Abstracts
A greenhouse study was conducted to evaluate the effects of ethylenediamine tetraacetic acid (EDTA) addition to soils on the lead (Pb) phytoextraction potential of jack beans (Canavalia ensiformis L.). In a pot experimentSoil samples (dystrophic Rhodic Hapludox) were treated with six Pb rates (0, 100, 200, 350, 1,200, and 2,400 mg kg-1 soil) applied as Pb(NO3)2 without and with EDTA application (0 and 0.5 g kg-1, respectively). Lead, Cl-, NO3-, NH4+, SO4(2-), H2PO4-, Zn2+, Cu2+, Fe3+, Al3+, Ca2+, Mg2+, K+ and DOC (dissolved organic carbon) concentrations obtained in a saturation soil extract (soil:water ratio of 1:0.3) were used for Pb speciation by means of the software Visual-Minteq 2.30. Soil Pb-availability was assessed with Diethylene triamine pentaacetic acid (DTPA) extraction. EDTA treated soils showed higher Pb (as PbEDTA2-), and Fe (as FeEDTA-) concentrations in soil solution leading to higher uptake of these elements by the jack bean. On the other hand, it decreased the concentration of stable complexes as Pb-DOC and Fe-DOC. EDTA also induced better nutrition to plants building up the concentration of non target metals (Ca, K, Mg, Zn, Cu, Fe and Mn) in shoots. Shoot dry matter yield remained constant even at the highest Pb rates after EDTA treatment. Jack bean can be considered as a potential Pb-phytoextractor. In addition, the DTPA solution was effective to assess Pb availability to the plants at all applied Pb rates.
ionic speciation; phytoextraction; soil pollution; soil solution; chelate
Este estudo teve como objetivo avaliar, em condições de casa de vegetação, os efeitos do ácido etilenodiamino tetraacético (EDTA) no potencial fitoextrator do feijão-de-porco (Canavalia ensiformis L.). Amostras de um Latossolo Vermelho distrófico foram tratadas com seis doses de Pb (0, 100, 200, 350, 1.200 e 2.400 mg kg-1 de solo) aplicadas como Pb(NO3)2 com e sem a aplicação de EDTA (0 e 0,5 g kg-1, respectivamente) e colocadas em vasos. A concentração de Pb2+, Cl-, NO3-, NH4+, SO4(2-), H2PO4-, Zn2+, Cu2+, Fe3+, Al3+, Ca2+, Mg2+, K+ e COD (carbono orgânico dissolvido) foram determinadas no extrato de saturação (razão solo:água de 1:0,3) e utilizadas para especiação iônica através do software Visual-Minteq 2.30. A disponibilidade de Pb foi avaliada com solução extratora de ácido dietilenotriamino pentacético (DTPA). O solo tratado com EDTA apresentou maior concentração de Pb (como PbEDTA2-) e Fe (como FeEDTA-) na solução do solo levando a maior absorção destes elementos pelo feijão de porco. Por outro lado reduziu a concentração das espécies Pb-COD e Fe-COD. O EDTA também contribuiu com melhor nutrição da planta devido ao aumento da concentração de outros nutrientes (Ca, K, Mg, Zn, Cu, Fe e Mn) na parte aérea. A produção de matéria seca foi constante mesmo para as doses mais altas de Pb no solo. O feijão-de-porco possui potencial fitorremediador. O extrator DTPA foi efetivo em avaliar a disponibilidade de Pb para as plantas para todas as doses de Pb aplicadas.
especiação iônica; fitoextração; poluição do solo; solução do solo; quelante
SOILS AND PLANT NUTRITION
Phytoremediation of lead by jack beans on a Rhodic Hapludox amended with EDTA
Fitorremediação de chumbo por feijão-de-porco em um Latossolo Vermelho Distrófico tratado com EDTA
Bruno Fernando Faria PereiraI,III; Cleide Aparecida de AbreuII,* * Corresponding author < cleide@iac.sp.gov.br> ; Uwe HerpinIII; Mônica Ferreira de AbreuII; Ronaldo Severiano BertonII
IUSP/ESALQ Programa de Pós Graduação em Solos e Nutrição de Plantas, C.P. 09 13418-900 Piracicaba, Sp Brasil
IIIAC, Av. Barão de Itapura, 1.481 C.P. 28 13020-902 Campinas, SP Brasil
IIIUSP/ESALQ/NUPEGEL, Alameda das Sibipirunas, 44, C.P. 09 13418-900 Piracicaba, Sp Brasil
ABSTRACT
A greenhouse study was conducted to evaluate the effects of ethylenediamine tetraacetic acid (EDTA) addition to soils on the lead (Pb) phytoextraction potential of jack beans (Canavalia ensiformis L.). In a pot experimentSoil samples (dystrophic Rhodic Hapludox) were treated with six Pb rates (0, 100, 200, 350, 1,200, and 2,400 mg kg-1 soil) applied as Pb(NO3)2 without and with EDTA application (0 and 0.5 g kg-1, respectively). Lead, Cl-, NO3-, NH4+, SO42-, H2PO4-, Zn2+, Cu2+, Fe3+, Al3+, Ca2+, Mg2+, K+ and DOC (dissolved organic carbon) concentrations obtained in a saturation soil extract (soil:water ratio of 1:0.3) were used for Pb speciation by means of the software Visual-Minteq 2.30. Soil Pb-availability was assessed with Diethylene triamine pentaacetic acid (DTPA) extraction. EDTA treated soils showed higher Pb (as PbEDTA2-), and Fe (as FeEDTA-) concentrations in soil solution leading to higher uptake of these elements by the jack bean. On the other hand, it decreased the concentration of stable complexes as Pb-DOC and Fe-DOC. EDTA also induced better nutrition to plants building up the concentration of non target metals (Ca, K, Mg, Zn, Cu, Fe and Mn) in shoots. Shoot dry matter yield remained constant even at the highest Pb rates after EDTA treatment. Jack bean can be considered as a potential Pb-phytoextractor. In addition, the DTPA solution was effective to assess Pb availability to the plants at all applied Pb rates.
