Abstracts
Micronutrient availability can be affected by the increase of the soil pH due to surface liming. A field trial was carried out on a loamy, kaolinitic, thermic Typic Hapludox at Ponta Grossa, Paraná State, Brazil. The main objective was to evaluate the effects of surface liming and re-liming on the availability of micronutrients [copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn)] for wheat (Triticum aestivum L.) cropped under a no-till system. A randomized complete block design was used in a split-plot arrangement. The main plots received surface lime applications (2, 4, and 6 Mg ha-1) in July 1993. In the subplots, surface lime (3 Mg ha-1) was applied again in June 2000. In 2003, before the wheat sowing, soil samples were taken at 0-5, 5-10, and 10-20 cm layers. Soil cationic micronutrients concentrations using different extractants (DTPA-TEA, Mehlich-1, HCl, and Mehlich-3) and solution/soil ratios were determined. Application of lime increased soil pH at 0-5, 5-10, and 10-20 cm. The increase in soil pH by liming did not affect soil organic carbon content. The Mehlich-3 solution had a greater capacity in extracting soil micronutrients. Increasing solution/soil ratio of the DTPA-TEA, Mehlich-1, and HCl solutions generally increased the extraction of Cu, Fe, Mn, and Zn. Liming and re-liming caused a decrease in Mn concentration in the wheat leaves. Leaf concentrations of Cu, Fe and Zn were not affected by liming treatments. The solutions of DTPA-TEA, Mehlich-1, HCl, and Mehlich-3 were ineffective to predict the soil cationic micronutrients availability for a wheat crop after surface application of lime.
Triticum aestivum L; soil acidity; dolomitic lime; multinutrient extractants; soil with variable charge
A disponibilidade de micronutrientes pode ser alterada pelo aumento no pH do solo proporcionado pela calagem superficial. Os efeitos da calagem e da reaplicação de calcário na superfície sobre a disponibilidade de micronutrientes [cobre (Cu), ferro (Fe), manganês (Mn) e zinco (Zn)] para o trigo (Triticum aestivum L.) foram estudados em um Latossolo Vermelho textura média sob plantio direto, em Ponta Grossa (PR). O delineamento experimental empregado foi o de blocos completos ao acaso com parcelas subdivididas. As parcelas receberam calagem superficial (2, 4 e 6 Mg ha-1) em julho de 1993. O calcário (3 Mg ha-1) foi reaplicado nas subparcelas em junho de 2000. Antes da semeadura do trigo em 2003, retiraram-se amostras de solo nas camadas de 0-5, 5-10 e 10-20 cm e determinaram-se os micronutrientes catiônicos com diferentes extratores (DTPA-TEA, Mehlich-1, HCl e Mehlich-3) e relações solução/solo. A calagem aumentou o pH do solo nas camadas de 0-5, 5-10 e 10-20 cm, mas não alterou o carbono orgânico. O extrator Mehlich-3 apresentou maior capacidade de extração de micronutrientes catiônicos. O aumento da relação solução/solo dos extratores DTPA-TEA, Mehlich-1 e HCl geralmente aumentou a extração de Cu, Fe, Mn e Zn. A calagem e a reaplicação do calcário diminuíram a concentração de Mn nas folhas de trigo. Os teores foliares de Cu, Fe e Zn não foram alterados pela calagem. As soluções de DTPA-TEA, Mehlich-1, HCl e Mehlich-3 foram ineficientes para prever a disponibilidade de micronutrientes catiônicos para o trigo, após aplicação superficial de calcário.
Triticum aestivum L; acidez do solo; calcário dolomítico; extratores multinutrientes; solo com carga variável
SOILS AND PLANT NUTRITION
Extraction methods and availability of micronutrients for wheat under a no-till system with a surface application of lime
Métodos de extração e disponibilidade de micronutrientes para o trigo cultivado em plantio direto com calagem na superfície
Adriel Ferreira da FonsecaI; Eduardo Fávero CairesI, * * Corresponding author < efcaires@uepg.br> ; Gabriel BarthII
IUEPG - Depto de Ciência do Solo e Engenharia Agrícola -84030-900 - Ponta Grossa, PR - Brasil
IIFUNDAÇÃO ABC - Setor de Fertilidade do Solo - 84165-980 - Castro, PR - Brasil
ABSTRACT
Micronutrient availability can be affected by the increase of the soil pH due to surface liming. A field trial was carried out on a loamy, kaolinitic, thermic Typic Hapludox at Ponta Grossa, Paraná State, Brazil. The main objective was to evaluate the effects of surface liming and re-liming on the availability of micronutrients [copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn)] for wheat (Triticum aestivum L.) cropped under a no-till system. A randomized complete block design was used in a split-plot arrangement. The main plots received surface lime applications (2, 4, and 6 Mg ha-1) in July 1993. In the subplots, surface lime (3 Mg ha-1) was applied again in June 2000. In 2003, before the wheat sowing, soil samples were taken at 0-5, 5-10, and 10-20 cm layers. Soil cationic micronutrients concentrations using different extractants (DTPA-TEA, Mehlich-1, HCl, and Mehlich-3) and solution/soil ratios were determined. Application of lime increased soil pH at 0-5, 5-10, and 10-20 cm. The increase in soil pH by liming did not affect soil organic carbon content. The Mehlich-3 solution had a greater capacity in extracting soil micronutrients. Increasing solution/soil ratio of the DTPA-TEA, Mehlich-1, and HCl solutions generally increased the extraction of Cu, Fe, Mn, and Zn. Liming and re-liming caused a decrease in Mn concentration in the wheat leaves. Leaf concentrations of Cu, Fe and Zn were not affected by liming treatments. The solutions of DTPA-TEA, Mehlich-1, HCl, and Mehlich-3 were ineffective to predict the soil cationic micronutrients availability for a wheat crop after surface application of lime.
