Abstracts
Sublethal adverse effects may result from exposure of aquatic organisms to insecticides at environmentally relevant concentrations. Fingerlings of the common carp (Cyprinus carpio, Linnaeus, 1758), grass carp (Ctenopharyngodon idella, Valenciennes, 1844), and bighead carp (Aristichthys nobilis, Richardson, 1845) were exposed to diafuran, an insecticide widely used during rice cultivation in Southern Brazil. The aim of this study was to verify the relationship between the lethal concentration (LC50) of diafuran and the acetylcholinesterase (AChE) activity in brain and muscle tissues of these species as a possible early biomarker of exposure to this insecticide. LC50 was determined for fish exposed to diafuran concentrations during 96 h (short term): common carp: control, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg L-1; grass carp: control, 1.0, 2.0, 3.0 and 3.5 mg L-1 and, bighead carp: control, 0.5, 1.0, 1.5, 2.0, 3.0 and 4.0 mg L-1, as well as the determination of AChE at concentrations near LC50 for these species. LC50 values (nominal concentrations) were 1.81 mg L-1 for the common carp, 2.71 mg L-1 for the grass carp and, 2.37 mg L-1 for the bighead carp. All carps exposed to diafuran were lethargic (lower concentrations) or immobile. Diafuran inhibited the acetylcholinesterase activity in brain (~38%) and muscle (~50%) of all species. Muscle of bighead carp under control treatment showed higher specific AChE activity than brain (14.44 against 5.94 µmol min-1 g protein-1, respectively). Concentrations of diafuran used for rice cropping may affect Cyprinus carpio, Ctenopharyngodon idella and Aristichthys nobilis behaviors and the AChE activities in brain and muscle of these species may be an early biomarker of toxicity of this insecticide.
Cyprinus carpio; Ctenopharyngodon idella; Aristichthys nobilis; pesticide; lethal concentration
Exposição a inseticidas em concentrações elevadas no ambiente podem ocasionar efeitos adversos subletais em organismos aquáticos. Alevinos de carpa húngara (Cyprinus carpio, Linnaeus, 1758), carpa capim (Ctenopharyngodon idella, Valenciennes, 1844) e carpa cabeça grande (Aristichthys nobilis, Richardson, 1845) foram expostos ao diafuran, um inseticida utilizado na cultura do arroz no sul do Brasil. O objetivo deste estudo foi verificar a relação entre concentração letal mediana (CL50) do diafuran e a atividade da enzima acetilcolinesterase (AChE) em cérebro e músculo dessas espécies, como um possível biomarcador inicial da exposição a este inseticida. A CL50 foi determinada com peixes expostos a concentrações de diafuran em 96 h: carpa húngara: controle; 0,5; 1,0; 1,5; 2,0; 2,5 e 3,0 mg L-1; carpa capim: controle; 1,0; 2,0; 3,0 e 3,5 mg L-1 e carpa cabeça grande: controle; 0,5; 1,0; 1,5; 2,0; 3,0 e 4,0 mg L-1, bem como a determinação da AChE em concentrações próximas da CL50 para essas espécies. Valores de CL50 (concentrações nominais) foram de 1,81 mg L-1 para carpa húngara, 2,71 mg L-1 para carpa capim e 2,37 mg L-1 para carpa cabeça grande. Todas as carpas expostas ao diafuran estavam letárgicas (menores concentrações) ou imóveis. Diafuran inibiu significativamente a atividade da AChE em cérebro (~38 %) e músculo (~50 %) de todas as espécies estudadas. Atividade da AChE em músculo para carpa cabeça grande foi mais alta que cérebro (14,44 contra 5,94 µmol min-1 g proteína-1, respectivamente). Este estudo demonstrou que concentrações de diafuran utilizadas na cultura do arroz podem afetar o comportamento de Cyprinus carpio, Ctenopharyngodon idella e Aristichthys nobilis, e a atividade da acetilcolinesterase cerebral e muscular pode ser um biomarcador inicial de toxicidade deste inseticida.
