Abstracts
Barium salts are used extensively for industrial purposes, generating residues that, if not appropriately disposed, can increase soil Ba content. The aim of the present work was to evaluate Ba extraction potential of mustard (Brassica juncea Czern.), sunflower (Helianthus annuus L.), and castor bean (Ricinus communis L.), grown in a soil artificially contaminated with increasing Ba additions. A greenhouse experiment was carried out by adding BaSO4 to a Rhodic Hapludox sample, at the 0, 150 and 300 mg kg-1 rates. After harvesting, the pot soil material was also analyzed for exchangeable Ba by CaCl2 extraction and by an ion exchange resin method. None of the plant species tested presented toxicity symptoms, decreased nutrient accumulation or decreased dry matter production in response to Ba treatments. The accumulation of Ba, in decreasing capacity was: sunflower> mustard> castor bean. The largest accumulation was with sunflower at 300 mg kg-1 of Ba added to the soil. When evaluated by the transference factor, none of the species tested was an efficient Ba accumulator, up to 47 days after emergence. The ion exchange resin method was not adequate to evaluate Ba availability to these plants.
toxicity; phytoextraction; fransference factor; ion exchange resin; exchangeable Ba
Sais de bário (Ba) são freqüentemente utilizados em diversas atividades industriais gerando resíduos que, se não dispostos adequadamente, podem elevar os teores desse metal no solo. Neste estudo, avaliou-se o potencial de extração de bário das espécies mostarda (Brassica juncea Czern.), girassol (Helianthus annuus L.) e mamona (Ricinus communis L.) com a finalidade de serem empregadas na fitorremediação de solos contaminados com esse elemento. Após o corte da parte aérea, o solo foi amostrado para determinação do Ba solúvel em CaCl2 e Ba trocável pelo método da resina trocadora de íons. Todas as espécies testadas não apresentaram sintomas de toxidez de Ba ou diminuição na produção da massa seca da parte aérea. Também não foram observadas alterações significativas na absorção de nutrientes pelas três espécies. Com relação à capacidade de extração de Ba do solo, as espécies se comportaram na seguinte ordem decrescente de acúmulo desse elemento na parte aérea: girassol > mostarda > mamona. Nenhuma das três espécies mostrou-se eficiente em extrair Ba do solo, até aos 47 dias após a germinação. O método da resina trocadora de íons não foi eficiente em indicar as quantidades fitodisponíveis de Ba presentes no solo.
toxidez; fitoextração; fator de transferência; resina de troca iônica; bário trocável
SOILS AND PLANT NUTRITION
Barium extraction potential by mustard, sunflower and castor bean
Potencial de extração de bário por mostarda, girassol e mamona
Aline Renée Coscione* * Corresponding author < aline@iac.sp.gov.br> ; Ronaldo Severiano Berton
Instituto Agronômico - Centro de P&D de Solos e Recursos Ambientais - C.P. 13020-902 - Campinas, SP - Brasil
ABSTRACT
Barium salts are used extensively for industrial purposes, generating residues that, if not appropriately disposed, can increase soil Ba content. The aim of the present work was to evaluate Ba extraction potential of mustard (Brassica juncea Czern.), sunflower (Helianthus annuus L.), and castor bean (Ricinus communis L.), grown in a soil artificially contaminated with increasing Ba additions. A greenhouse experiment was carried out by adding BaSO4 to a Rhodic Hapludox sample, at the 0, 150 and 300 mg kg1 rates. After harvesting, the pot soil material was also analyzed for exchangeable Ba by CaCl2 extraction and by an ion exchange resin method. None of the plant species tested presented toxicity symptoms, decreased nutrient accumulation or decreased dry matter production in response to Ba treatments. The accumulation of Ba, in decreasing capacity was: sunflower> mustard> castor bean. The largest accumulation was with sunflower at 300 mg kg1 of Ba added to the soil. When evaluated by the transference factor, none of the species tested was an efficient Ba accumulator, up to 47 days after emergence. The ion exchange resin method was not adequate to evaluate Ba availability to these plants.