Key words:Triticum aestivum L., soil acidity, dolomitic lime, multinutrient extractants, soil with variable charge.
RESUMO
A disponibilidade de micronutrientes pode ser alterada pelo aumento no pH do solo proporcionado pela calagem superficial. Os efeitos da calagem e da reaplicação de calcário na superfície sobre a disponibilidade de micronutrientes [cobre (Cu), ferro (Fe), manganês (Mn) e zinco (Zn)] para o trigo (Triticum aestivum L.) foram estudados em um Latossolo Vermelho textura média sob plantio direto, em Ponta Grossa (PR). O delineamento experimental empregado foi o de blocos completos ao acaso com parcelas subdivididas. As parcelas receberam calagem superficial (2, 4 e 6 Mg ha-1) em julho de 1993. O calcário (3 Mg ha-1) foi reaplicado nas subparcelas em junho de 2000. Antes da semeadura do trigo em 2003, retiraram-se amostras de solo nas camadas de 0-5, 5-10 e 10-20 cm e determinaram-se os micronutrientes catiônicos com diferentes extratores (DTPA-TEA, Mehlich-1, HCl e Mehlich-3) e relações solução/solo. A calagem aumentou o pH do solo nas camadas de 0-5, 5-10 e 10-20 cm, mas não alterou o carbono orgânico. O extrator Mehlich-3 apresentou maior capacidade de extração de micronutrientes catiônicos. O aumento da relação solução/solo dos extratores DTPA-TEA, Mehlich-1 e HCl geralmente aumentou a extração de Cu, Fe, Mn e Zn. A calagem e a reaplicação do calcário diminuíram a concentração de Mn nas folhas de trigo. Os teores foliares de Cu, Fe e Zn não foram alterados pela calagem. As soluções de DTPA-TEA, Mehlich-1, HCl e Mehlich-3 foram ineficientes para prever a disponibilidade de micronutrientes catiônicos para o trigo, após aplicação superficial de calcário.
Palavras-chave: Triticum aestivum L., acidez do solo, calcário dolomítico, extratores multinutrientes, solo com carga variável
Introduction
Surface application of lime without incorporation has been the usual practice to control soil acidity in no-till (NT) systems (Caires et al., 2005). However, surface liming can decrease the cationic micronutrients availability as a consequence of increasing soil pH at the most superficial layers of the soil (Caires & Da Fonseca, 2000; Godsey et al., 2007).
Studies related to bioavailability of cationic micronutrients in Brazilian soils have been carried out in pots under greenhouse conditions or in field trials under conventional tillage systems (Camargo et al., 1982; Bataglia & Raij, 1989; Abreu et al., 1994, 1996, 1998, 2002; Rodrigues et al. 2001; Nascimento et al., 2006). In no-till systems, soil organic matter and cationic micronutrients concentrations have been higher up to 10 cm depth (Zanão Júnior et al., 2007). Because the surface-applied lime under NT systems has been more effective in increasing pH near the soil surface (Caires et al., 2005), more information is necessary concerning the effects of liming on cationic micronutrient bioavailability in this cropping system. In addition, well-known extractants, i.e. chelating and/or acid solutions reported in Norvell (1984) and Abreu et al. (2007), must be more studied under NT systems to identify the more appropriated soil micronutrient extractant.
From the economic point of view wheat is, one of the most important winter crops in Southern Brazil. Therefore, the wheat crop was chosen to be used in this study to evaluate the effects of surface liming and re-liming in a long term NT system on availability of cationic micronutrients (Cu, Fe, Mn, and Zn). Different extractants and soil/solution ratios were evaluated, as well as the micronutrients concentrations in wheat leaves to determine the most appropriated soil extractants for available cationic micronutrients in this crop production system.
Material and Methods
The experiment was carried out in Ponta Grossa, PR, Brazil (25º05'58"S, 50º09'30"W), on a loamy, kaolinitic, thermic Typic Hapludox. Before the establishment of the experiment (May 1993 ) soil chemical and particle size analyses of the topsoil (0-20 cm) showed the following results: pH [1:2.5 soil: 0.01 mol L-1 calcium chloride (CaCl2) ] : 4.5; exchangeable Al3+, Ca2+, Mg2+, and K+ : 6, 16, 10, and 1.4 mmolc dm-3, respectively; total acidity pH 7.0 (H + Al): 58 mmolc dm-3; P (Mehlich-1): 9.0 mg dm-3; soil organic carbon (SOC): 19 g dm-3; base saturation 32%; clay, silt, and sand: 295, 240, and 465 g kg-1 respectively. The clay fraction of the soil had 265.8 g kg-1 of kaolinite, 26.8 g kg-1 goethite, and 2.4 g kg-1 hematite. At the beginning of the experiment, the field site had been used for grain cropping under NT system during the previous 15 yr.
A randomized complete block design, with three replications in a split-plot arrangement was used. The main plots (8.0 m × 6.3 m) treatment consisted of a surface dolomitic lime broadcasting at the rates of 0, 2, 4, and 6 Mg ha-1 in July 1993. The lime rates were calculated to raise the base saturation in the topsoil (0-20 cm) to 50, 70 and 90% base saturation. The dolomitic lime used contained 176 g kg-1 Ca, 136 g kg-1 Mg, and 84% effective calcium carbonate equivalent (ECCE). Approximately, seven years later (in June 2000) the main plots were subdivided in two subplots (4.0 m × 6.3 m) to study the influence of surface re-liming (196 g kg-1 Ca, 130 g kg-1 Mg, and 90% ECCE) at the rates of 0 and 3 Mg ha-1. The re-liming highest rate was calculated to raise the topsoil base saturation to 65% (Caires et al., 2000) regarding the treatment on which were applied 4 Mg ha-1 of lime in July 1993 [pH 0.01 mol L-1 CaCl2 of 4.6; cation exchange capacity (CEC) at pH 7.0 of 110.8 mmolc dm-3; and 41% of base saturation].