Cyprinus carpio; Ctenopharyngodon idella; Aristichthys nobilis; agroquímico; concentração letal
ANIMAL SCIENCE AND PASTURES
Acetylcholinesterase enzyme activity in carp brain and muscle after acute exposure to diafuran
Atividade da enzima acetilcolinesterase em cérebro e músculo de carpas após exposição aguda ao diafuran
Jaqueline Ineu GolombieskiI, * * Corresponding author < jgolombieski@yahoo.com.br > ; Enio MarchesanI; Edinalvo Rabaioli CamargoI; Joseânia SalbegoII; Joele Schmitt BaumartI; Vania Lucia LoroII; Sérgio Luiz de Oliveira MachadoIII; Renato ZanellaIV; Bernardo BaldisserottoV
IUFSM - Depto. de Fitotecnia, Campus Universitário - 97105-900 - Santa Maria, RS - Brasil
IIUFSM - Depto. de Química - Lab. de Bioquímica Adaptativa
IIIUFSM - Depto. de Defesa Fitossanitária - Lab. de Plantas Daninhas
IVUFSM - Depto. de Química - Lab. de Análises de Resíduos de Pesticidas
VUFSM - Depto. de Fisiologia e Farmacologia - Lab. de Fisiologia de Peixes
ABSTRACT
Sublethal adverse effects may result from exposure of aquatic organisms to insecticides at environmentally relevant concentrations. Fingerlings of the common carp (Cyprinus carpio, Linnaeus, 1758), grass carp (Ctenopharyngodon idella, Valenciennes, 1844), and bighead carp (Aristichthys nobilis, Richardson, 1845) were exposed to diafuran, an insecticide widely used during rice cultivation in Southern Brazil. The aim of this study was to verify the relationship between the lethal concentration (LC50) of diafuran and the acetylcholinesterase (AChE) activity in brain and muscle tissues of these species as a possible early biomarker of exposure to this insecticide. LC50 was determined for fish exposed to diafuran concentrations during 96 h (short term): common carp: control, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg L-1; grass carp: control, 1.0, 2.0, 3.0 and 3.5 mg L-1 and, bighead carp: control, 0.5, 1.0, 1.5, 2.0, 3.0 and 4.0 mg L-1, as well as the determination of AChE at concentrations near LC50 for these species. LC50 values (nominal concentrations) were 1.81 mg L-1 for the common carp, 2.71 mg L-1 for the grass carp and, 2.37 mg L-1 for the bighead carp. All carps exposed to diafuran were lethargic (lower concentrations) or immobile. Diafuran inhibited the acetylcholinesterase activity in brain (~38%) and muscle (~50%) of all species. Muscle of bighead carp under control treatment showed higher specific AChE activity than brain (14.44 against 5.94 µmol min-1 g protein-1, respectively). Concentrations of diafuran used for rice cropping may affect Cyprinus carpio, Ctenopharyngodon idella and Aristichthys nobilis behaviors and the AChE activities in brain and muscle of these species may be an early biomarker of toxicity of this insecticide.