Key words: toxicity, phytoextraction, fransference factor, ion exchange resin, exchangeable Ba
RESUMO
Sais de bário (Ba) são freqüentemente utilizados em diversas atividades industriais gerando resíduos que, se não dispostos adequadamente, podem elevar os teores desse metal no solo. Neste estudo, avaliou-se o potencial de extração de bário das espécies mostarda (Brassica juncea Czern.), girassol (Helianthus annuus L.) e mamona (Ricinus communis L.) com a finalidade de serem empregadas na fitorremediação de solos contaminados com esse elemento. Após o corte da parte aérea, o solo foi amostrado para determinação do Ba solúvel em CaCl2 e Ba trocável pelo método da resina trocadora de íons. Todas as espécies testadas não apresentaram sintomas de toxidez de Ba ou diminuição na produção da massa seca da parte aérea. Também não foram observadas alterações significativas na absorção de nutrientes pelas três espécies. Com relação à capacidade de extração de Ba do solo, as espécies se comportaram na seguinte ordem decrescente de acúmulo desse elemento na parte aérea: girassol > mostarda > mamona. Nenhuma das três espécies mostrou-se eficiente em extrair Ba do solo, até aos 47 dias após a germinação. O método da resina trocadora de íons não foi eficiente em indicar as quantidades fitodisponíveis de Ba presentes no solo.
Palavras-chave: toxidez, fitoextração, fator de transferência, resina de troca iônica, bário trocável
INTRODUCTION
Barium is an earth alkaline metal with similar geochemistry to calcium. Although often highly insoluble in water and mineral acids the Ba salts are extensively used for industrial purposes. Barium salts used on domestic utensils can lead to its accumulation in urban residues, such as sewage sludge industrial residues that include materials such as petroleum perforation mud. For both residues, the final disposition is commonly the soil (Ippolito & Barbarick, 2006; Brewer et al., 2004). Ba salts solubilization and their cation release can occur under specific conditions of pH, in absence of oxygen, or due to microbial action, (Baldi et al., 1996; Carbonell et al., 1999; Davidson et al., 2005; Phillips et al., 1998; Ghode et al., 1995).
A large number of plants has small quantities of Ba (4 a 50 mg kg1) in their tissues. When larger amounts are accumulated this element can be toxic inhibiting plant growth (Chaudhry et al., 1977; Llugany et al., 2000; Kuperman et al., 2006). Also, in animals and men, the ingestion of Ba in soluble forms is highly toxic. Thus, the monitoring of Ba accumulation in soil and water has deserved attention in local and international environmental legislation (Cetesb, 2005).
Phytoextraction, one of the phytoremediation techniques, consists of the use of metal-accumulating plants to remove undesirable metals from a contaminated soil by the harvesting and remotion of their shoots. This alternative is promising since it could reduce costs and is more environmental friendly than others. Mustard (Brassica juncea Czern.) has been used successfully to remove lead from soils (Blaylock et al., 1997), and sunflower (Helianthus annuus L.) has been shown also to act as a phytoaccumulating species. On the other hand, castor bean plant (Ricinus communis L.) is a robust grower, with high biomass production and hence with potential as a phytoaccumulator. The aim of the present work was to evaluate Ba extraction from soils for these three species, grown in a soil contaminated with increasing Ba contents.
MATERIAL AND METHODS
A greenhouse experiment was carried out from March to May 2004 at Campinas, State of São Paulo, Brazil (22º53'S; 47º03'W; 674 m), to evaluate Ba extraction potential by mustard, sunflower, and castor bean.
Soil sampling and characterization - A surface (0-20 cm) sample of a Rhodic Hapludox was used in a greenhouse experiment. It was collected at Campinas, SP, Brazil, and tested for soil fertility (Raij et al., 2001) resulting: pH in 0.01 M CaCl2 (1:2 soil solution ratio): 6.1; organic matter: 3.7g dm3; P (resin): 12 mg dm3; V: 89%; and the following attributes in mmolc dm3: K: 2.5; Ca: 83; Mg: 31; H+Al: 15; CEC: 131.3. Particle size (Camargo et al., 1986) presented: 537; 173 and 290 g kg1, of clay, silt and sand, respectively. Barium soil background concentration was 27.5 mg kg1 as determinated by the EPA 3051 method (US EPA, 1995).