The experimental area has been fertilized with N, P, and K, without addition of cationic micronutrients. Soybean [Glycine max (L.) Merr.] or corn (Zea mays L.) in the spring-summer season, and wheat or triticale (× Triticosecale) or black oat (Avena strigosa Schreb.) in the autumn-winter season has been cultivated. More details about the historic of cropping and fertilization and the effects of surface application of lime in the soil-plant system was reported elsewhere (Caires et al. 2005).
Wheat, cv. CD 104 (moderately susceptible to the soil exchangeable Al3+) was sown in June 2003, with 17 cm between rows and 140 kg of seed per hectare, for a population between 250 and 300 plants m-2. Fertilizers were applied by top dressing at the rates of 80 kg ha-1 N and 35 kg ha-1 K, as ammonium nitrate and potassium chloride, respectively.
Wheat plants flowered fully 84 days after emergence, and the crop maturation occurred 126 days after emerging. Air temperature was adequate for wheat growing and rainfall was on a considerable amount before sowing (55 mm) and before the plant flowering stage (56 mm). However, there was an extended water deficit during the vegetative development stage. Rainfall was 434 mm during the wheat crop cycle. More details about the wheat crop management are in Caires et al. (2006b).
During the flowering period of the wheat crop, leaves were sampled from 30 plants (flag leaf standard) of each subplot (Bataglia et al., 1978). These samples were washed in de-ionized water, dried in a forced-air oven at 60ºC until constant mass was achieved, and ground in a Wiley type mill to pass a 0.75 mm screen. After nitric-perchloric acid digestion of the plant tissues, Cu, Fe, Mn, and Zn concentrations were determined by atomic absorption spectrophotometry, according to Malavolta et al. (1997).
In May 2003, before wheat sowing ten years after surface liming and three years after surface re-liming soil samples were taken at 0-5, 5-10, and 10-20 cm layers. Twelve soil core samples per subplot were taken with a soil probe sampler and mixed to obtain a composite sample, which was air dried, and crushed to pass a 2-mm sieve. Soil pH and SOC were determined according to procedures suggested by Pavan et al. (1992). Soil available contents of Cu, Fe, Mn and Zn were extracted using the following procedures:
a)Method-1 [DTPA-TEA solution (2:1)]: Ten cm3 of air dried soil + 20 mL of extracting solution were shaken during 2 h. Soil extracts (2:1 extractant/soil ratio) were obtained with DTPA-TEA (0.005 mol L-1 diethylenetriaminepentaacetic acid + 0.1 mol L-1 triethanolamine + 0.01 mol L-1 CaCl2) solution at pH 7.3 as described by Lindsay & Norvell (1978). This is the method used by some Brazilian laboratories, adopting the "IAC System of Soil Analysis" (Abreu et al., 1998)].
b) Method-2 [DTPA-TEA solution (5:1)]: Ten cm3 of air dried soil + 50 mL of extracting solution were shaken for 2 h. Soil extracts [5:1 extractant/soil ratio, according to Norvell (1984)] were obtained with DTPA-TEA solution at pH 7.3 (Lindsay & Norvell, 1978).
c) Method-3 [Mehlich-1 solution (5:1)]: Five cm3 of air dried soil + 25 mL of extracting solution were shaken for 5 min. Soil extracts (5:1 extractant/soil ratio) were obtained with Mehlich-1 [0.05 mol L-1 hydrochloric acid (HCl) + 0.0125 mol L-1 sulfuric acid (H2SO4)] solution. This procedure is widely used by many laboratories in Brazil for determination of micronutrients (Bernardi et al., 2002).
d) Method-4 [Mehlich-1 solution (10:1)]: Two and one-half cm3 of air dried soil + 25 mL of extracting solution were shaken for 5 min. Soil extracts (10:1 extractant/soil ratio) were obtained with Mehlich-1 solution. This procedure is widely used by many laboratories in Brazil as a multinutrient extractant (Abreu et al., 1998).
e) Method-5 [HCl solution (5:1)]: Five cm3 of air dried soil + 25 mL of extracting solution were shaken for 30 min. Soil extracts (5:1 extractant/soil ratio) were obtained with 0.1 mol L-1HCl solution as described by Osiname et al. (1973).
f) Method-6 [HCl solution (10:1)]: Two and one-half cm3 of air dried soil + 25 mL of extracting solution were shaken for of 30 min. Soil extracts (10:1 extractant/soil ratio) were obtained with 0.1 mol L-1 HCl solution as described by Osiname et al. (1973).
g) Method-7 [Mehlich-3 solution]: Two and one-half cm3 of air dried soil + 25 mL of extracting solution were shaken for 5 min. Soil extracts (10:1 extractant/soil ratio) were obtained with Mehlich-3 [0.2 mol L-1 acetic acid (CH3COOH) + 0.25 mol L-1 NH4NO3 + 0.015 mol L-1 ammonium fluoride (NH4F) + 0.013 mol L-1 nitric acid (HNO3) + 0.001 mol L-1 ethylenediaminetetraacetic acid (EDTA)] solution at pH 2.5 as described by Mehlich (1984).
All soil extracting solution suspensions were shaken on a horizontal-circular shaking machine at 220 rpm. After this step, all suspensions were filtered through Whatman #42 filter paper and the concentration of micronutrients were measured using a PerkinElmer Optima 3000 XL simultaneous inductively coupled plasma optical emission spectrometry (ICP-OES), under routine operating conditions, at the 324.754 nm, 259.940 nm, 257.610 nm, and 213.856 nm atomic lines for Cu, Fe, Mn, and Zn, respectively.