Key words: ionic speciation, phytoextraction, soil pollution, soil solution, chelate
RESUMO
Este estudo teve como objetivo avaliar, em condições de casa de vegetação, os efeitos do ácido etilenodiamino tetraacético (EDTA) no potencial fitoextrator do feijão-de-porco (Canavalia ensiformis L.). Amostras de um Latossolo Vermelho distrófico foram tratadas com seis doses de Pb (0, 100, 200, 350, 1.200 e 2.400 mg kg-1 de solo) aplicadas como Pb(NO3)2 com e sem a aplicação de EDTA (0 e 0,5 g kg-1, respectivamente) e colocadas em vasos. A concentração de Pb2+, Cl-, NO3-, NH4+, SO42-, H2PO4-, Zn2+, Cu2+, Fe3+, Al3+, Ca2+, Mg2+, K+ e COD (carbono orgânico dissolvido) foram determinadas no extrato de saturação (razão solo:água de 1:0,3) e utilizadas para especiação iônica através do software Visual-Minteq 2.30. A disponibilidade de Pb foi avaliada com solução extratora de ácido dietilenotriamino pentacético (DTPA). O solo tratado com EDTA apresentou maior concentração de Pb (como PbEDTA2-) e Fe (como FeEDTA-) na solução do solo levando a maior absorção destes elementos pelo feijão de porco. Por outro lado reduziu a concentração das espécies Pb-COD e Fe-COD. O EDTA também contribuiu com melhor nutrição da planta devido ao aumento da concentração de outros nutrientes (Ca, K, Mg, Zn, Cu, Fe e Mn) na parte aérea. A produção de matéria seca foi constante mesmo para as doses mais altas de Pb no solo. O feijão-de-porco possui potencial fitorremediador. O extrator DTPA foi efetivo em avaliar a disponibilidade de Pb para as plantas para todas as doses de Pb aplicadas.
Palavras-chave: especiação iônica, fitoextração, poluição do solo, solução do solo, quelante
Introduction
In the São Paulo State, Brazil, guiding values for soil Pb contamination were proposed by the Environmental and Sanitation Technology Company (CETESB, 2001) which define: i) guiding value for enhanced monitoring (100 mg kg-1 soil); ii) intervention values (defined as concentration limits causing potential risks for human health) for agricultural areas (200 mg kg-1 soil ), for residential areas (350 mg kg-1 soil); and iii) for industrial areas (1,200 mg kg-1 soil). In this State, 197 heavy metal contaminated locations have been located including Pb contaminated sites according to CETESB (CETESB, 2006). Different strategies have been developed to remediate heavy metal contaminated sites. One of such strategies is phytoextraction, or the use of specialized plants called phytoextractors. In particular Pb-phytoextractors are plants able to accumulate more than 1,000 mg of Pb per kg of plant dry matter (Raskin et al., 1994). The phytoextraction, a type of phytoremediation, has the advantage of not being a destructive technique and it is a well accepted practice within the scope of 'green technologies' (Robinson et al., 2003). A successful phytoextraction system depends on several factors such as concentration of metal in soil solution and the ability of plants to accumulate metals in top parts (Ernst, 1996; Schmidt, 2003).
Phytoextractor species are desired to have several characteristics such as high ability of metal absorption and translocation to upper plant parts and high biomass production (Blaylock et al., 1997; Huang et al., 1997). However, although the biomass production of tropical species is generally high, only few studies have been carried out with the objective to identify tropical species with good phytoextraction potential. In addition few studies have been carried out with tropical soils and phytoextraction (Gabos et al., 2009).
Lead availability to plants is very limited with less than 0.1% of the total soil Pb concentration in soil solution (Huang et al., 1997). Enhancing Pb soil solution concentration is crucial for successful Pb phytextraction (Blaylock et al., 1997; Huang et al., 1997; Epstein et al., 1999; Jarvis and Leung, 2001; Shen et al., 2002).
The use of chelating agents constitutes a promising tool for Pb desorption from the soil matrix by forming soluble complexes leading to increasing plant absorption (Shen et al., 2002). There are several chelating agents such as ethylenediamine tetraacetic acid (EDTA), hydroxyethylethylenediaminetriacetic acid (HEDTA), ethylene glycol tetraacetic acid (EGTA), diethylene triamine pentaacetic acid (DTPA), ethylenediamine-n,n'-bis (2-hydroxyphenylacetic acid) (EDDHA), ethylenediamine-N,N'-disuccinic acid (EDDS) (Huang et al., 1997; Grcman et al., 2003; Meers et al., 2005), which are usually applied to soils for remediation purposes. In this context EDTA is considered the most effective one (Huang et al., 1997; Shen et al., 2002; Saifullah et al., 2009), although a main problem of its use is the leaching of Pb-EDTA with subsequent risk of groundwater contamination. On the other hand, because the major part of Pb-EDTA is taken up in short time (6 h) (Shen et al., 2002), various studies have reported significant leaching reduction by splitting the EDTA application (Grcman et al., 2001; Wenzel et al., 2003; Gabos et al., 2009). Brazilian soils are highly weathered with deep profiles (> 20 m) dominated by hydrous oxides of Fe and Al (Brady and Weil, 1999) that form inner-sphere complex with Pb2+ (Sparks, 1995).
Studies on Pb-phytoremediation on highly weathered soils rich in iron oxides are still recent in Brazil. The objectives of this study were to evaluate: (i) the potential of jack bean as Pb-phytoextractor; (ii) the effects of EDTA on plant Pb accumulation and ionic speciation of soil solution; and, (iii) the potential of DTPA solution to predict Pb availability to plants in soils with high Pb levels.
