Abstracts
The growing demand for high quality soybean [Glycine max (L.) Merrill] seeds requires a precise seed quality control system from the seed industry. One way to accomplish this is by improving vigor testing. Cold test has been traditionally employed for corn seeds. However, it has also been used for other seed crops such as cotton (Gossypium spp.), soybean (Glycine Max), dry bean (Phaseolus vulgaris) and pea (Pisum sativum). This study was carried out with the objective of adjusting an alternative procedure for the cold test to determine soybean seed vigor. Six commercial soybean seed lots of the cultivar BRS 133 were used. The physiological potential of the seed lots was evaluated by germination on paper towel and sand box, seedling field emergence, tetrazolium, accelerated aging and electrical conductivity tests. Seed moisture content was also determined. The temperature used for the cold test procedures was 10ºC during five days. Four cold test procedures were evaluated: i) plastic boxes with soil; ii) rolled paper towel with soil; iii) rolled paper towel without soil, and iv) an alternative procedure, using rolled paper towel without soil under cold water. A completely randomized experimental design with eight replications was used and the means were compared by the Tukey test (p = 0.05). To verify the dependence between the alternative test and others single linear correlation was used. All cold test procedures had similar coefficients of variation (CV), highlighting that rolled paper towel with soil and the alternative procedure had the best performance, with an average of 94% and 93% normal seedlings and CV of 3.2% and 3.6%, respectively. The alternative procedure has satisfactory results for estimating soybean seed vigor, yielding consistent results compared to the traditional procedure.
Glycine max (L.) Merrill; physiological potential; emergence; vigor tests
A crescente demanda por semente de soja [Glycine max (L.) Merrill] de alta qualidade tem requerido da indústria de sementes um controle de qualidade mais preciso. Uma maneira de conseguir isso é pela melhoria dos testes de vigor. O teste de frio tem sido empregado em sementes de milho, no entanto, tem sido usado também para sementes de algodão (Gossypium spp.), soja (Glycine Max), feijão ((Phaseolus vulgaris) e ervilha (Pisum sativum). Este estudo foi conduzido com objetivo de ajustar o procedimento do teste para determinar o vigor de semente de soja. Foram utilizados seis lotes comerciais de semente de soja, cultivar BRS 133. O potencial fisiológico dos lotes foi avaliado usando-se os testes de germinação em rolo de papel toalha, tipo germitest, e em caixa com areia, de emergência de plântulas em campo, de tetrazólio, de envelhecimento acelerado e de condutividade elétrica. Determinou-se também o teor de água das sementes. Para composição dos procedimentos do teste de frio foi utilizada temperatura de 10ºC, tempo de permanência no frio por cinco dias, e quatro procedimentos: caixa com terra, rolo de papel com terra, rolo de papel sem terra e metodologia alternativa, rolo de papel sem terra sob água refrigerada. O delineamento experimental utilizado foi inteiramente casualizado com oito repetições e as médias foram comparadas pelo teste de Tukey (p = 0,05). Para verificar a dependência entre o método alternativo e os demais usou-se o coeficiente de correlação linear simples. Os procedimentos para condução do teste de frio apresentaram coeficientes de variação muito próximos (CV) com destaque para a metodologia do rolo de papel com terra e procedimento alternativo, em média, com 94% e 93% de plântulas normais e CV de 3,2% e 3,6% respectivamente. O procedimento alternativo teve resultado satisfatório para estimar vigor da semente de soja, produzindo resultados consistentes em comparação ao procedimento tradicional.