Key words:Cyprinus carpio, Ctenopharyngodon idella, Aristichthys nobilis, pesticide, lethal concentration
RESUMO
Exposição a inseticidas em concentrações elevadas no ambiente podem ocasionar efeitos adversos subletais em organismos aquáticos. Alevinos de carpa húngara (Cyprinus carpio, Linnaeus, 1758), carpa capim (Ctenopharyngodon idella, Valenciennes, 1844) e carpa cabeça grande (Aristichthys nobilis, Richardson, 1845) foram expostos ao diafuran, um inseticida utilizado na cultura do arroz no sul do Brasil. O objetivo deste estudo foi verificar a relação entre concentração letal mediana (CL50) do diafuran e a atividade da enzima acetilcolinesterase (AChE) em cérebro e músculo dessas espécies, como um possível biomarcador inicial da exposição a este inseticida. A CL50 foi determinada com peixes expostos a concentrações de diafuran em 96 h: carpa húngara: controle; 0,5; 1,0; 1,5; 2,0; 2,5 e 3,0 mg L-1; carpa capim: controle; 1,0; 2,0; 3,0 e 3,5 mg L-1 e carpa cabeça grande: controle; 0,5; 1,0; 1,5; 2,0; 3,0 e 4,0 mg L-1, bem como a determinação da AChE em concentrações próximas da CL50 para essas espécies. Valores de CL50 (concentrações nominais) foram de 1,81 mg L-1 para carpa húngara, 2,71 mg L-1 para carpa capim e 2,37 mg L-1 para carpa cabeça grande. Todas as carpas expostas ao diafuran estavam letárgicas (menores concentrações) ou imóveis. Diafuran inibiu significativamente a atividade da AChE em cérebro (~38 %) e músculo (~50 %) de todas as espécies estudadas. Atividade da AChE em músculo para carpa cabeça grande foi mais alta que cérebro (14,44 contra 5,94 µmol min-1 g proteína-1, respectivamente). Este estudo demonstrou que concentrações de diafuran utilizadas na cultura do arroz podem afetar o comportamento de Cyprinus carpio, Ctenopharyngodon idella e Aristichthys nobilis, e a atividade da acetilcolinesterase cerebral e muscular pode ser um biomarcador inicial de toxicidade deste inseticida.
Palavras-chave: Cyprinus carpio, Ctenopharyngodon idella, Aristichthys nobilis, agroquímico, concentração letal
INTRODUCTION
Insecticides are used extensively in agriculture, but their levels in superficial waters generally range far below lethal concentrations for aquatic organisms. However, sublethal adverse effects may result from exposure of aquatic organisms to insecticides at environmentally relevant concentrations (Das & Mukherjee, 2003; Saglio et al., 1996). Diafuran (carbamate) is used in rice fields to control pests and the contamination of water bodies adjacent to rice fields by carbofuran, mainly through run off, is quite possible (Adhikari et al., 2004). Pesticides used in pest control programs seem to produce many physiological and biochemical changes in freshwater organisms by influencing the activities of several enzymes (Sancho et al., 1998).
Acetylcholinesterase (AChE, EC 3.1.1.7) activity is routinely used as a biomarker of the exposure to certain groups of contaminants, such as organophosphate and carbamate insecticides (Grue et al., 1997). Low concentrations of the compounds can inhibit AChE, which leads to an accumulation of acetylcholine at central cholinergic synapses and neuromuscular junctions (Sancho et al., 1997; Varó et al., 2003). The inhibition of the acetylcholinesterase by pesticides can affect locomotion and equilibrium of exposed organisms (Saglio & Trijasse, 1998; Bretaud et al., 2000). In fish, previous studies on carbofuran have focused on the effects of high concentrations on inhibition of AChE activity (Health et al., 1997).
Freshwater aquaculture constitutes one-third of the total fish production in Southern Brazil, with carps being the dominant species. Thus, the aim of this study was to verify the relationship between the lethal concentration (LC50) of diafuran used during rice cropping and the AChE enzyme activity in brain and muscle tissue of the common carp (Cyprinus carpio, Linnaeus, 1758), the grass carp (Ctenopharyngodon idella, Valenciennes, 1844), and the bighead carp (Aristichthys nobilis, Richardson, 1845), as a possible early biomarker of the exposure of these organisms to the insecticide.
MATERIAL AND METHODS
Chemicals
All reagents used in the experiments were of the highest analytical grade Acetylthiocholine, DTNB (5,5'dithio-bis 2 nitrobenzoic acid), bovine serum albumin and Carbofuran (2,3-dihidro-2,2-dimetyl-7-benzofuranil-n-metylcarbamate) were obtained commercially, the last as Diafuran (50% purity).
Exposures
Common carp (5.5 ± 0.5 g and 7.7 ± 2.2 cm), grass carp (11.7 ± 3.3 g and 10.4 ± 3.1 cm), and bighead carp (11.3 ± 3.4 g and 10.2 ± 3.0 cm) fingerlings were obtained from a commercial fish grower near Santa Maria, Rio Grande do Sul State, Brazil. Fish were acclimated to laboratory conditions for 7 days. They were kept in tanks (250 L) and the water was constantly aerated by a static system. Fingerlings were then transferred to continously aerated 40 L boxes and maintained in an air conditioned room. Groups of ten fish/box (three replicates each species) (n = 3) were exposed for 96 h to different diafuran concentrations (dissolved in water) (mg L-1): common carp (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0), grass carp (1.0, 2.0, 3.0, and 3.5), and bighead carp (0.5, 1.0, 1.5, 2.0, 3.0, and 4.0). Control fish were maintained under the same conditions.