Soil and plant data were submitted to variance and regression analyses. Regression equations were adjusted to the obtained data according to lime rates (0, 2, 4, and 6 Mg ha-1), adopting the magnitude of coefficients of determination (p < 0.05) as the criteria of choice. The effects of re-liming at 0 and 3 Mg ha-1 were compared by the Tukey test (p = 0.05). When a no significant interaction of the liming versus the re-liming treatment was observed, the effects of treatments were compared by using their means. Simple linear correlation analyses (Pearson correlation, p < 0.05) were obtained for soil pH and cationic micronutrients extracted by different procedures. All statistical analyses were performed using the SAS program, version 8.02 (SAS Institute, 1999).
Results and Discussion
Soil organic carbon and soil reaction
No interactions were observed between the surface liming rates (0, 2, 4, and 6 Mg ha-1) and surface re-liming (0 and 3 Mg ha-1) for SOC and soil pH. Surface liming and re-liming did not cause changes in the SOC concentrations. The mean concentrations of SOC at 0-5, 5-10 and 10-20 cm layers were 25.1, 19.2 and 17.7 g dm-3, respectively. Caires et al. (2006a) reported similar results for other Oxisol (clayey, kaolinitic, thermic Rhodic Hapludox) under a NT system.
Soil pH ( ) increased linearly with increasing surface liming rate (x, in Mg ha-1) in the folowing layers: 0-5 cm (
) increased linearly with increasing surface liming rate (x, in Mg ha-1) in the folowing layers: 0-5 cm ( =4.85 + 0.14x, R2 = 0.98), 5-10 cm (
=4.85 + 0.14x, R2 = 0.98), 5-10 cm ( = 4.31 + 0.13x, R2 = 0.99) and 10-20 cm (
= 4.31 + 0.13x, R2 = 0.99) and 10-20 cm ( = 4.32 + 0.07x, R2 = 0.97). Surface re-liming increased significantly soil pH from 4.9 to 5.7, 4.6 to 4.8, and 4.5 to 4.6 at the 0-5, 5-10 and 10-20 cm layers, respectively. More details about the effects of surface liming on the soil chemical attributes are related elsewhere (Caires et al., 2006b).
= 4.32 + 0.07x, R2 = 0.97). Surface re-liming increased significantly soil pH from 4.9 to 5.7, 4.6 to 4.8, and 4.5 to 4.6 at the 0-5, 5-10 and 10-20 cm layers, respectively. More details about the effects of surface liming on the soil chemical attributes are related elsewhere (Caires et al., 2006b).
The availability of micronutrients to the crops is controlled by many soil factors such as pH, soil organic matter, temperature, and moisture (Fageria et al., 2002). In this study, because the wheat crop presented adequate growth and yield (Caires et al., 2006b) and SOC concentrations were unchanged due to liming treatments, soil pH was assumed to be the major factor on determining the bioavailability of Cu, Fe, Mn, and Zn.
Extractable cationic micronutrients
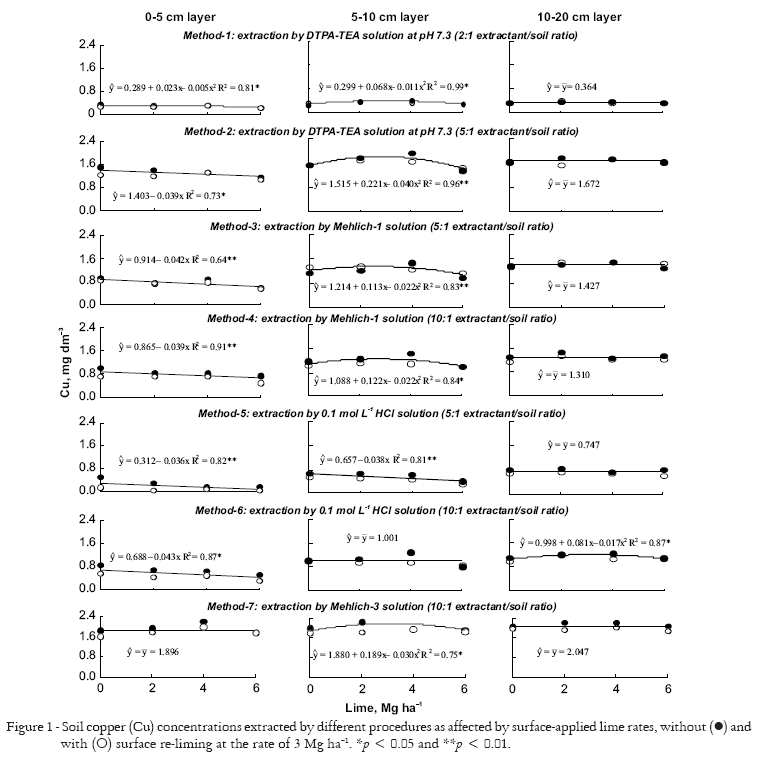
Soil extractable Cu, for all extraction methods, was not influenced by the interaction between lime rates (0, 2, 4, and 6 Mg ha-1) and re-liming (0 and 3 Mg ha-1).Copper concentrations decreased linearly with increasing lime application rates for DTPA-TEA solution (5:1), Mehlich-1 solution (5:1 and 10:1) and HCl solution (5:1 and 10:1) at 0-5 cm layer; and also by 0.1 mol L-1 HCl solution (5:1) at 5-10 cm layer (Figure 1). Negative correlations were observed between concentrations of extractable Cu and soil pH, mainly, at 0-5 cm layer (Table 1). Solubility of Cu2+ is soil pH dependent and decreases 100-fold for each unit increase in pH (Fageria et al., 2002). Moreover, these authors also state that increases in soil pH above 6.0 induces hydrolysis of hydrated Cu which can lead to a stronger Cu adsorption by the clay minerals and organic matter.
Unusual effects were also observed and associated with liming; e.g. quadratic increase of the Cu concentrations extracted by DTPA-TEA solution (2:1) at 0-5 cm layer; by DTPA-TEA solution (2:1 and 5:1), Mehlich-1 solution (5:1 and 10:1) and Mehlich-3 solution at 5-10 cm layer; and, by 0.1 mol L-1 HCl solution (10:1) at 10-20 cm layer (Figure 1). A possible explanation for these effects is that an increase in soil pH as a consequence of liming can have enhanced microbial activity, increasing dissolved organic matter (Filep et al., 2003) and, consequently, improving solubility of Cu bounded to the low molecular-weight organic compounds.