Glycine max (L.) Merrill; potencial fisiológico; emergência; testes de vigor
CROP SCIENCE
Alternative procedure for the cold test for soybean seeds
Procedimento alternativo para o teste de frio em semente de soja
Bruno Guilherme Torres Licursi VieiraI; Roberval Daiton VieiraI,* * Corresponding author < rdvieira@fcav.unesp.br> ; Francisco Carlos KrzyzanowskiII; José de Barros França NetoII
IUNESP/FCAV - Depto. de Produção Vegetal, Via de Acesso Prof. Paulo Donato Castellane, s/n - 14884-900 - Jaboticabal - SP - Brasil
IIEmbrapa Soja, C.P. 231 - 86001-970 - Londrina, PR - Brasil
ABSTRACT
The growing demand for high quality soybean [Glycine max (L.) Merrill] seeds requires a precise seed quality control system from the seed industry. One way to accomplish this is by improving vigor testing. Cold test has been traditionally employed for corn seeds. However, it has also been used for other seed crops such as cotton (Gossypium spp.), soybean (Glycine Max), dry bean (Phaseolus vulgaris) and pea (Pisum sativum). This study was carried out with the objective of adjusting an alternative procedure for the cold test to determine soybean seed vigor. Six commercial soybean seed lots of the cultivar BRS 133 were used. The physiological potential of the seed lots was evaluated by germination on paper towel and sand box, seedling field emergence, tetrazolium, accelerated aging and electrical conductivity tests. Seed moisture content was also determined. The temperature used for the cold test procedures was 10ºC during five days. Four cold test procedures were evaluated: i) plastic boxes with soil; ii) rolled paper towel with soil; iii) rolled paper towel without soil, and iv) an alternative procedure, using rolled paper towel without soil under cold water. A completely randomized experimental design with eight replications was used and the means were compared by the Tukey test (p = 0.05). To verify the dependence between the alternative test and others single linear correlation was used. All cold test procedures had similar coefficients of variation (CV), highlighting that rolled paper towel with soil and the alternative procedure had the best performance, with an average of 94% and 93% normal seedlings and CV of 3.2% and 3.6%, respectively. The alternative procedure has satisfactory results for estimating soybean seed vigor, yielding consistent results compared to the traditional procedure.
Key words:Glycine max (L.) Merrill, physiological potential, emergence, vigor tests
RESUMO
A crescente demanda por semente de soja [Glycine max (L.) Merrill] de alta qualidade tem requerido da indústria de sementes um controle de qualidade mais preciso. Uma maneira de conseguir isso é pela melhoria dos testes de vigor. O teste de frio tem sido empregado em sementes de milho, no entanto, tem sido usado também para sementes de algodão (Gossypium spp.), soja (Glycine Max), feijão ((Phaseolus vulgaris) e ervilha (Pisum sativum). Este estudo foi conduzido com objetivo de ajustar o procedimento do teste para determinar o vigor de semente de soja. Foram utilizados seis lotes comerciais de semente de soja, cultivar BRS 133. O potencial fisiológico dos lotes foi avaliado usando-se os testes de germinação em rolo de papel toalha, tipo germitest, e em caixa com areia, de emergência de plântulas em campo, de tetrazólio, de envelhecimento acelerado e de condutividade elétrica. Determinou-se também o teor de água das sementes. Para composição dos procedimentos do teste de frio foi utilizada temperatura de 10ºC, tempo de permanência no frio por cinco dias, e quatro procedimentos: caixa com terra, rolo de papel com terra, rolo de papel sem terra e metodologia alternativa, rolo de papel sem terra sob água refrigerada. O delineamento experimental utilizado foi inteiramente casualizado com oito repetições e as médias foram comparadas pelo teste de Tukey (p = 0,05). Para verificar a dependência entre o método alternativo e os demais usou-se o coeficiente de correlação linear simples. Os procedimentos para condução do teste de frio apresentaram coeficientes de variação muito próximos (CV) com destaque para a metodologia do rolo de papel com terra e procedimento alternativo, em média, com 94% e 93% de plântulas normais e CV de 3,2% e 3,6% respectivamente. O procedimento alternativo teve resultado satisfatório para estimar vigor da semente de soja, produzindo resultados consistentes em comparação ao procedimento tradicional.
Palavras-chave:Glycine max (L.) Merrill, potencial fisiológico, emergência, testes de vigor
Introduction
Various techniques of seed vigor tests have been developed to estimate field performance. The cold test is one of the oldest and most popular seed vigor test. It was developed to evaluate the physiological potential of corn seeds, seeking to simulate adverse soil conditions (excessive water, low temperatures and presence of fungi in the soil) that frequently occur during the sowing sea son in the US Corn Belt (AOSA, 2002). The efficiency of the cold test has been tested experimentally by sev eral researchers as reported by Barros et al. (1999) and AOSA (2002). However, cold test procedures have not been standardized among seed laboratories, which generally conduct their own versions of the test.