Diafuran was added to the water only at the beginning of the experiment and the water quality did not change throughout the experimental period: temperature: 20.5 ± 0.63°C, pH: 8.3 ± 0.02, dissolved oxygen: 4.9 ± 0.17 mg L-1, total alkalinity: 156.5 ± 7.93 mg L-1 CaCO3, hardness: 41.4 ± 3.91 mg L-1 CaCO3, total ammonia nitrogen: 0.9 ± 0.12 mg L-1, and nitrite: 0.01 ± 0.004 mg L-1. The quantification of waterborne carbofuran was performed by high performance liquid chromatography with photodiode array detection after a solid-phase extraction step as described by Gándara et al. (2002). Measured values were similar to nominal values (~5% variation).
Mortality for each insecticide concentration was recorded (12 h) to estimate LC50. Throughout the experimental period the swimming activity (normal, erratic swimming, lethargy, immobility) of the fish was observed, recorded and compared to the control.
Sampling and enzyme assay
At the end of the exposure period eight surviving fish from each treatment were killed and placed on ice and tissues (brain and muscle) were removed, frozen in liquid nitrogen and then stored at -20°C until AChE assay. For determination of enzyme activity the lower diafuran concentrations used in the LC50 experiment were used (mg L-1): common carp (0.5, 1.0, 1.5, and 2.0), grass carp (1.0, 2.0, and 3.0), and bighead carp (1.0, 1.5, 2.0, and 3.0). All enzyme tests were made in triplicate. Brain and muscle tissues were weighed and homogenized in 150 mM NaCl (15 mL) using a Potter-Elvejhem glass/Teflon homogenizer. The homogenates were centrifuged for 15 min at 3000 g at 5°C and the supernatant was used as the enzyme source. AChE activity was measured as described by Ellman et al. (1961) and modified by Villescas et al. (1981). Aliquots of the supernatant (50-100 mL) (brain and muscle, respectively) were incubated at 25°C for 2 min with 0.1 M phosphate buffer, pH 7.5; 1 mM DTNB as chromogen. After 2 min, the reaction was initiated by the addition of acethylthiocholine (AcSCh) (0.08 M) as substrate for the reaction mixture. The final volume was 2.0 mL. Absorbances were determined at 412 nm during 2 min. Enzyme activity was expressed as mmoL of AcSCh hydrolyzed per min and per gram of protein.
Statistics analysis
Means of LC50 for 96 h were calculated using probit analysis as described by Finney (1971). The AChE activity data were analyzed using one-way analysis of variance followed by the Tukey-Kramer test and expressed as mean ± standard error. The differences between treatments and controls were tested (p < 0.05).
RESULTS
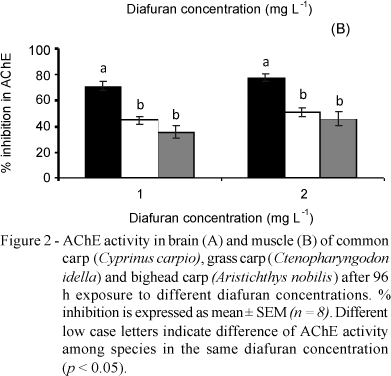
The 96h-LC50 of diafuran were: 1.81 mg L-1 for the common carp (confidence interval: 1.67 to 1.96), 2.71 mg L-1 for the grass carp (confidence interval: 2.50 to 2.89), and 2.37 mg L-1 for the bighead carp (confidence interval: 2.07 to 2.76). The AChE activity in brain and muscle of the unexposed control of common and grass carps was similar, however the muscle of the unexposed control of bighead carp presented higher specific AChE activity than the brain (14.44 against 5.94 mmol/AcSCh/min/ g protein, respectively) (Figure 1).