Material and Methods
Soil sampling and preparation
A greenhouse experiment was carried out using jack bean (Canavalia ensiformis L.) as test plant. Plastic pots (3 dm3) were filled with uncontaminated agricultural soil samples of a dystrophic Rhodic Hapludox, collected in the São Paulo State (22º53'30.40" S; 47º03'53.35"W; 671 m asl), Brazil. The uncontaminated soil samples were taken from the 0-20 cm soil layer and analyzed for physical and chemical parameters according to van Raij and Quaggio (1983) and Camargo et al. (1986) (Table 1). Soil samples were oven dried at 60ºC, sieved through a 2 mm-mesh screen, and then limed with Ca(OH)2 and MgCO3·Mg(OH)2·1H 2O (p.a.) at a ratio of 4:1 (Ca:Mg) to raise pH to 5.5. After liming, the samples were incubated for 30 days. Soil moisture was maintained using distilled water whenever necessary to keep 70% of the total soil pores filled with water in order to ensure complete lime reaction with the soil.
Experimental design, soil and plant preparation
The experiment was completely randomized in a (6 × 2) factorial design (six Pb rates, with and without EDTA application) with three replications. The Pb rates were applied as Pb(NO3)2 at rates of: 0; 100; 200; 350; 1,200 and 2,400 mg kg-1 soil. The rates were chosen following guiding values for soil contamination proposed by CETESB (2001) which corresponded to the guiding value for enhanced monitoring (100 mg kg-1 soil), intervention values (defined as concentration limits causing potential risks for human health) for agricultural areas (200 mg kg-1 soil), for residential areas (350 mg kg-1 soil) and for industrial areas (1,200 mg kg-1 soil). Twice as much as the intervention value for industrial areas (2,400 mg kg-1 soil) was additionally applied to simulate extreme soil Pb contamination. At the same time different rates of NH4NO3 were added to compensate the different N-contents of the Pb(NO3)2 rates applied by considering the highest Pb rate as reference. The NH4NO3 rates added were 324, 310, 297, 276, 162 and 0 mg kg-1 for the Pb rates of 0, 100, 200, 350, 1,200 and 2,400 mg kg-1, respectively. The treated soil samples remained incubated for 5 months.
Soil samples were fertilized with P, K, S, B, Cu, Mn and Zn as follows (in mg kg-1): 360 (P-P2O5); 100 (K-KCl); 30 (S); 0.5 (B-Na2B4O7); 1.0 (Cu-CuSO4); 8.0 (Mn-MnCl2) and 2.0 (Zn-ZnSO4). Phosphate (supertriple - granules) and sulfur (elemental - powder) were applied in solid form and the other elements in liquid form. Subsequently, the soil was then homogenized and splint in two equal portions. One half of the soil samples were treated with 0.5 g of (C10H14N2Na2 O82H2O) disodium-EDTA kg-1 soil for each Pb rate, added as salt and homogenized in soil. The other half remained untreated (control).
Jack beans were seeded in 3 dm3 plastic pots filled with the treated soil. Each pot received 24 seeds and after emergence six seedlings were left per pot. The soil moisture was monitored by weighing pots daily. Distilled water was used to keep 70% of total pores filled with water. During the experiment the natural insecticide 'Neem' (Azadirachta indica) was utilized to control caterpillars. Shoots were harvested 45 days after sowing.
Analytical procedures
After harvest, the jack bean shoots were washed in tap water, 1% HCl solution, and then rinsed in distilled water. Dry matter yield was determined after shoots were dried in a forced-air oven at 70ºC until constant weight was reached.
For P, Ca, Mg, Cu, Fe, Mn, Zn, Al, Cd, Cr, Ni and Pb determinations, the plant samples were ground in a Wiley type mill and digested following the procedure: 0.5 g of plant samples were soaked overnight with 5.0 mL of HNO3 (65%). Samples were then digested in a block digester for four more hours at 180ºC. After the samples were cooled to room T (25ºC), 1.3 mL of perchloric acid (70%) was added and the samples were heated again (210ºC) until the solution was colorless. After cooling (25ºC), the solution was brought to volume (25 mL) with distilled water (Bataglia et al., 1983). Determinations were carried using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) (Jobin Yvon 40P, Longjumeau, France). The concentration of elements in plant shoots were calculated based on the 0.5 g of digested tissue. Lead accumulation per pot was calculated multiplying the Pb shoot concentration (mg kg-1) by the total dry matter yield per pot.
After soil incubation and before jack bean sowing, soil samples were collected from every pot and analyzed for available Pb and other ionic species using the following procedure:
DTPA Extraction: 0.005 mol L-1 diethylene triamine pentaacetate (DTPA) + 0.1 mol L-1 triethanolamine (TEA) + 0.01 mol L-1 calcium chloride (CaCl2) at pH 7.3. Procedure according to Lindsay and Norvel (1978) 10 cm3 of soil + 20 mL of extracting solution and 2 hour-shaking. The extracting solution was analyzed for DTPA-extractable Pb by ICP-OES.