Experimental design - The treatments were arranged as a completely randomized design, in a 3 × 3 factorial experiment (three species and three Ba rates), with three replications. Air-dried soil samples were sieved trough a 3-mm-mesh screen and portions of 1.65 dm3 (equivalent to 2 kg) were amended with Ba. The Ba rates in mg kg1 were: 0, 150, and 300 added as BaSO4. These last two rates are equivalent to the levels of alert and intervention in agricultural lands, respectively (Cetesb, 2005). After the contaminant addition, the soil was fertilized (mg per pot) as follows: 500 of P (simple superphosphate); and a solution prepared with KCl, (NH4)2SO4, H3BO4, ZnSO4, MnSO4.3H2O, CuSO4.5H2O and Na2MoO4.2H2O to provide 400 of K; 57 of S; 50 of N; 1.5 of B; 4.0 of Zn; 2.0 of Mn; 0.5 of Cu, and 0.4 of Mo, respectively. Following, the soil was homogenized and transferred to 2 dm3 plastic pots. Soil water was monitored daily during the course of the experiment, by weighting the pots and adding deionized water to the soil surface up to 60% of soil maximum water retention capacity. Maximum water retention capacity was determined previously by weighting the pots before and after the saturated soil had been freely drained for two hours. Each pot received six castor bean, ten mustard or ten sunflower seeds and after seedling emergence, three plants per pot for mustard and two plants per pot were left for castor bean and sunflower. At 7, 14, 21, and 28 days after emergence, a total of 700 mg of N per pot was added by a solution containing NH4NO3 (300 mg), Ca(NO3)2.4H2O (200 mg), and Mg(NO3)2.6H2O (200 mg). At 47 days after emergence shoots were harvested.
Analytical determinations - After harvesting the shoots were washed with tap water and oven dried at 60ºC to constant weights. In sequence, the shoot dry matter yields were determined, ground in a Wiley mill, and submitted to oven digestion (incineration) according to Bataglia et al. (1983) for determination of P, Mg, Ca, K, Zn, Mn, Fe, Cu, and B by inductively coupled plasma emission spectrometry (ICP-OES). Nitrogen content was determined by a modified Kjeldahl method (Raij et al., 2001).
Soil samples from each pot were analyzed for Ba using the ion exchange resin method commonly employed for routine soil fertility analysis (Raij et al., 2001) and by extraction with 0.1 mol L1 CaCl2 solution, using a 1:2.5 soil solution ratio, with ICP-OES determination.
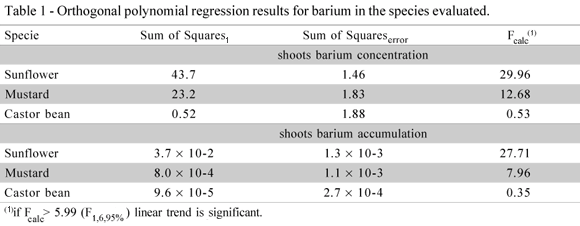
Results for shoots barium concentration and shoots barium accumulation per pot (Table 1) were submitted to orthogonal polynomial regression for evaluation of linear or deviation of linear trends using F test, and shoots dry matter were submitted to average comparison by student t test (using spooled and four degrees of freedom), both at 5% significance level (Gomes, 2000; Conagin et al., 2006). In addition, Figures were added in order to show the separation of means and the trends assessed by the statistical analysis.
