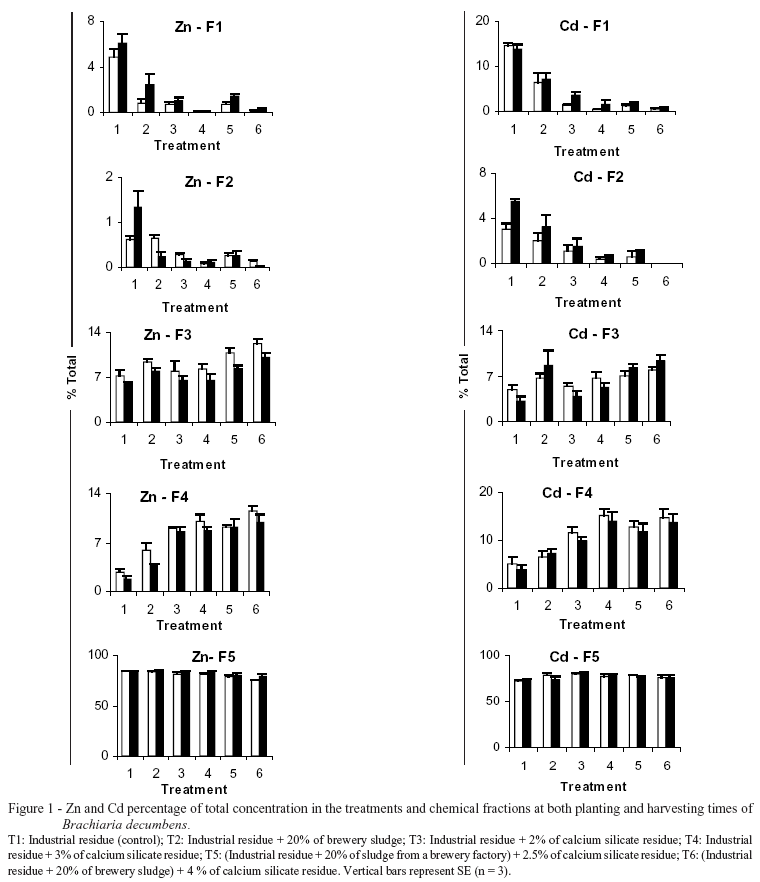
Differences in Zn and Cd concentrations in extracts allowed to estimate the concentrations of these elements in the most likely chemical forms they are found in residues. The chemical fractions were defined as: F1: extraction with water  fraction soluble in water; F2: extracted with NaNO3 extracted with water
fraction soluble in water; F2: extracted with NaNO3 extracted with water  exchangeable fraction; F3: extracted with DTPA extracted with NaNO3
exchangeable fraction; F3: extracted with DTPA extracted with NaNO3 fraction predominantly associated to the surface of Fe oxides and to more stable organic compounds, due to the formation of inner-sphere complexes; F4: extracted with acetic acid extracted with NaNO3
fraction predominantly associated to the surface of Fe oxides and to more stable organic compounds, due to the formation of inner-sphere complexes; F4: extracted with acetic acid extracted with NaNO3  fraction predominantly associated to carbonate precipitates; F5: total -
fraction predominantly associated to carbonate precipitates; F5: total - of fractions (F1; F2; F3 and F4)
of fractions (F1; F2; F3 and F4)  fraction associated to Fe compounds with high crystallinity degree (Residual fraction). After harvest, shoots and roots were removed and dried in an oven at 70ºC for 48h. Cd and Zn contents were determined by digestion of dried plant material in concentrated HNO3-HClO4 6:1 (Tedesco et al., 1995). Metals-ion concentrations were determined by atomic absorption spectrophotometry.
fraction associated to Fe compounds with high crystallinity degree (Residual fraction). After harvest, shoots and roots were removed and dried in an oven at 70ºC for 48h. Cd and Zn contents were determined by digestion of dried plant material in concentrated HNO3-HClO4 6:1 (Tedesco et al., 1995). Metals-ion concentrations were determined by atomic absorption spectrophotometry.
The experiment was performed in a completely randomized statistical design involving four replicates. Statistical analyses were performed using SAEG (Sistema para Análises Estatísticas e Genéticas), version 5.0. Differences among means were determined by analyses of variance. Pearson's correlation coefficients were used to express the associations of quantitative variables.
RESULTS AND DISCUSSION
High total concentrations of Zn, Cd, Fe, Mn and Pb were found in the industrial residue (Table 1). Soluble concentrations of Zn and Cd were found to be high as well, since these are elements that can be more easily leached, with a high risk in contaminating the local environment, especially the groundwater.
The leaching test presented high Cd concentrations that can be lost in great amounts. According to the ABNT norms, a hazard ranking could be adopted for this residue based on Cd and Pb concentrations. In the ABNT ranking, a non-hazard residue should have a Cd leaching concentration below 0.5 mg kg 1. Therefore, as the Cd residue concentration found here is almost ninety-fold the established limit in the leaching test, the residue should be classified as belonging to the hazard class I residue hazardous (ABNT, 1987d), and should be stored adequately. Remediation techniques should also be adopted to reduce residue toxicity.