After diafuran exposure, the AChE activity decreased (p < 0.05) for all concentrations in both tissues in relation to the control. Maximum inhibition of the AChE activity for all species was reached when exposed to 1 mg L-1 diafuran. Maximum percentage AChE activity for 1 mg L-1 of diafuran in brain and muscle tissue compared to control was 28.92 and 28.89% for common carp, 30.17 and 55.45% for grass carp, and 55.22 and 64.54% for bighead carp (Figure 1). AChE inhibition was higher in the brain of common and grass carps exposed to 1 and 2 mg L-1 of diafuran than in bighead carp (Figure 2A). In addition, the highest AChE inhibition in fish muscle exposed to 1 and 2 mg L-1 diafuran was observed for the common carp (Figure 2B).
Swimming activity was normal only for control fish. At the lowest diafuran concentrations fish were lethargic (0.5 mg L-1 for common carp, 1.0 and 2.0 mg L-1 for grass carp and 0.5 and 1.0 mg L-1 for bighead carp), and at higher concentrations they remained immobile in the boxes (1.0, 1.5, 2.0, and 2.5 mg L-1 for common carp, 3.0 mg L-1 for grass carp and 1.5, 2.0, and 3.0 mg L-1 for bighead carp).
DISCUSSION
Diafuran (50% carbofuran) LC50 for the three carps species (common, grass and bighead carp) was 1.81, 2.71 and 2.37 mg L-1, respectively. As the recommended concentration of this insecticide in the rice field is 0.75 mg L-1 (SOSB AI, 2003), its use could be harmful for these species. In the field there is a faster decomposition of carbofuran (Plese et al., 2005), but in their study fish were not exposed to optimal water conditions (dissolved oxygen levels, temperature, pH) as in the laboratory. The 96-h LC50 for carbofuran (dissolved in water) in common carp larvae was 1.55 mg L-1 (2.5 ± 0.5 cm) (Kaur & Dhawan, 1993), similar to the value of the present study. On the other hand, according to the British Crop Protection Council (1991) and Resgalla et al. (2002) the LC50 of carbofuran for common carp fingerlings (2-8 cm) was 0.5 and 0.612 mg L-1 respectively, indicating that this species is sensible to this insecticide. However, both studies (British Crop Protection Council, 1991; Resgalla et al., 2002) used pure carbofuran (99%) dissolved in acetone and in experimental conditions without aeration. Pure carbofuran is very toxic to fish, and usually fish LC50 values are below 1 mg L-1 (Trotter et al., 1991).
AChE activity is frequently used as a biomarker of insecticide and pesticide toxicity. The activity of this enzyme is extremely important for many physiological functions, such as prey location, predator evasion and orientation toward food (Miron et al., 2005). When AChE activity decreases, ACh is not broken and accumulates within synapses which therefore cannot function in a normal way (Dutta & Arends, 2003).
In unexposed bighead carp muscle, the AChE-specific activity was two-fold higher than that observed in brain tissue and for common and grass carps the values were similar. Higher muscle AChE activity compared to that of brain was also observed in juvenile goldfish (Carassius auratus) (Bretaud et al., 2000). However, in channel catfish (Ictalurus punctatus) the AChE-specific activity was higher in the brain than in the muscle (Straus & Chambers, 1995). For all diafuran concentrations, for the three species, there was a decrease in the AChE activity in brain and muscle tissue. In the same way, common carp (6-10 cm) exposed to carbofuran (99%) presented lower brain AChE activity (Dembelé et al., 2000). Three different size groups (fry: 3-4, fingerlings: 6-8 and sub-adults: 10-12cm) of Nile tilapia (Oreochromis niloticus) exposed to carbosulfan (carbamate) 1, 4, 8 e 10 µg L-1 for 48 h presented lower brain AChE activity with the increasing concentration of carbosulfan (Chandrasekara & Pathiratne, 2007).