For orthogonal polynomial regression evaluation of linear trend, considering when no barium was applied to the soil (To); for 150 mg kg1 Ba added to the soil (T1) and for 300 mg kg1 (T2), the average of treatments was used to obtain the sum of squares (SS) for the evaluation of linear (l) or deviation of linear (dl) trends by:
SSl = [1T2 1T0]2 / 3(12 + (1)2) = (T2 T0)2 / 6
SSdl = [1T2 + 1T0 2T1]2 / [12 + 12 + ( 2)2] 3=(T2 + T0 2T1)2 / 6
where the mean of square (MS) will be given by the SS divided by 1 and F can be calculated as: Fcalc = (MSl or MSdi) / MSerror and compared to the tabulated values of F1,6,95%.
The phytoextraction potential of species was be evaluated by the transference factor (T), defined as the ratio of the contaminant concentration in the plant tissue and its total concentration in the soil (Accioly & Siqueira, 2000). The desirable values for phytoaccumulating plants are above 1.
RESULTS AND DISCUSSION
No toxicity symptoms were observed in shoots for the species tested during the experiment. Ba did not affect the development of the plants since there was no difference between dry matter productions in soil with or without Ba for each species (Figure 1), when compared using t test (p < 0.05). Also based on these results effects on plant growth that might be caused by the sulphate salt can be discarded.
In spite of no effect of Ba toxicity on plant growth was observed, the t test comparisons confirmed that sunflower had a higher dry matter production than castor bean or mustard and that mustard had a higher dry matter production than castor bean. Sunflower dry matter was 161% and 71% higher than castor bean and mustard productions, respectively. This higher production could result in a concordant higher accumulation of Ba by sunflower if the concentrations of Ba were equal among the species.
For the N, P, Mg, Ca, K, Zn, Mn, Fe, Cu and B concentrations in the plants tissues (data not tabulated), no differences between treated or not treated plants with Ba were found, an indication that the addition of Ba to the soil, at the levels studied, imparted in no change in nutrient accumulation by plants, although examples of imbalances between Ca or K and Ba or deficiency of S in the presence of elevated contents of Ba were reported (Chaudry et al., 1997; Llugany et al., 2000).
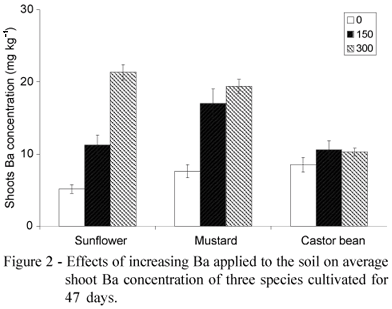
The analysis by orthogonal polynomial regression, at the significance level adopted, showed a difference among Ba levels for sunflower and mustard, with a linear trend (Figure 2). However, the same trend did not occur for castor bean, with low accumulation occurring in soils treated with Ba (Table 1). No deviation of linearity was observed for the species tested. Thus, the former two are more promising as good phytoaccumulating plants in the remediation of areas contaminated with this metal, as its maximum accumulation was not reached in the present study. The highest concentration of Ba was obtained for sunflower, with 21.3 mg kg1, followed by mustard (19.4 mg kg1), and castor bean (10.6 mg kg1).
The three plant species evaluated presented distinct behavior under Ba stress (Figure 3). The analysis by orthogonal polynomial regression showed a similar result to that of Barium concentrations in shoots, with differences among Ba levels for sunflower and mustard, (a linear trend) and it was not significant for accumulation of barium in castor bean (Table 1). Decreasing capacity of Ba accumulation was: sunflower> mustard> castor bean. The larger accumulation was in sunflower, at the rate of 300 mg kg1 of Ba added to the soil. At this contaminant level the sunflower also presented a Ba content 50% higher than mustard accumulated in shoots, in addition to the larger dry matter production. A small response, corresponding to a small increase in Ba shoots accumulation, was observed for castor bean, with the increase of soil contamination.
The highest transference factor (T) values here were 0.071; 0.113, and 0.075, for castor bean, mustard, and sunflower, respectively, under the application of 150 mg kg1 of Ba. Those values suggest that the three species evaluated are not suited for Ba accumulation.