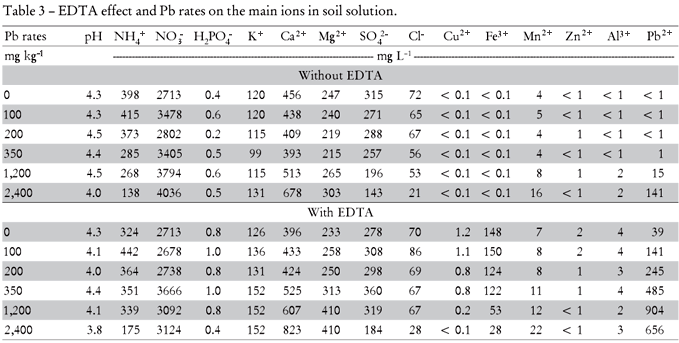
Saturation Extract: for ionic speciation calculation was obtained according to the procedure of Wolt (1994). De-ionized water was added to soil samples to obtain a soil:water ratio of 1:0.3. After that, the soil paste was filtered (Whatman # 42) through a Buchner funnel with slow-filtering paper by using a vacuum pump. The saturation extracts were analyzed for Pb, Cl-, NO3-, NH4+, S, P, Zn, Cu, Fe, Al, Ca, Mg, K, DOC (dissolved organic carbon), electrical conductivity (EC) and pH, as follows: Cl- was determined by using a selective electrode (3 mL soil solution + 9 mL buffer at pH 4.7) (Orion 710-A, Environmental Instruments Water - Thermo Electron Corporations, Beverly, USA). NO3- and NH4+, were measured by the Kjeldahl method as described by Cantarella and Trivelin (2001); S, P, Zn, Cu, Fe, Pb and Al by ICP-OES. As the most common anionic forms of P and S in soil solution are H2PO4- and SO42- (Brady and Weil, 2008) equivalent calculations of S as SO42- and P as H2PO4-were carried out for the ionic speciation input of data. Calcium and Mg concentrations were obtained by atomic absorption spectrometry; K by flame photometry; DOC by catalytic combustion oxidation; EC by using an electrical conductivimeter, and pH by using a pH-meter.
For ionic speciation calculation of soil solution, the EDTA concentration was estimated considering a soil:water ratio of 1:0.3, the same ratio used for saturation extract, and 0.5 g kg-1 EDTA completely dissolved, hence our estimate of 1,666 mg L1 of EDTA in soil solution. The concentration of EDTA and of all other ions in the saturation extracts were transferred to a geochemical model using the software visual-MINTEQ 2.30 (Gustafsson, 2005) in order to obtain the percentage distribution of ionic species in the soil solution.
Statistical analysis
Analysis of variance according to the proposed model was analyzed in order to test the main effects (Pb and EDTA) and the interaction between these independent variables. Linear and quadratic regressions were also carried out between Pb rates applied (as independent variable) and: shoot dry matter yield, shoot Pb concentrations, shoot Pb accumulation, shoot non target metal (Ca, K, Mg, Zn, Cu, Fe and Mn) concentration, as well as between Pb-DTPA extractable shoot Pb concentrations (as dependent variable). The models were chosen according to a significant level p < 0.05 by using analysis of variance (ANOVA). All statistical analyses were made using the SAS Proc GLM, Version 9.12 (SAS Institute, 2004).
Results and Discussion
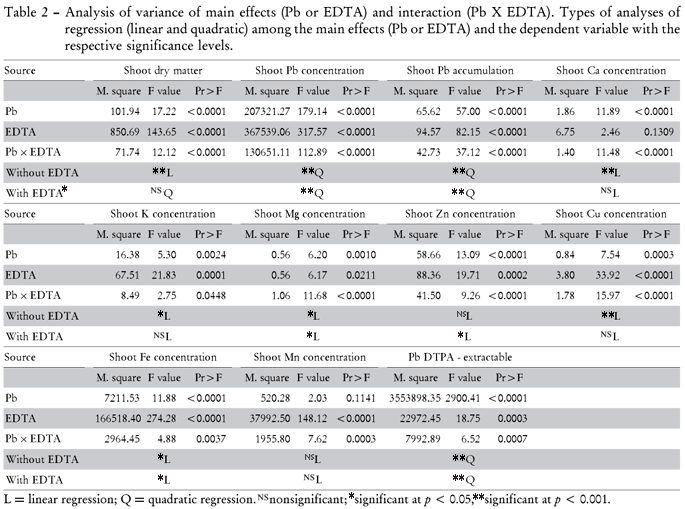
Regarding the statistical analysis, the independent variables (Pb and EDTA) have influenced all studied dependent variables as well as presenting interaction effects. The proposed statistical analysis also shows significant difference for the major part of the studied models of interactions among the dependent variable (Pb rates added to the soil) and the independent variables of the soil, soil solution and plant nutrients, and toxic elements (Table 2). These interactions will be discussed in some detail below:
Jack Bean Phytoremediation Potential
The dry matter yield of jack bean shoots was lower under the EDTA treatment as compared to the control (Figure 1). The yield reduction could be due to a chelate-induced heavy metal availability or to toxic effects of the chelant itself, although there were no obvious symptoms of chelate toxicity. Previous studies (Geebelen et al., 2002) reported clear physiological effects of increasing Pb-EDTA concentration in plants such as: i) induction of peroxidases, enzymes of the ascorbate-glutathione cycle and ii) Nicotinamide adenine dinucleotide phosphate (NADP+) reduction enzyme in roots and primary leaves indicating oxidative stress, iii) reduction of chlorophyll a (-26%) and b (-33%) content in leaves as well as iv) visible effects on root morphology and shoot length. Negative symptoms caused by EDTA were reported to lower photosynthetic rate, transpiration and stomata conductance in artichoke (Cynara cardunculus L.) (Hernandez-Allica et al., 2003). Vassil et al. (1998) observed lower leaf water content in mustard plants grown in hydroponics and treated with 0.5 mmol L-1 Pb(NO3)2 and 2.5 mmol L-1 EDTA. Furthermore, Grcman et al. (2001) showed that EDTA application decreased the soil arbuscular mycorrhiza fungi population. These findings may also have contributed to the lower jack bean growth observed in the present study.