Changes in brain and muscle AChE activity observed in common, grass and bighead carps exposed to diafuran probably reflected in movement disturbances, with fish lethargic and immobile in the boxes, help to explain behavoir alterations induced by insecticides. Erratic swimming, convulsions and lethargy were also observed in fathead minnows (Pimephales promelas) exposed to carbofuran (0.2 g L-1) (Health et al., 1997) and European eel (Anguilla anguilla) (Sancho et al., 1997; Fernández-Vega et al., 2002) after exposure to fenitrothion and thiobencarb, respectively, and silver catfish (Rhamdia quelen) fingerlings exposed to 10 mg L-1 clomazone for 96 h (Miron et al., 2005).
Cholinesterase inhibition in brain and muscle produce adverse effects in movement because the AChE participates in neuronal and neuromuscular transmissions (Fernández-Vega et al., 1999, 2002). Diafuran provoked high AChE inhibition in brain and muscle for all carps. Common carp exposed to 0.05 mg L-1 of carbofuran for 48 h showed 80% inhibition in brain AChE activity (Bertrand et al., 1998), and exposure to 50 µg L-1 in goldfish inhibited 23% AChE activity in skeletal muscle (Bretaud et al., 2000). Common carp exposed to 0.1, 0.22 µg L-1 carbofuran (99%) showed 27.8 and 75% AChE activity inhibition, respectively, after 24 h (Dembelé et al., 2000). In addition, AChE activity inhibition in brain of Nile tilapia exposed to 10 µg L-1 carbofuran (48 h) was 59% (Chandrasekara & Pathiratne, 2007). Brain AChE inhibition was also observed in European eels exposed to diazinon (0.042 mg L-1 inhibition higher than 75%) (Cerón et al., 1996), and Lepomis macrochirus exposed to endosulfan (0.001 mg L-1 for 96 h, inhibition of 16%) (Dutta & Arends, 2003).
Carbofuran elicits acute excessive intoxication by virtue of reversible inhibition (carbamylation) of AChE, which hydrolyses acetylcholine (ACh), a neurohumoral transmitter. The inhibition of AChE consequently leads to excessive ACh accumulation at the synapses and neuromuscular juntions, resulting in overstimulation of ACh receptors, which could ultimately end in death due to respiratory failure (Gupta, 1994). Carbamate insecticides possess inhibitory effects on AChE activity at low concentrations in various freshwater fish species (Silva Filho et al., 2004; Liu et al., 2007)
Different results reported in the literature may depend on data such as chemical formulations of pesticides, their application and degradation rates, absorption estimative, and residues in run off water in the aquatic environment. Additional research is necessary to explain the species-specific differences in the relationship between AChE inhibition and mortality, and the physiologic perturbations associated with AChE inhibition (Fulton & Key, 2001). Although there is some controversy in the literature regarding the extent of the AChE inhibition required to cause death in aquatic animals, most estimatives lie in the 70-85% range. It seems that the extent of AChE inhibition leading to death is dependent upon the species and the type of tissue examined (Bretaud et al, 2000; Fulton & Key, 2001). Therefore, the in vivo response of carp brain and muscle AChE is a promising biomarker to demostrate the presence of anticholinesterase pesticides such as carbofuran in tropical waters at very low concentrations.
CONCLUSIONS
Concentrations of diafuran used during rice cropping may affect Cyprinus carpio, Ctenopharyngodon idella and Aristichthys nobilis behavior and the AChE activity in brain and muscle tissues of these species may be an early biomarker of the toxicity of this insecticide.
ACKNOWLEDGEMENTS
To CNPq for financial support and for fellowships to J.I. Golombieski and B. Baldisserotto.
Received March 27, 2007
Accepted January 07, 2008
- ADHIKARI, S.; SARKAR, B.; CHATTERJEE, A.; MAHAPATRA, C.T.; AYYAPPAN., S. Effects of cypermethrin and carbofuran on certain hematological parametres and prediction of their recovery in a freshwater teleost Labeo rohita (Hamilton). Ecotoxicology and Environmental Safety, v.58, p.220-226. 2004.
- BRITISH CROP PROTECTION COUNCIL. The pesticide manual. 9 ed. Surey: BCPC, 1991. p.126-127.