Soil extractable Fe byDTPA-TEA solution (2:1 and 5:1), Mehlich-1 solution (10:1), and 0.1 mol L-1 HCl solution (5:1 and 10:1) at 0-5 cm layer was influenced by the interactions between lime rates (0, 2, 4, and 6 Mg ha-1) and re-liming (0 and 3 Mg ha-1) (Figure 2). Lower concentrations of soil extractable Fe was observed on the re-limed plots. For the other studied extractants and layers, liming caused linear decrease of the extractable Fe concentrations (Figure 2). In addition, negative correlations were observed between concentrations of extractable Fe and soil pH for all studied layers (Table 1). Ferric (Fe3+) and ferrous (Fe2+) activity in the soil solution decrease 1000-fold and 100-fold, respectively, for each unit increase in soil pH (Lindsay, 1979; Fageria et al., 2002). Alleoni et al. (2005) also observed a decrease in Fe concentrations extracted by DTPA-TEA (2:1 extractant/soil ratio) at the 10 cm soil layer after surface liming application under NT system.
No interactions were observed between lime rates (0, 2, 4, and 6 Mg ha-1) and re-liming (0 and 3 Mg ha-1) to Mn extracted by different procedures (Figure 3). All extractants indicate that increasing surface lime rate increased quadratically extractable Mn concentration at 0-5 cm layer (Figure 3). Surface liming probably caused an increase on microbial activity and on dissolved organic matter (Filep et al., 2003) at the soil surface. Increased microbial activity can also result in a decrease in the oxidation-reduction potential of the soil, increasing Mn availability (Stevenson, 1986); consequently, manganese (II) forms only relatively weak bounds with organic ligands (Marschner, 1995). In all extraction methods, no reductions in extractable Mn at the soil surface layer (0-5 cm) were detected from surface application of lime. However, increasing surface lime rate decreased linearly Mn concentration extracted by DTPA-TEA solution (5:1) at 5-10 cm layer, and by DTPA-TEA solution (2:1 and 5:1), 0.1 mol L-1 HCl solution (5:1 and 10:1) and Mehlich-3 solution at 10-20 cm layer. In these cases, negative correlations were generally observed between concentrations of extractable Mn and soil pH, mainly, below the 5 cm depth (Table 1). According to Fageria et al. (2002), the main ionic Mn species in a soil solution is Mn2+, and its concentrations decrease 100-fold for each unit increase in soil pH.
The various soil extractable Zn concentrations (Method-1 to Method-7) were not influenced by the interaction between lime rates (0, 2, 4, and 6 Mg ha-1) and re-liming (0 and 3 Mg ha-1) (Figure 4). Increasing surface lime rate increased linearly Zn concentrations extracted by DTPA-TEA solution (5:1) at 5-10 cm layer. This can be interpreted as a direct effect of the high chelation capacity as proportioned by 5:1 solution/soil ratio used in Method-2. Norvell (1984) proposed to use a greater solution/soil ratio to compensate the limitations of extractants, mainly of their chelation capacity in soil at low pH. For the other depths and procedures, changes on extractable Zn concentrations after liming treatments were not observed. Acid extractants have normally not been efficient to detect slight changes in extractable Zn as a consequence of liming, becoming a hard task to select an adequate extractant for this micronutrient (Abreu et al., 2007).
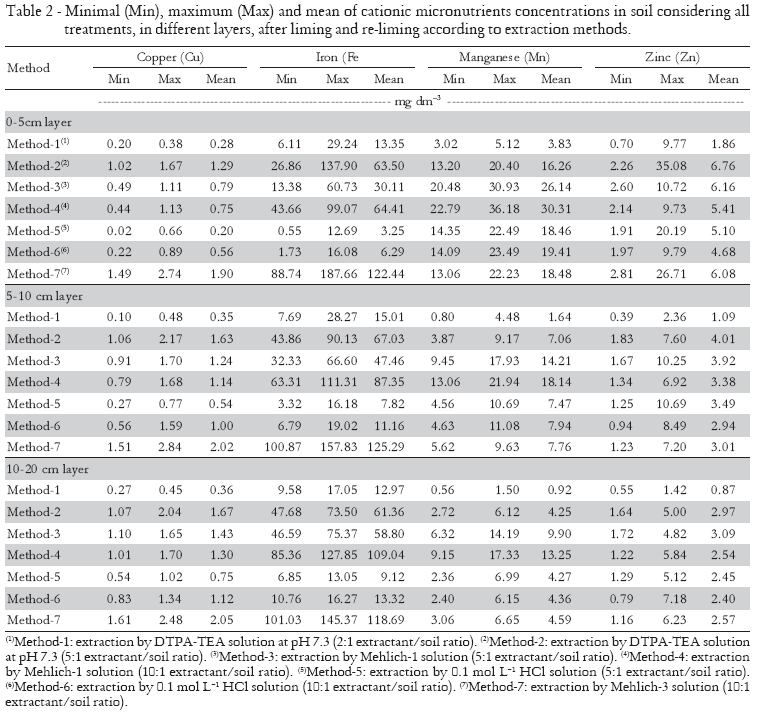
The mean capacity of the evaluated solutions to extract soil Cu followed this order at 0-5 cm layer (Table 2): Mehlich-3 solution > DTPA-TEA solution (5:1) > Mehlich-1 solution (5:1)  Mehlich-1 solution (10:1) > 0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1) > 0.1 mol L-1 HCl solution (5:1). At the 5-10 and 10-20 cm layers this order was similar; only the 0.1 mol L-1 HCl solution (5:1) extracted more Cu than the DTPA-TEA solution (2:1). The Mehlich-3 solution demonstrated a greater capacity of extraction in comparison to the other extractants, which is in agreement with Abreu et al. (1996). Because of the acid reagents and chelates, such as EDTA, it is expected higher amounts of micronutrients when they are extracted by the Mehlich-3 solution than by the DTPA-TEA (Vidal-Vázquez et al., 2005) and by diluted acids (e.g. Mehlich-1 and HCl) (Abreu et al., 2002). Diluted acid solutions may only partially solubilize soil's Cu, while chelating agents such as DTPA or EDTA reduce the Cu activity in solution by complexation, causing the dissolution of the labile forms of Cu in soils (Abreu et al., 1998). Also, a greater solution/soil ratio induces an increase in extraction capacity, except for Mehlich-1 solution (Table 2).