No effects of the different soil Pb rates on shoot dry matter yields were found after EDTA addition (Figure 1). This positive findings disagree with Geebelen et al. (2002) and Wang et al. (2007) who found reduction in shoot length and weight of bean (Phaseolus vulgaris L.) and Bibens maximowicziana L. after increasing Pb rates and EDTA addition. Results of this study indicate a good potential of the jack bean as a Pb phytoextractor plant due to its high dry matter yield on tropical soils, which was confirmed by Gabos et al. (2009). On the other hand, for treatments without EDTA, shoot dry matter yields decreased with increasing Pb rates and presented the lowest yield at the highest Pb rate (2,400 mg kg-1).
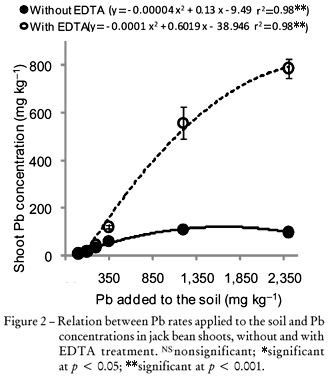
Without EDTA application the shoot Pb concentration was about 10 mg kg-1 for the lowest Pb rate and reached around 105 mg kg-1 for the 1,673 mg kg-1 soil Pb-rate (Figure 2). Toxic Pb concentrations for many plants range between 30 and 300 mg kg-1 (Kabata-Pendias and Mukherjee, 2007). On the other hand, EDTA treatment induced higher Pb concentrations in jack bean shoots with increasing Pb rates (Figure 2). The Pb concentrations in plant shoots without EDTA showed a maximum concentration of around 100 mg kg-1 whereas after EDTA application the Pb concentrations increased up to seven fold (866 mg kg-1) in pots with highest Pb additions (Figure 2). However, no differences in shoot dry matter yield at the highest Pb rate were found showing almost the same values with and without EDTA (Figure 1). This result may be attributed to specific physiological mechanisms of plants with high potential of phytoremediation such as detoxification of metals by distributing to the apoplast by binding to cell walls and chelation of metals in the cytoplasm, as well as sequestration of metals in the vacuole by tonoplast-located transporters (Yang et al., 2005).
The relative increases in the extraction efficiencies after EDTA treatment, as compared to the control (without EDTA) were 27, 29, 100, 415 and 692% higher Pb accumulation rates for the applied Pb doses of 100, 200, 350, 1,200 and 2,400 mg kg-1, respectively (Figure 3). Shen et al. (2002) reported an increase in Pb concentration from 127 (control) to 1,170 mg kg-1 in bean shoots grown on a soil containing 10,600 mg kg-1 of Pb and treated with 3 mmol kg-1 EDTA. This was attributed to the chelate effectiveness in enhancing Pb availability to plants. EDTA reacts generally with metals of the soil matrix by forming soluble complexes in the soil solution (Vassil et al., 1998) which may have also caused the increasing Pb availability and the higher plant uptake in the present study.
The amount of Pb extracted was calculated and the number of harvests necessary to extract all applied Pb from the soil (Figure 3). Generally, the EDTA greatly reduced the number of harvests to remove the total amount of Pb applied Except for Pb rates of 100 and 200 mg kg-1, what may be related to the lower shoot dry matter. Under EDTA application, the number of harvests necessary to completely remove the Pb from soil ranges from 300 to 1,000 harvests. On the other hand, it ranges between 500 and 4,000 harvests without EDTA. Even with EDTA application it could take a long time to remediate the area. However, the plants were cultivated under greenhouse conditions during 45 days at the beginning of flowering what may lead to a lower shoot dry mass production. Under field conditions the flowering starts around 75 days (Favero et al., 2001).
The potential of plant species to remediate heavy metal contaminated soils can be evaluated by its hyperaccumulation potential and by calculating transfer factors (t) between plant and soil. The transfer factor (t) is defined as the ratio between the total element concentration in the plant and in the soil (Kumar et al., 1995; Henry, 2000). The higher the t value the more effective is the phytoextractor potential. However, based on these criteria, jack beans could not be considered as a high potential phytoextractor, since the t values obtained were lower than 1.7 (Raskin et al., 1994). Without EDTA the t values were 0.16, 0.17, 0.17, 0.09, 0.04 for the rates of Pb 100, 200, 350, 1,200 and 2,400 mg kg-1, respectively. On the other hand for the soil EDTA treated the t values were 0.21, 0.22, 0.35, 0.47, 0.33 for the rates of Pb 100, 200, 350, 1,200 and 2,400 mg kg-1, respectively. The reference t value (1.7) was proposed for mustard plants (Brassica sp.) considered as high potential Pb phytoextractors (Henry, 2000). Furthermore, jack bean shoot Pb concentrations were lower than the reference concentration (>1,000 mg Pb kg-1) for Pb-hyperaccumulators (Raskin et al., 1994). Nevertheless, although jack beans are not fulfilling these criteria, they had comparatively high dry matter yields under tropical environmental conditions that could compensate the general drawback of 'real' metal hyper-accumulators with generally low biomass production (Meers et al., 2005). This fact should also be taken into account for the evaluation of the phytoextraction potential of plants. For instance, jack beans showed about three-fold higher shoot dry matter yields with mean values of 28.1 and 18.4 g per pot compared to 10.0 and 2.3 g per pot for maize under same growth conditions without and with EDTA, respectively (Pereira et al., 2007).
EDTA effects on non target metal concentration in jack bean shoots
Concentration of non target metals (Ca, Mg, K, Mg, Cu, Fe and Mn) in shoots of jack bean increased or did not change in response to the EDTA treatment (Figure 4). From zero until the rate of 1,200 mg kg-1 of Pb, Ca shoot concentration was higher in the soil without EDTA. After this rate, Ca concentration was higher in EDTA treated plants. Without EDTA, shoot Ca concentrations kept decreasing with increasing Pb rates (Figure 4) showing the lowest concentration for the treatment with 2,400 mg kg-1 Pb. Similar results were observed by Huang and Cunningham (1996), who related decreasing Ca uptake following increasing soil Pb concentrations to both blocking of the Ca channels in the plasma membrane or competitive transport of Pb and Ca within the Ca channels. Also Kabata Pendias and Mukherjee (2007) reported the antagonistic interaction between Ca and Pb. With EDTA treatments the shoot Ca concentrations remained constant with increasing Pb rates applied (Figure 4). It appears that the EDTA contributes to the plant nutrition keeping adequate Ca concentrations in shoots during the phytoextraction process even for higher Pb rates.