In relation to Pb, despite the high total Pb content in the residue, the leaching concentration remained below 5 mg kg-1, which is considered within acceptable limits (ABNT, 1987c). Both the brewery sludge and the calcium silicate used as chemical amendments to the industrial residue presented low heavy metal concentrations (Table 2) and are not considered dangerous.
Residue Inertization
There was a reduction in Zn and Cd percentages (Figure 1) in fractions of high bioavailability, F1 (soluble in water) and F2 (exchangeable). The reduction was more significant for treatments with pH 7.0 (treatments 4 and 6) (Table 3), especially when organic matter was added (treatment 6), suggesting that pH and organic matter content affected Zn and Cd complexation and precipitation in this residue.
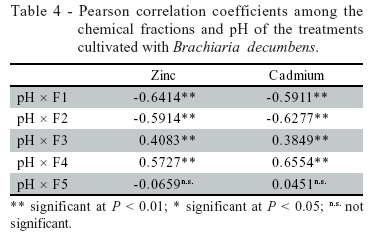
The residue pH tended to decrease from planting to harvesting stages (Table 3), probably due to the exudation of compounds by the root system. The decrease in fractions F1 and F2 was followed by increases on fractions of lower solubility, especially F3 and F4 (Figure 1). Since heavy metal precipitation, complexation and adsorption are favored by both the pH raising and the presence of organic matter (Walker et al., 2003), the addition of both alkaline and organic products probably enhanced the transference of Zn and Cd from the more soluble to the less soluble fractions (Table 4). Fractions F1 and F2 were negatively correlated with pH, while fractions F3 and F4 were positively and significantly correlated with pH (Table 4).
In fraction F3, Zn and Cd percentages were found to be higher when organic matter and the alkaline products were simultaneously added (T5 and T6), suggesting that organic matter forms more stable complexes (inner-sphere) and higher pH enhances residue cation exchange capacity (CEC). Zn and Cd percentages were higher in fraction F3 than in fractions F1 and F2, when organic matter and the alkaline product were simultaneously added. The organic matter may also form soluble complexes (chelates) with heavy metals, which may either precipitate at high pH. A reduction on heavy metal solubility, as Zn and Cd, by organic matter addition was also observed by Yamada et al. (1984) and Mesquita (2002). Studying the Cu complexation in humic substances, Grimm et al. (1991) verified that at low pH (2.5), the surface was mostly occupied by H+; however, at higher pH (4.5), bonding sites were rather occupied by Cu, due to the reduction on the competition with protons. High H+concentration at low pH values resulted in less Hg2+ complexed by humic substances (Yin et al., 1997).
The addition of calcium silicate significantly increased Zn and Cd percentages in fraction F4, whether the organic matter was added or not. On the other hand, there was no difference in these element contents between treatments in which only calcium silicate was added (T3 and T4) and treatments in which the organic product was simultaneously added (T 5 and T6), suggesting that pH increases were mostly responsible for the increase on the Zn and Cd contents in this fraction. The residue pH was important for Zn precipitation. Both Zn and Cd present in this residue were mostly found in fraction F5. As there was no significant difference among treatments and the control, the formation of more stable complexes was suggested not to be the only form to retain Zn and Cd in the residue. Addition of amendments had beneficial effects on the growth of Brachiaria decumbens plants because all treatments with amendments had a higher yield in relation to the control (Table 5).
The phytotoxicity symptoms increased in severity as plants grew, as already described by Alkorta et al. (2004) and reduced with both the addition of alkaline reaction and organic products. Plants of the control treatment died five days after transplantation. In T3, plants were gradually affected by the contaminated residue. Toxicity symptoms included severe chlorosis, and plant death. Treatments with pH 7.0 (T4 and T6) showed high dry matter yield of B. decumbens, indicating that the pH value was important to reduce the solubility of the heavy metals. The treatment of organic matter and the alkaline residue simultaneously added (T6) presented the highest dry matter yield, suggesting that the organic matter was also important in reducing the bioavailability of heavy metals and improving the physical characteristics of the residue, like structure and porosity, preventing residue compaction, and promoting a better development of roots and a higher crop yield.
The amendment addition affected the extraction of Zn and Cd by plants in the different treatments (Table 5). The increment in dry matter for plants growing on substrate with alkaline and organic amendments simultaneously added (T6) increased the Zn and Cd accumulation in plants, corroborating to the amortization effect of the alkaline reaction (calcium silicate) and organic (brewery sludge) products on the phytotoxicity of the heavy metals, thus promoting plant growth. Despite the lower solubility of Zn and Cd in treatments with higher addition of alkaline and organic amendments (Figure 1), the accumulation of these elements was higher in T4 and T6 (Table 5), probably due to a better plant development under these treatments and a better established root system, which have resulted in a higher heavy metal uptake. Plants may regulate the solubility of heavy metals in the rhizosphere through acidification due either to the H+ extrusion or to the exudation of organic compounds from roots (Yang at al., 2005). Despite the lower root mass production (Table 5), Zn accumulated in roots more than in aboveground biomass, or even more, particularly when Zn concentration in the roots was higher.