Mehlich-1 solution (10:1) > 0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1) > 0.1 mol L-1 HCl solution (5:1). At the 5-10 and 10-20 cm layers this order was similar; only the 0.1 mol L-1 HCl solution (5:1) extracted more Cu than the DTPA-TEA solution (2:1). The Mehlich-3 solution demonstrated a greater capacity of extraction in comparison to the other extractants, which is in agreement with Abreu et al. (1996). Because of the acid reagents and chelates, such as EDTA, it is expected higher amounts of micronutrients when they are extracted by the Mehlich-3 solution than by the DTPA-TEA (Vidal-Vázquez et al., 2005) and by diluted acids (e.g. Mehlich-1 and HCl) (Abreu et al., 2002). Diluted acid solutions may only partially solubilize soil's Cu, while chelating agents such as DTPA or EDTA reduce the Cu activity in solution by complexation, causing the dissolution of the labile forms of Cu in soils (Abreu et al., 1998). Also, a greater solution/soil ratio induces an increase in extraction capacity, except for Mehlich-1 solution (Table 2).
Soil Fe concentrations occurred in the following order at 0-5 and 5-10 cm layers, considering the extraction mean capacity of various solutions (Table 2): Mehlich-3 solution > Mehlich-1 solution (10:1) > DTPA-TEA solution (5:1) > Mehlich-1 solution (5:1) > DTPA-TEA solution (2:1) > 0.1 mol L-1 HCl solution (10:1) > 0.1 mol L-1 HCl solution (5:1). At the layer of 10-20 cm this order was similar; only the 0.1 mol L-1 HCl solution (10:1) extracted slightly more Fe than the DTPA-TEA solution (2:1). Abreu et al. (2004) verified that extracting the Fe followed this order: Mehlich-3 > Mehlich-1 (5:1 extractant/soil ratio) > DTPA-TEA (2:1 extractant/soil ratio). Similar results were also observed by Rodrigues et al. (2001).
The order of the Mn extraction, according to the mean capacity of the various solutions and to the 0-5, 5-10, and 10-20 cm layers was the following (Table 2): Mehlich-1 solution (10:1) > Mehlich-1 solution (5:1) > 0.1 mol L-1 HCl solution (10:1)  Mehlich-3 solution
Mehlich-3 solution  0.1 mol L-1 HCl solution (5:1)
0.1 mol L-1 HCl solution (5:1)  DTPA-TEA solution (5:1) > DTPA-TEA solution (2:1). Abreu et al. (2004) also observed that Mehlich-1 (5:1 extractant/soil ratio) solution extracted more Mn than Mehlich-3 solution. On the other hand, Rodrigues et al. (2001) found that the Mehlich-3 solution have a higher Mn extraction capacity than the DTPA-TEA (2:1 extractant/soil ratio) solution and Mehlich-1 (5:1 extractant/soil ratio) solution.
DTPA-TEA solution (5:1) > DTPA-TEA solution (2:1). Abreu et al. (2004) also observed that Mehlich-1 (5:1 extractant/soil ratio) solution extracted more Mn than Mehlich-3 solution. On the other hand, Rodrigues et al. (2001) found that the Mehlich-3 solution have a higher Mn extraction capacity than the DTPA-TEA (2:1 extractant/soil ratio) solution and Mehlich-1 (5:1 extractant/soil ratio) solution.
The mean capacity of the evaluated solutions when extracting the Zn from the soil followed this order at 0-5 cm layer: DTPA-TEA solution (5:1)  Mehlich-1 solution (5:1)
Mehlich-1 solution (5:1)  Mehlich-3 solution > Mehlich-1 solution (10:1)
Mehlich-3 solution > Mehlich-1 solution (10:1)  0.1 mol L-1 HCl solution (5:1) > 0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). At the layer of 5-10 cm this order was: DTPA-TEA solution (5:1) Mehlich-1 solution (5:1)
0.1 mol L-1 HCl solution (5:1) > 0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). At the layer of 5-10 cm this order was: DTPA-TEA solution (5:1) Mehlich-1 solution (5:1)  0.1 mol L-1 HCl solution (5:1)
0.1 mol L-1 HCl solution (5:1)  Mehlich-1 solution (10:1)
Mehlich-1 solution (10:1)  Mehlich-3 solution
Mehlich-3 solution  0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). At the layer of 10-20 cm this order was: Mehlich-1 solution (5:1)
0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). At the layer of 10-20 cm this order was: Mehlich-1 solution (5:1)  DTPA-TEA solution (5:1)
DTPA-TEA solution (5:1)  Mehlich-3 solution
Mehlich-3 solution  Mehlich-1 solution (10:1)
Mehlich-1 solution (10:1)  0.1 mol L-1 HCl solution (5:1)
0.1 mol L-1 HCl solution (5:1)  0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). Wang et al. (2004) also observed that Mehlich-3 solution extracted more Zn than DTPA-TEA (2:1 solution/soil ration). Abreu et al. (2002) also verified that extracting the Zn followed this order: Mehlich-1 (5:1 extractant/soil ratio) > Mehlich-3 > DTPA-TEA (2:1 extractant/soil ratio). Diluted strong acids (as with Mehlich-1 and Mehlich-3 solutions) apparently dissolved partially the Zn contained in the soil solid phase (Abreu et al., 2002). Moreover, HCl solution may preferentially remove Zn from mineral surfaces while EDTA and DTPA may remove Zn preferentially from the soil organic matter (Haynes, 1997).