EDTA addition to soil increased shoot K concentration for all Pb rates applied to the soil, except the highest rate (Figure 4). With EDTA the plants had an increasing tendency for Mg and Zn shoot concentrations with increasing soil Pb rates. For Cu and Mn higher concentration in plant shoots were also found after EDTA treatment. On the other hand, there was no tendency of increasing Cu and Mn concentrations with EDTA (Figure 4). However, the EDTA ability to increase metal concentration in plant shoots was reported in various other studies (Evangelou et al., 2007; Saifullah et al., 2009).
The effect of added Pb was negative on the Fe shoot concentration with or without EDTA. However, Fe concentrations in plant shoots growing with EDTA were more than twice as higher for all Pb rates when compared with no EDTA treated plants. This may be associated with the predominant ionic specie as FeEDTA2- available in soil solution after EDTA application. In contaminated sites, increasing Fe concentration in shoots are desirable as a nutrient amendment for phytoextraction approaches for maintaining plant health by increasing the amounts of chlorophyll (Keller and Hammer, 2004). On the other hand there was a decreasing tendency of Fe in shoots with increasing Pb rates probably due to Fe and Pb competition to the biding sites in the root cell wall (January, 2008). In such case, EDTA can amend Pb-induced Fe deficiency in the plants by increasing Fe in shoot during the Pb phytoextraction process.
Soil Available Pb
The present study revealed good correlations between the applied (x) Pb rates and the Pb concentrations in the DTPA extracted solution (y) with highly significant coefficients without EDTA (r2 = 0.99**, y = - 0.00007 x2 + 0.96 x 20.10) and after EDTA treatment (r2 = 0.99**, y = - 0.00006 x2 + 1.02 x 0.002). This suggests that the DTPA extraction is an efficient method to predict Pb concentrations in the soil, also at high Pb rates. Moreover, the relation between Pb extracted with DTPA and Pb concentrations in plants had a significant correlation with or without EDTA treatment (Figure 5). This result confirms the DTPA efficiency to predict Pb availability to the plants even for high soil Pb levels (1,200 and 2,400 mg kg-1).
The relation between the available Pb fraction in solution after DTPA extraction and Pb in the plant shoots presented different results at the EDTA treated and untreated soils (Figure 5). Without EDTA shoot Pb concentrations varied slightly with increasing Pb concentrations in the DTPA extracts. On the other hand, at the EDTA treated soils the Pb concentrations in plant shoots increased with increasing Pb concentrations in the DTPA extracts, reaching approximately 800 mg kg-1 dry matter.
Ionic Speciation in Soil Solution
Lead and iron were the most influenced ions in the soil solution (saturation extract) after EDTA treatment (Table 3), but they had different concentration patterns. Lead increased in the soil solution with increasing Pb rates until 1,200 mg kg-1 and decreased subsequently, whereas inverse tendencies were found for Fe (Figure 6). The increase in Pb in soil solution resulted in higher plant Pb accumulation, and showed similar results to the DTPA extraction method (Figure 4)
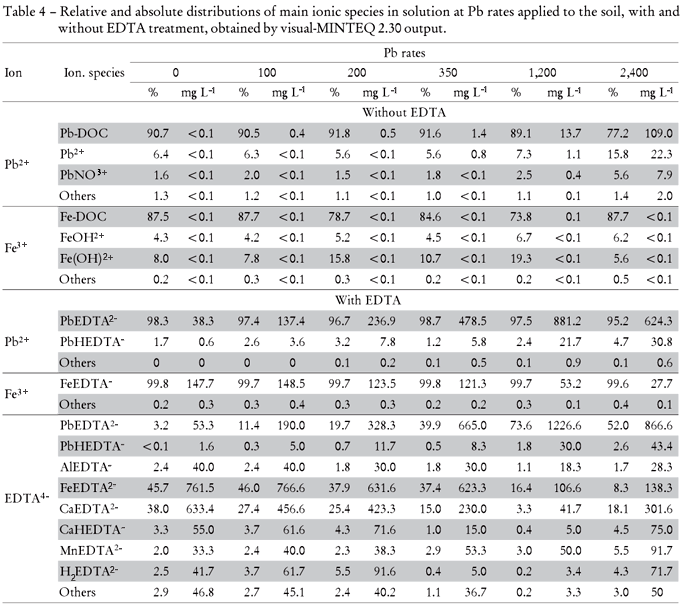
The differences between Pb and Fe concentrations in the soil solution after EDTA treatment are suggested to be a result of both competition to the same binding sites at the chelating agent and different soil contents. Due to the high complex stability of Fe with EDTA, Fe likely competes at high concentrations with Pb (target metal) when Pb concentrations in the soil are lower (Thayalakumaran et al., 2003). Using a model, Nowack et al. (2006) estimated that Fe (III) would complex almost 100% of EDTA up to pH 7. Therefore, the EDTA efficiency to form Pb-EDTA chelates may be reduced in this pH range as well as in the presence of other metals with high affinity to EDTA such as Fe2+, Al3+, Cd2+, Zn2+ and Co2+ (Geebelen et al., 2002) and Ca (Thayalakumaran et al., 2003). Macro and micronutrients as well as toxic elements in soil may reduce EDTA efficiency. These effects agree with Neugschwandtner et al. (2009) who found competition of other elements as Ca, K, Mg, P, Mn and especially Fe, with the target cations heavy metals, as Pb2+, during the phytoextraction process. As mentioned above, the low Pb soil concentrations in the present study are suggested to favor the formation of the FeEDTA- complex and to diminish Pb-EDTA2- complexation. Under high Pb soil concentrations, higher portions of Pb-EDTA2- were found. These interrelations are relevant for many tropical soils, rich in iron oxides and Fe3+ contents as in the used Rhodic Hapludox.