- BERTRAND, C.; COUSIN, X.; HAUBRUGE, E.; TOUTANT, J.P.; CHATONNET, A. L' acétylcholinestérase des poissons, cible des organophosphorés et des carbamates. Caractérisation du gene et des formes moléculaires de l'enzime chez Danio rerio. Effets des anticholinestérasiques. Bulletin Français de la Pêche et de la Pisciculture, v.350, p.535-546, 1998.
- BRETAUD, S.; TOUTANT, J.P.; SAGLIO, P. Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus) Ecotoxicology and Environmental Safety, v.47, p.117-124, 2000.
- CHANDRASEKARA, L.W.H.; PATHIRATNE, A. Body size-related differences in the inhibition of brain acetylcholinesterase activity in juvenile Nile tilapia (Oreochromis niloticus) by chlorpyrifos and carbosulfan. Ecotoxicology and Environmental Safety, v.67, p.109-119, 2007.
- CERÓN, J.J.; FERRANDO, M.D.; SANCHO, E.; GUTIERREZ-PANIZO, C.; ANDREU-MOLINER, E. Effects of diazinon exposure on cholinesterase activity in different tissues of European eel (Anguilla anguilla). Ecotoxicology and Environmental Safety; v.35, p.222-225, 1996.
- DAS, B.K.; MUKHERJEE, S.C. Toxicity of cypermethrin in Labeo rohita fingerlings: biochemical, enzymatic and haematological consequences. Comparative Biochemistry and Physiology. Part C, v.134, p.109-121, 2003.
- DEMBELÉ, K.; HEUBRUGE, E.; GASPAR, C. Concentration effects of selected insecticides on brain acetylcholinesterase in the common carp (Cyprinus carpio L.). Ecotoxicology and Environmental Safety, v.45, p.49-54, 2000.
- DUTTA, H.M.; ARENDS, D.A., Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environmental Research v.91, p.157-162, 2003.
- ELLMAN, G.L.; COURTNEY, D.; ANDRES, V.J.; FEATHERSTONE, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, v.7, p.88, 1961.
- FERNÁNDEZ-VEGA, C.; SANCHO, E.; FERRANDO, M.D.; ANDREU, E. Thiobencarb toxicity and plasma AChE inhibition in the European eel. Journal of Toxicology and Environmental Health B, v.34, p.6273, 1999.
- FERNÁNDEZ-VEGA, C.; SANCHO, E.; FERRANDO, M.D.; ANDREU, E. Thiobencarb-induced changes in acetylcholinesterase activity of the fish Anguilla anguilla. Pesticide Biochemistry and Physiology, v.72, p.55-63, 2002.
- FINNEY, D.J. Probit analysis. Cambridge: Cambridge University Press, 1971. 144p.
- FULTON, M.H.; KEY, P.B. Acetylcholinesterase inhibition in estuarine fish and invertebrates and indicator of organophosphorus insecticide exposure and effects. Environmental Toxicology Chemistry, v.20, p.37-45, 2001.
- GÁNDARA, S.; CANCHO-GRANDE, B.; LÓPEZ-BLANCO, M.C. Comparison of solid-phase extraction for carbofuran in water analyzed by high-performance liquid chromatography-photodiode-array detection. Journal of Chromatography A, v.963, p.117-123, 2002.
- GRUE, C.E.; GILBERT, P.L.; SEELEY, M.E. Neurophysiological and behavioral changes in non-target wildlife exposed to organophosphate and carbamate pesticide: thermoregulation, food consumption and reproduction. American Zoologist, v.37, p.369-388, 1997.
- GUPTA, R.C. Carbofuran toxicity: invited review. Journal of Toxicology and Environmental. Health, v.43, p.383-418, 1994.
- HEALTH, A.G.; CECH, J.J.; BRINK, L.; MOBERG, P.; ZINKL, J.G. Physiological responses of fathead minnow larvae to rice pesticides. Ecotoxicology and Environmental Safety, v.37, p.280-288, 1997.