In nature, Ba content of soils is around 100 to 3000 mg kg1 (Pais & Jones Jr., 1998). The Environmental Agency of the State of São Paulo (Cetesb) has recently set Ba contamination standard values in agricultural lands for São Paulo State, Brazil, adopting 150 and 300 mg kg1 as alert and intervention values, respectively (Cetesb, 2005). In spite of that, concentrations of 200 mg kg1 have already been reported as toxic to plants (Pais & Jones Jr., 1998). Chaudry et al (1977), using 500, 1000 and 2000 mg kg1 of Ba, added as Ba(NO3)2, observed accumulation on shoots and decrease in productions of bush beans and barley. Deleterious effects on root system seem to occur at very low concentrations of free Ba cation in solutions, as reported for hydroponics (Llugany et al., 2000).
In the present study Ba was applied to soil as BaSO4 (barite) considering the contamination caused by petroleum perforation mud and the possibility of solubilization from this source as reported elsewhere indicating that Ba may not be as immobile as expected (Baldi et al., 1996; Carbonell et al., 1999). However, since toxicity and accumulation Ba is dependent on its availability in the soil, it was also evaluated in the soil material from the pots' experiment.
The CaCl2 extraction, probably corresponding to Ba soluble in soil, presented a small increase in soils treated with barium sulphate (Table 2). Such low values may explain the lack of symptoms in the plant species testes as well as the low accumulation verified. The evaluation of Ba using the ion exchange resin (Raij et al., 2001), probably corresponding to exchangeable Ba in soil, lead to surprisingly high contents. The recovery varied from 26% for sunflower pots, at the 300 mg kg1 level, to 33 % for castor bean pots under the same dosage while for the CaCl2 extraction, it was 1.0% and 0.4%, respectively. Even for the soil without Ba addition high exchangeable values where found, such as 78 to 50 % of the total Ba content (27.5 mg kg1). Since Ba salts are sparingly soluble, this unusual extraction might be attributed to the " infinite sink" behavior of ion exchangeable resin, which removed Ba from soil solution thereby promoting BaSO4 dissolution due to the chemical equilibrium. In addition, since Ba absorbed by the species tested was very low (not more than 0.1% of the Ba added) it seems that this method is not adequate to evaluate Ba availability to plants.
CONCLUSIONS
According to their extraction capacity of Ba from the soil, the species can be classified in following order: sunflower> mustard> castor bean. None of the three species studied was efficient as a Ba accumulator, up to 47 days after emergence. The ion exchange resin method was not suitable in predicting the phytoavailability of Ba present in the soil.
Received May 02, 2007
Accepted April 16, 2008
- ACCIOLY, A.M.A.; SIQUEIRA, J.O. Contaminação química e biorremediação do solo. In: NOVAIS, R.F.; ALVAREZ V, V.H.; SCHAEFER, C.E.G.R. (Ed.) Tópicos em ciência do solo Viçosa: SBCS, 2000. p.299-352.
- BALDI, F.; PEPI, M.; BURRINI, D.; KNIEWALD, G.; SCALI, D.; LANCIOTTI, E. Dissolution of Ba from barite in sewage sludges and cultures of Desulfovibrio desulfurican Applied Environmental Microbiology, v.62, p.2398-2404, 1996.
- BATAGLIA, O.C.; FURLANI, A.M.C.; TEIXEIRA, J.P.F.; FURLANI, P.R.; GALLO, J.R. Métodos de análise de plantas Campinas: Instituto Agronômico, 1983. 48p. (Boletim técnico, 78)
- BLAYLOCK, M.J.; SLAT, D.E.; DULSHENKOV, S.; ZAKHAROVA, O.; GUSSMAN, C.; KAPULNIK, Y.; ENSLEY, B.D.; RASKIN, I. Enhanced accumulation of lead in Indian Mustard by soil-applied chelating agent. Environmental Science and Technology, v.31, p.860-865, 1997.
- BREWER, E.; STEVENSON, A.G.; HOWE, J.A.;CARROL, J.; SHIMMIELD, G.B. Drill cutting accumulations in the Northern and Central North Sea: a review of environmental interactions and chemical fate. Marine Pollution Bulletin, v.48, p. 12-25, 2004.