Considering the different Pb and Fe ionic species in soil solution (Table 4) without EDTA application the predominant Pb and Fe forms were on average 88% of Pb-DOC and 83.3% of Fe-DOC. On the other hand, in the presence of EDTA mainly PbEDTA2- (97%) was formed, whereas free Pb+2 was not detectable. In the same way, 99.7% of total Fe was bound to EDTA (FeEDTA-). With regard to all complex forms of EDTA4-, PbEDTA2- increased from 3.2 to 52% in the soil solution from the lowest to the highest (0 to 2,400 mg kg-1) Pb rates added, respectively. The opposite trend was observed for the AlEDTA-, FeEDTA- and CaEDTA2- complex species, whose soil solution contents decreased with increasing Pb rates.
Conclusions
Jack beans can be considered as a potential plant for remediation of Pb contaminated soils by its high dry matter yield even when cultivated in soils with high Pb concentrations (1,200 and 2,400 mg kg-1). The addition of EDTA greatly enhances the process as it led to a 7-fold increase in shoot Pb concentration.
The EDTA use builds up the concentration of non target metals (Ca, K, Mg, Zn, Cu, Fe and Mn) in jack bean shoots. This interrelation among Fe, Pb and EDTA is strongly relevant for tropical soils usually rich in iron oxides and Fe3+ contents as in the studied Rhodic Hapludox. Iron competition with Pb on EDTA binding sites (FeEDTA-) reduces the formation of EDTA- complexes with the target metal (Pb-EDTA) and consequently its uptake.
The DTPA solution was effective to assess Pb availability to the jack bean plant. Soil Pb extracted by DTPA displayed high correlation with the Pb concentration in leaf tissue for all tested Pb rates, regardless of the addition of EDTA.
EDTA was efficient to increase Jack bean Pb phytoextraction. However, before adopting this method it is necessary to evaluate the leaching of metal-EDTA complexes in tropical soils.
Acknowledgement
To FAPESP for Grant number 03/03311-5.
Received December 04, 2008
Accepted February 02, 2010
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. 1983. Methods of Plant Analysis. Instituto Agronômico, Campinas, SP, Brazil. (Technical Bulletin, 78). (in Portuguese).
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakharova, O.; Gussman, C.; Kapulnik, Y.; Ensley, B.D.; Raskin, I. 1997. Enhanced accumulation of Pb in Indian Mustard by soil-applied chelating agents. Environmental Science and Technology 31: 860-865.
- Brady, N.C.; Weil, R.R. 1999. The Nature and Properties of Soils. Prentice Hall, Englewood Cliffs, NJ, USA.
- Brady, N.C.; Weil, R.R. 2008. The nature and properties of soils. New Jersey, Prentice Hall, NJ, USA.
- Camargo, O.A.; Moniz, A.C.; Jorge, J.A.; Valadares, J.M.A.S. 1986. Physical and Chemical Methods in Soil Analysis of Instituto Agronômico. Instituto Agronômico, Campinas, SP, Brazil (in Portuguese).
- Cantarella, H.; Trivelin, P.C.O. 2001. Inorganic nitrogen analysis by distilation method, In: IAC. Chemical analysis for evaluation of tropical soils fertility. Instituto Agronômico, Campinas, SP, Brazil (in Portuguese).
- Companhia de Tecnologia de Saneamento Ambiental [CETESB]. 2006. Contaminated Sites Inventory in São Paulo State. CETESB, São Paulo, SP. Brazil. (in Portuguese).
- Companhia de Tecnologia de Saneamento Ambiental [CETESB]. 2001. Benchmarks for soil and groundwater in São Paulo State. Available at: http://www.cetesb.sp.gov.br/Solo/relatorios/aguas_final.zip [Accessed Sep. 04, 2001] (in Portuguese).
- Epstein, A.L.; Gussman, C.D.; Blaylock, M.J.; Yermiyahu, U.; Huang, J.W.; Kapulnik, Y.; Oser, C.S. 1999. EDTA and Pb-EDTA accumulation in Brassica juncea grown in Pb-amended soil. Plant and Soil 208: 87-94.
- Ernst, W.H.O. 1996. Bioavailability of heavy metals and descontamination of soils by plants. Applied Geochemistry 11: 163-167.
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. 2007. Chelate assisted phytoextraction of heavy metals from soil effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68: 989-1003.
- Favero, C.; Jucksch, I.; Alvarenga, R.C.; Costa, L.M. 2001. Modification in the population of spontaneous plants in the presence of Green manure. Pesquisa Agropecuária Brasileira 36: 1355-1362. (in Portuguese, with abstract in English).
- Gabos, M.B.; Abreu, C.A.; Coscione, A.R. 2009. EDTA assisted phytoremediation of a Pb contamined soil: metal leaching and uptake by jack beans. Scientia Agricola 66: 506-514.
- Geebelen, W.; Vangronsveld, J.; Adriano, D.C.; Poucke, L.C.V.; Clijsters, H. 2002. Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris Physiologia Plantarum 115: 377-384.
- Grcman, H.; Velikonja-Bolta, S.; Vodnik, D.; Kos, B.; Lestan, D. 2001. EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant and Soil 235: 105-114.
- Grcman, H.; Vodnik, D.; Velikonja-Bolta, S.; Lestan, D. 2003. Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. Journal of Environmental Quality 32: 500-506.