0.1 mol L-1 HCl solution (10:1) > DTPA-TEA solution (2:1). Wang et al. (2004) also observed that Mehlich-3 solution extracted more Zn than DTPA-TEA (2:1 solution/soil ration). Abreu et al. (2002) also verified that extracting the Zn followed this order: Mehlich-1 (5:1 extractant/soil ratio) > Mehlich-3 > DTPA-TEA (2:1 extractant/soil ratio). Diluted strong acids (as with Mehlich-1 and Mehlich-3 solutions) apparently dissolved partially the Zn contained in the soil solid phase (Abreu et al., 2002). Moreover, HCl solution may preferentially remove Zn from mineral surfaces while EDTA and DTPA may remove Zn preferentially from the soil organic matter (Haynes, 1997).
Micronutrients in the wheat leaves
The 1993 liming and the 2000 re-liming treatments did not affect the Cu, Fe and Zn concentrations in the wheat leaves in 2003 (Figure 5). However, Mn concentration in the leaves was influenced by the interaction between lime rates (0, 2, 4, and 6 Mg ha-1) and re-liming (0 and 3 Mg ha-1). Increasing the surface liming rate decreased linearly Mn concentration in the wheat leaves both in the plots with or without re-liming. Surface re-liming caused a decrease in Mn concentration in the wheat leaves, mainly in the plots not limed in 1993. However, these observed Mn concentrations were adequate for wheat, according to Malavolta et al. (1997).
Surface liming application under NT systems has been shown to decrease the plant's Mn uptake (Caires & Da Fonseca, 2000; Godsey et al., 2007; Caires et al., 2008). This may be due to a decrease its concentration due to a decrease on the bioavailable form in the soil solution due to a soil pH increase. In all studied methods, no decreases were detected in extractable Mn at the 0-5 cm soil layer due to an increase in soil pH as a consequence of a surface liming (Figure 3). Increasing surface lime rate only decreased Mn concentration extracted by DTPA-TEA solution (5:1) at 5-10 cm layer, and by DTPA-TEA solution (2:1 and 5:1), 0.1 mol L-1 HCl solution (5:1 and 10:1) and Mehlich-3 solution at 10-20 cm layer. So, the solutions of DTPA-TEA, Mehlich-1, Mehlich-3, and HCl were ineffective to detect important decreases in soil Mn availability pursuant to the surface-applied lime.
Changes in cationic micronutrients availability at the soil most superficial layers after liming affect the mineral nutrition of plants, mainly under NT systems (Caires & Da Fonseca, 2000; Godsey et al., 2007; Caires et al., 2008).However, the cationic micronutrients concentrations extracted by the DTPA-TEA, Mehlich-1, Mehlich-3, and HCl solutions after the surface lime application under a NT system cannot represent the available amount to the crops. Our study highlight the difficulty of selecting a procedure for the extraction of these micronutrients that would be more appropriated to predict their cationic micronutrients bioavailability. This is in agreement with the results obtained in other studies in Brazilian soils (Vidal-Vázquez et al., 2005; Nascimento et al., 2006; Abreu et al., 2007).
Acknowledgements
To FAPESP, CNPq, and CAPES for providing scholarships to the authors. To Department of Soil Science from USP/ESALQ because of allowing the use of ICP-OES.
Received November 24, 2008
Accepted July 27, 2009
- Abreu, C.A.; Novais, R.F.; Raij, B. van; Ribeiro, A.C. 1994. Influência da reação do solo na extração de manganês por diferentes extratores químicos. Revista Brasileira de Ciência do Solo 18: 91-99.
- Abreu, C.A.; Raij, B. van; Abreu, M.F.; Santos, W.R.; Andrade, J.C. 1996. Efficiency of multinutrient extractants for the determination of available copper in soils. Communications in Soil Science and Plant Analysis 27: 763-771.
- Abreu, C.A.; Abreu, M.F.; Andrade, J.C.; Raij, B. van. 1998. Restrictions in the use of correlation coefficients in comparing methods for the determination of the micronutrients in soils. Communications in Soil Science and Plant Analysis 29: 1961-1972.
- Abreu, C.A.; Raij, B. van; Gabe, U.; Abreu, M.F.; Paz-González, A. 2002. Efficiency of multinutrient extractants for the determining of available zinc in soils. Communications in Soil Science and Plant Analysis 33: 3313-3324.
- Abreu, C.A.; Raij, B. van; Abreu, M. F.; Paz-González, A. 2004. Avaliação da disponibilidade de manganês e ferro em solos pelo uso do método modificado da resina de troca iônica. Revista Brasileira de Ciência do Solo 28: 579-584.
- Abreu, C.A.; Lopes, A.S.; Santos, G.C.G. 2007. Micronutrientes. p.645-736. In: Novais, R.F.; Alvarez V., V.H.; Barros, N.F.; Fontes, R.L.F.; Cantarutti, R.B.; Neves, J.C.L., eds. Fertilidade do solo. Sociedade Brasileira de Ciência do Solo, Viçosa, MG, Brazil.
- Alleoni, L.R.F.; Cambri, M.A.; Caires, E.F. 2005. Atributos químicos de um Latossolo de Cerrado sob plantio direto de acordo com doses e formas de aplicação de calcário. Revista Brasileira de Ciência do Solo 29: 923-934.
- Bataglia, O.C.; Raij, B. van. 1989. Eficiência de extratores de micronutrientes na análise de solo. Revista Brasileira de Ciência do Solo 13: 205-212.