- CAMARGO, O.A.; MONIZ, A.C.; JORGE, J.A.; VALADARES, J.M.A.S. Métodos de análise química e física de solos do Instituto Agronômico. Campinas: Instituto Agronômico, 1986. 94p.
- CARBONELL, A.A.; PUBLIDO, R.; DELAUNE, R.R.; PATRICK JR., W.H.J. Soluble Ba in barite and phosphogypsum amended Mississipi River alluvial sediment. Journal of Environmental Quality, v.28, p.316-321, 1999.
- CHAUDHRY, F.M.; WALLACE, A.; MUELLER, R.T. Ba toxicity in plants. Communications in Soil Science and Plant Analysis, v.8, p.795797, 1977.
- COMPANHIA DE TECNOLOGIA DE SANEAMENTO AMBIENTAL - CETESB. Relatório de estabelecimento de valores orientadores para solos e águas subterrâneas no Estado de São Paulo São Paulo: CETESB, 2005. 73 p.
- CONAGIN, A.; NAGAI, V.; AMBROSIO, L.A. Princípios de técnica experimental e análise estatística de experimentos Campinas: Instituto Agronômico, 2006. E-book, versão1.
- DAVIDSON, C.C.; GIBSON, M.D.; HAMILTON, E.; MACGILLIVRAY, B.H.; REGLINSKI, J.; REBAZAL, E. The long-term environmental of strontium and Ba released from former mine workings in the granites of the Sunart region of Scotland, UK. Chemosphere, v.58, p.793798, 2005.
- GHODE, R.; MULLEY, R. SARIN, R. Operationally determined chemical speciation of barium and chromium in drilling fluid wastes by sequential extraction. Chemical Speciation and Bioavailability, v.7, p.133-137, 1995.
- GOMES, F.P. Curso de estatística experimental. 14 ed. Piracicaba: F.P. Gomes, 2000. 473p
- IPPOLITO, J.A.; BARBARICK, K.A. Biossolids affect soil Ba in a dryland wheat ecosystem. Journal of Environmental Quality, v.35, p.23332341, 2006.
- KUPERMAN, R.G.; CHECKAL, R.T.; SIMINI, M.; PHILLIPS, C.I.; SPEICHER, J.A. BARCLIFT, D.J. Toxicity benchmarks for antimony, Ba and beryllium determined using reproduction endpoints for Folsomia candida, Eisenia fetida and Enchytraeus crypticus. Environmental Toxicity and Chemistry, v.25, p.754762, 2006.
- LLUGANY, M.; POSCHENRIEDER, C.; BARCELO, J. Assessment of Ba toxicity to bush beans. Archives of Environmental Contamination and Toxicology, v.39, p. 440-444, 2000.
- PAIS, I.; JONES JR., J.B. The handbook of trace elements Boca Raton: St. Lucie Press, 1998. 223p.
- PHILLIPS, C.; EVANS, J.; HOM, W.; CLAYTON, J. Long-term changes in sediment Ba inventories associated with drilling-rellates discharges in the Santa Maria basin, California, USA. Journal of Environmental Toxicology, v.17, p.1653-1661, 1998.
- RAIJ, B. van.; ANDRADE, J.C.; CANTARELLA, H.; QUAGGIO, J.A. Análise química para avaliação da fertilidade de solos tropicais Campinas: Instituto Agronômico, 2001. 285p.
- UNITED STATES ENVIRONMENTAL PROTECTION AGENCY - US EPA. Test methods for evaluating solid waste: physical/chemical methods. 3 ed. Washington,DC: U.S. EPA, 1995. (SW-846, Method 3051). Available at: http://www.epa.gov/epaoswer/hazwaste/test/main.htm Accessed 12 August 2008.
Publication Dates
-
Publication in this collection
13 Feb 2009 -
Date of issue
Feb 2009
History
-
Accepted
16 Apr 2008 -
Received
02 May 2007