- KAUR, K.; DHAWAN, A. Variable sensitivity of Cyprinus carpio eggs, larvae, and fry to pesticides. Bulletin of Environmental Contamination Toxicology, v.50, p.593-599, 1993.
- LIU, H.; YI, M.; SHI, X.; LIANG, P.; GAO, X. Sobstrate specificity of brain acetylcholinesterase and its sensitivity to carbamate insecticides in Carassius auratus. Fish Physiology and Biochemistry, v.33, p.29-34, 2007.
- MIRON, D.S.; CRESTANI, M.; SHETTINGER, M.R.; MORSCH, V.M.; BALDISSEROTTO, B.; TIERNO, M.A.; MORAES, G.; VIEIRA, V.L.P. Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Heptapteridae). Ecotoxicology and Environmental Safety, v.61, p.98-403, 2005.
- PLESE, L.P.; PARAIBA, L.C.; FOLONI, L.L.; TREVIZAN, L.R.P. Kinetics of carbosulfan hydrolysis to carbofuran and the subsequent degradation of this last compound in irrigated rice fields. Chemosphere, v.60, p.149-156, 2005.
- RESGALLA JR., C.; NOLDIN, J.A.; SANTOS, A.L.; SATO, G.; EBERHARDT, D.S. Toxicidade aguda de herbicidas e inseticidas utilizados na cultura do arroz irrigado sobre juvenis de carpa (Cyprinus carpio). Pesticidas: Revista Ecotoxicologia e Meio Ambiente, v.12, p.59-68, 2002.
- SAGLIO, P.; TRIJASSE, S.; AZAM, D. Behavioral effects of waterborne carbofuran in goldfish. Archives of Environmental Contamination and Toxicology, v.31, p.232-238, 1996.
- SAGLIO, P., TRIJASSE, S. Behavioural responses to atrazine and diuron in goldfish. Archives of Environmental Contamination and Toxicology, v.35, p.484-491, 1998.
- SANCHO, E.; FERRANDO, M.D.; ANDREU, E. Response and recovery of brain acetylcholinesterase in the European eel, Anguilla anguilla, exposed to feniltrothion. Ecotoxicology and Environmental Safety; v.38, p.205-209, 1997.
- SANCHO, E., FERRANDO, M.D., FÉRNANDEZ, C., ANDREU, E. Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotoxicology and Environmental Safety. v.41, p.168-175, 1998.
- SILVA FILHO, M.V.; OLIVEIRA, M.M.; SALLES, J.B.; BASTOS, V.I.F.C.; CASSANO, V.P.F.; BASTOS, J.C. Methyl-paraoxon comparative inhibition kinetics for acetylcholinesterase from barin of neotropical fishes. Toxicology Letters, v.153, p.247-254, 2004.
- SOCIEDADE SUL-BRASILEIRA DE ARROZ IRRIGADO - SOSBAI. Arroz irrigado: recomendações técnicas da pesquisa para o sul do Brasil. Itajaí:, SOSBAI, 2003. 126p.
- STRAUS, D.L.; CHAMBERS, J.E. Inhibition of acetylcholinesterase and aliesterases of fingerlings channel catfih by chlorpyrifos, parathion, and S,S,-tributyl-phosphorotrithioate (DEF). Aquatic Toxicology, v.33, p.311-324, 1995.
- TROTTER, D.M.; KENT, R.A.; WONG, P. Aquatic fate and effect of carbofuran. Critical Reviews in Environmental Control, v.21, p.137-176, 1991.
- VARÓ, I.; NAVARRO, J.C.; AMAT, F.; GUILHERMINO, L. Effect of dicholorvos on cholinesterase activity of the European sea bass (Dicentrarchus labrax). Pesticide Biochemistry and Physiology, v.75, p.61-72, 2003.
- VILLESCAS, R.; OSWALD, R.; MARIMOTO, H., Effects of neonatal undernutrition and cold stress on behavior and biochemical brain parameters in rats. Journal of Nutrition, v.111, p.1103-1110, 1981.
Publication Dates
-
Publication in this collection
21 July 2008 -
Date of issue
2008
History
-
Received
27 Apr 2007 -
Accepted
07 Jan 2008