- Gustafsson, J.P. 2005. Visual MINTEQ version 2.30. Department of Land and Water Resources Engineering, Stockholm, Sweden. Available at: www.lwr.kth.se/English/OurSoftware/vminteq/ [Accessed Sep. 27, 2005]
- Henry, J.R. An overview of the phytoremediation of lead and mercury. 2000. National Network of Environmental Management Studies (NNEMS). Available at: www.clu-in.org/download/remed/henry.pdf U.S. Environmental Protection Agency, Washington, DC, USA. [Accessed Sep. 04, 2008]
- Hernandez-Allica, J.; Barrutia, O.; Becerril, J.M.; Garbisu, C. 2003. EDTA reduces the physiological damage of lead on cardoon plants grown hydroponically. Journal de Physique IV 107: 613-616.
- Huang, J.W.; Chen, J.; Berti, W.R.; Cunningham, S.D. 1997. Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environmental Science and Technology 31: 800-805.
- Huang, J.W.; Cunningham, S.D. 1996. Lead phytoextraction: species variation in lead uptake and translocation. New Phytologist 134: 75-84.
- January, M.C.; Cutright, T.J.; van Keulen, H.; Wei, R. 2008. Hydroponic phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus hyperaccumulate multiple heavy metals? Chemosphere 70: 531-537.
- Jarvis, M.D.; Leung, D.W.M. 2001. Chelated lead transport in Chamaecytisus proliferus (L.f) link ssp. proliferus var. palmensis (H. Christ): an ultrastructural study. Plant Science 161: 433-441.
- Kabata-Pendias, A.; Mukherjee, A.B. 2007. Trace elements from soil to human. Springer New York, NY, USA.
- Keller, C.; Hammer, D. 2004. Efficiency of repeated phytoextraction with Thlaspi caerulescens in Cd and Zn contaminated soils on soil toxicity and metal availability. Environmental Pollution 131: 243-254.
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. 1995. Phytoextraction: the use of plants to remove heavy metals from soils. Environmental Science and Technology 29: 1232-1238.
- Lindsay, W.L.; Norvell, W.A. 1978. Development of DTPA soil test for zinc, iron, manganese and copper. Soil Science Society of American Journal 42: 421-428.
- Meers, E.; Ruttens, A.; Hopgood, M.J.; Samson, D.; Tack, F.M. 2005. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58: 1011-1022.
- Neugschwandtner, R.W.; Tlusto, P.; Komárek, M.; Száková, J. 2009. Nutrient mobilization and nutrient contents of Zea mays in response to EDTA additions to heavy-metal-contaminated agricultural soil. Journal of Plant Nutrition and Soil Science 172: 520-527.
- Nowack, B.; Schulin, R.; Robinson, B.H. 2006. A critical assessment of chelant-enhanced metal phytoextraction. Environmental Science and Technology 40: 5225-5232.
- Pereira, B.F.F.; Abreu, C.A.; Romeiro, S.; Lagôa, A.M.M.A.; Paz-González, A. 2007. Pb-Phytoextraction by maize in a Pb-EDTA treated oxisol. Scientia Agricola 64: 52-60.
- Raij, B. van; Quaggio, J.A. 1983. Soil Analysis Methods for Soil Fertility. Instituto Agronômico, Campinas, SP, Brazil (in Portuguese).
- Raskin, I.; Kumar, P.N.; Dushenkov, S.; Salt, D.E. 1994. Bioconcentration of heavy metals by plants. Current Opinion in Biotechnology 5: 285-290.
- Robinson, B.; Fernandez, J.E.; Madejón, P.; Marañhón, T.; Murillo, J.M.; Green, S.; Brent, C. 2003. Phytoextraction: an assessment of biochemical and economic viability. Plant and Soil 249: 117-125.
- Saifullah, M.E.; Qadir, M.; de Caritat, P.; Tack, F.M.G.; du Laing, G.; Zia, M.H. 2009. EDTA-assisted Pb phytoextraction. Chemosphere 74: 279-1291.
- SAS Institute. 2004. SAS System: Version 9.1.2. SAS Institure, Cary, NC, USA.
- Schmidt, U. 2003. Enhancing phytoextraction: the effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. Journal of Environmental Quality 32: 1939-1954.
- Shen, Z.G.; Li, X.D.; Wang, C.C.; Chen, H.M.; Chua, H. 2005. Lead phytoextraction from contaminated soil with high-biomass plant species. Journal of Environmental Quality 31: 1893-1900.
- Sparks, D.L. 1995. Environmental Soil Chemistry. Academic Press, San Diego, CA, USA.
- Thayalakumaran, T.; Vogeler, I.; Scotter, D.R.; Percival, H.J.; Robinson, B.H.; Clothier, B.E. 2003. Leaching of copper from contaminated soil following the application of EDTA. I. Repacked soil and a model. Australian Journal of Soil Research 41: 323-333.
- Vassil, A.D.; Kapulnik, Y.; Raskin, I.; Salt, D.E. 1998. The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiology 117: 447-453.
- Wang, H.; Lu, S.; Li, H.; Yao, Z. 2007. EDTA-enhanced phytoremediation of lead contaminated soil by Bidens maximowicziana, Journal of Environmental Sciences 17: 1496-1499.
- Wenzel, W.W.; Unterbrunner, R.; Sommer, P.; Sacco, P. 2003. Chelate-assisted phytoextraction using canola (Brassica napus L.) in outdoors pot and lysimeter experiments. Plant Soil 249: 83-96.
- Wolt, J.D. 1994. Soil Solution Chemistry: Applications in Environmental Science and Agriculture. John Wiley, New York, NY, USA.
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. 2005. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. Journal of Trace Elements in Medicine and Biology 18: 339-353.
Publication Dates
-
Publication in this collection
14 June 2010 -
Date of issue
2010
History
-
Received
04 Dec 2008 -
Accepted
02 Feb 2010