- Bataglia, O.C.; Teixeira, J.P.F.; Furlani, P.R.; Furlani, A.N.C.; Gallo, J.R. 1978. Métodos de Análise Química de Plantas. Instituto Agronômico de Campinas [IAC], Campinas, SP, Brasil.
- Bernardi, A.C.C.; Silva, C.A.; Pérez, D.V.; Meneguelli, N.A. 2002. Analytical quality program of soil fertility laboratories that adopt Embrapa methods in Brazil. Communications in Soil Science and Plant Analysis 33: 2661-2672.
- Caires, E.F.; Da Fonseca, A.F. 2000. Absorção de nutrientes pela soja cultivada no sistema de plantio direto em função da calagem na superfície. Bragantia 59: 213-220.
- Caires, E.F.; Banzatto, D.A.; Da Fonseca, A.F. 2000. Calagem na superfície em sistema plantio direto. Revista Brasileira de Ciência do Solo 24: 161-169.
- Caires, E.F.; Alleoni, L.R.F.; Cambri, M.A.; Barth, G. 2005. Surface application of lime for crop grain production under a no-till system. Agronomy Journal 97: 791-798.
- Caires, E.F.; Barth, G.; Garbuio, F.J. 2006a. Lime application in the establishment of a no-till system for grain crop production in Southern Brazil. Soil & Tillage Research 89: 3-12.
- Caires, E.F.; Corrêa, J.C.L.; Churka, S.; Barth, G.; Garbuio, F.J. 2006b. Surface application of lime ameliorates subsoil acidity and improves root growth and yield of wheat in an acid soil under no-till system. Scientia Agricola 63: 502-509.
- Caires, E.F.; Garbuio, F.J; Barth, G. 2008. Surface application of lime and cover black oat and corn residues for no-till soybean production. Communications in Soil Science and Plant Analysis 39: 2102-2118.
- Camargo, O.A.; Valadares, J.M.A.S.; Dechen, A.R. 1982. Efeito do pH e da incubação na extração de manganês, zinco, cobre e ferro do solo. Revista Brasileira de Ciência do Solo 6: 83-88.
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. 2002. Micronutrients in crop production. Advances in Agronomy 77: 185-268.
- Filep, T.; Kincses, I.; Nagy, P.T. 2003. Dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) content of an Arenosol as affected by liming in a pot experiment. Archives of Agronomy and Soil Science 9: 111-117.
- Godsey, C.B.; Pierzynski, G.M.; Mengel, D.B.; Lamond, R.E. 2007. Management of soil acidity in no-till production systems through surface application of lime. Agronomy Journal 99: 764-772.
- Haynes, R.J. 1997. Micronutrient status of a group of soils in Canterbury, New Zealand, as measured by extraction with EDTA, DTPA and HCl, and its relationship with plant response to applied Cu and Zn. Journal of Agricultural Science 129: 325-333.
- Lindsay, W.L. 1979. Chemical Equilibria in Soils. John Wiley & Sons, New York, NY, USA.
- Lindsay, W.L.; Norvell, W.A. 1978. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal 42: 421-428.
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. 1997. Avaliação do Estado Nutricional das Plantas:Princípios e Aplicações.2ed. Potafos, Piracicaba, SP, Brazil.
- Marschner, H. 1995. Mineral Nutrition of Higher Plants. 2ed. Academic Press, San Diego, CA, USA:
- Mehlich, A. 1984. Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Communications in Soil Science and Plant Analysis 15: 1409-1416.
- Nascimento, C.W.A.; Oliveira, A.B.; Ribeiro, M.R.; Melo, E.E.C. 2006. Distribution and availability of zinc and copper in benchmark soils of Pernambuco State, Brazil. Communications in Soil Science and Plant Analysis 37: 109-125.
- Norvell, W.A. 1984. Comparison of chelating agents as extractants for metals in diverse soil materials. Soil Science Society of America Journal 48: 1285-1292.
- Osiname, O.A.; Schulte, E.E.; Corey, R.B. 1973. Soil tests for available copper and zinc in soils of Western Nigeria. Journal of the Science of Food and Agriculture 24: 1341-1349.
- Pavan, M.A.; Bloch, M.F.; Zempulski, H.C.; Miyazawa, M.; Zocoler, D.C. 1992. Manual de Análise Química do Solo e Controle de Qualidade. Instituto Agronômico do Paraná [IAPAR], Londrina, PR, Brazil.
- Rodrigues, M.R.L.; Malavolta, E.; Moreira, A. 2001. Comparação de soluções extratoras de ferro e manganês em solos da Amazônia. Pesquisa Agropecuária Brasileira 36: 143-149.
- SAS Institute. 1999. SAS System: Release 8.02. SAS Institute, Cary, NC, USA.
- Stevenson, F.J. 1986. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients. John Wiley &Sons, New York, NY, USA.
- Vidal-Vázquez, E.; Caridad-Cancela, R.; Taboada-Castro, M.M.; Paz-Donzález, A.; Abreu, C.A. 2005. Trace elements extracted by DTPA and Mehlich-3 from agricultural soils with and without compost additions. Communications in Soil Science and Plant Analysis 36: 717-727.
- Wang, J.J.; Harrell, D.L.; Henderson, R.E.; Bell, P.F. 2004. Comparison of soil-test extractants for phosphorus, potassium, calcium, magnesium, sodium, zinc, copper, manganese, and iron in Louisiana soils. Communications in Soil Science and Plant Analysis 35: 145-160.
- Zanão Júnior, L.A.; Lana, R.M.Q.; Guimarães, E.C. 2007. Variabilidade espacial do pH, teores de matéria orgânica e micronutrientes em profundidades de amostragem num Latossolo Vermelho sob semeadura direta. Ciência Rural 37: 1000-1007.
Publication Dates
-
Publication in this collection
16 Mar 2010 -
Date of issue
Feb 2010
History
-
Received
24 Nov 2008 -
Accepted
27 July 2009