As a conclusion, application of amendments to the contaminated residue promoted the growth of B. decumbens plants by reducing the availability of Cd and Zn in the residue. The present study emphasizes the inertizing action of both the calcium silicate and the brewery sludge on heavy metal toxicity, in soils cultivated with B. decumbens.
ACKNOWLEDGEMENTS
To CNPq for financial assistance.
Received January 31, 2006
Accepted April 20, 2004
- ALKORTA, I.; HERNÁNDEZ-ALLICA, J.; BECERRIL, J.M.; AMEZAGA, I.; ALBIZU, I.; GARBISU, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Reviews in Environmental Science & Biotechnology, v.03, p.71-90, 2004.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS - ABNT. NBR 10007: Amostragem de resíduos. Rio de Janeiro, 1987a. 21p.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS - ABNT. NBR 10006: Solubilização de resíduos. Rio de Janeiro, 1987b. 6p.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS - ABNT. NBR 10005: Lixiviação de resíduos. Rio de Janeiro, 1987c. 16p.
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS - ABNT. NBR-10004: Classificação de resíduos. Rio de Janeiro, 1987d. 71p.
- EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA. Manual de métodos de análise de solo. 2.ed. Rio de Janeiro: EMBRAPA, CNPS, 1997. 212p.
- GARBISU, C.; HERNÁNDEZ-ALLICA, J.; BARRUTIA, O.; ALKORTA, I.; BECERRIL, J.M. Phytoremediation: A technology using green plants to remove contaminants from polluted areas. Review in Environmental Health, v.17, p.75-90, 2002.
- GARBISU, C.; ALKORTA, I. Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresource Technology, v.77, p.229-236, 2001.
- GLEBA, D.; BORISJUK, N.V.; BORISJUK, L.G.; KNEER, R.; POULEV, A.; SKARZHINSKAYA, M.; DUSHENKOV, S.; LOGENDRA, S.; GLEBA, Y.Y.; RASKIN, I. Use of plant roots for phytoremediation and molecular farming. Proceedings of the National Academy of Sciences of the USA, v.96, p.5973-5977, 1999.
- GRIMM, D.M.; AZARRAGA, L.V.; CARREIRA, L.A.; SUSETYO, W. Continuous multiligand distribution model used to predict the stability constant of Cu (II) metal complexation with humic material from fluorescence quenching data. Environmental Science & Technology, v.25, p.1427-1431, 1991.
- KAMNEV, A.A. Phytoremediation of heavy metals: an overview. Recent Advances in Marine Biotechnology, v.8, p.269-317, 2003.
- KELLER, C.; VÉDY, J.C. Distribution of cadmium fractions in two forest soils. Journal of Environmental Quality, v.23, p.987-999, 1994.
- LINDSAY, W.L.; NORWELL, W.A. Development of a DTPA test zinc, iron, manganese and copper. Soil Science Society of America Proceedings, v.42, p.421 428, 1978.
- MESQUITA, A.A. Remediação de áreas contaminadas por metais pesados provenientes de lodo de esgoto. Seropédica: UFRRJ, 2002. 68p. (Dissertação - Mestrado).
- SCOTT, K. Cause and control of losses of chromium during nitric-perchloric acid oxidation of aquatic sediments. Analyst, v.103, p.754-758, 1978.
- TEDESCO, M.J.; GIANELLO, C.; BISSANI, C.A.; BOHNEN, H.; VOLKWEISS, S.J. Análise de solo, plantas e outros materiais 2.ed. Porto Alegre: UFRGS, Departamento de Solos, 1995. 174p. (Boletim Técnico, 5).
- URE, A.; QUEVAUVILLER, P.M.; MUNTAU, H.; GRIEPINK, B. Speciation of heavy metals in soils and sediments, an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. International Journal of Environmental Analytical Chemistry, v.51, p.135-151, 1993.
- WALKER, D.J.; CLEMENTE, R.; ROIG., A.; BERNAL, M.P. The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environmental Pollution, v.122, p.303-312, 2003.
- YAMADA, R.; IMAIUMI, M.; SANO, K. Behaviour of heavy metals in soil treated successively with sewage sludge. Ken Agricultural Research Centre, v.16, p.228 238, 1984.
- YANG, X.; FENG, Y.; HE, Z.; STOFFELLA, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. Journal of Trace Elements in Medicine and Biology, v.18, p.339-353, 2005.
- YEOMANS, J.C.; BREMNER, J.M. A rapid and precise method for routine determination of organic carbon in soil. Soil Science and Plant Analysis, v.19, p.1467-1476, 1988.
- YIN, Y.; ALLEN, H.E.; HUANG, C.P. Kinetics of mercury (II) adsorption and desorption on soil. Environmental Science & Technology, v.31, p.496-503, 1997.
Publication Dates
-
Publication in this collection
05 Oct 2007 -
Date of issue
Oct 2007
History
-
Accepted
20 Apr 2004 -
Received
31 Jan 2006