Some methodological variations have been proposed to simplify the test. The method developed by Hope cited by Fiala (1981) consists in using germination paper towel lined with soil, which reduces the amount of soil and space necessary as compared to the traditional method. Loeffler et al. (1985) suggested another procedure by using germination paper towel without soil, known as the cold test without soil. This procedure was sensitive enough to detect drying damage in corn seeds, as well as to provide greater reproducibility of results due to the simplicity of the method. Therefore, the cold test seeks to evaluate the effects of a combination of low temperature, microorganism action and high substrate moisture by identifying differences in physiological potential among seed lots (Caseiro and Marcos-Filho, 2000 and 2002). Thus, this study was conducted with the objective of adjusting an alternative procedure of the cold test to determine soybean seed vigor.
Material and Methods
Six commercial lots of soybean seeds [G. max (L.) Merrill], cultivar BRS 133 were used to determine the physiological potential, submitting them to the tests of tetrazolium, germination, seedling emergence in sand. The following tests were implemented to improve characterization of the seed lots: electrical conductivity (Vieira and Kryzanowski, 1999; AOSA, 2002), seedling emergence in the field (Nakagawa, 1999), and initial seed water content (AOSA, 2002). Seeds were stored in a cold chamber (10ºC and 50-60% air relative humidity) during the studies to preserve the seed physiological potential. Seed water content was determined by the oven method at 105 ± 3ºC for 24 h with results expressed in percentages (AOSA, 2002).
The germination test was conducted with eight replications of 50 seeds per treatment, using germination paper towel as substrate. The germination paper was moistened with distilled water until reaching 2.5 times its dry weight and placed in a germinator at 25ºC. Evaluation of normal seedlings was made with a single count on the fifth day after sowing (AOSA, 2002). The seedling emergence in sand test was conducted with eight replications (trays) of 50 seeds per treatment in plastic boxes (30:20:10cm), using river sand substrate moistened up to 60% of its retention capacity. Trays were maintained at room temperature, 25ºC-30ºC (AOSA, 2002). Normal seedlings were evaluated in a single count on the fifth day after sowing.
The tetrazolium test (TT) was conducted on two sub-samples of 50 seeds per treatment. Pre-conditioning was carried out on moist germination paper towel, for 16 hours at 25ºC. Later, the seeds were transferred to plastic cups with 0.075% solution of 2, 3, 5 triphenyl tetrazolium chloride (TTC) and stained in a dark chamber at 40ºC for 3 h. Following this period, seeds were washed in running water and each seed was analyzed individually by examining the location and extent of external and internal damages (mechanical damage, weathering and stink bug feeding damage) by cutting longitudinally the seeds along the embryonic axis and the seed coat was removed (França-Neto et al., 1999).
The electrical conductivity test (ECT) was performed by using eight replications of 50 seeds per lot. Seeds were weighed within two decimal points and then placed in 200 mL-plastic cups containing 75 mL distilled and deionized water for 24 hours at 25ºC. Electrical conductivity of the soaking solution was measured using an automated Digimed (DM-31) conductivity meter. Results were expressed in µS cm-1 g-1 (Vieira and Krzyzanowski, 1999; AOSA, 2002).
To measure seed vigor by the accelerated aging test, 42 g of seeds were spread in a single layer on a stainless steel screen inside a plastic germination box (11:11:3.5 cm) and kept at 42ºC and approximately 100% air relative humidity for 48 hours (Marcos-Filho, 1999; AOSA, 2002).
For seedling field emergence, eight replications of 50 seeds per lot were used. Before sowing, seeds were treated with carbendazin (methyl benzimidazol-2-ylcarbamate C9H9N3O2) + thiram (Tetramethylthiuram disulfide C6H12N2S4) at the concentration of 2.5 mL of the commercial product plus 2.5 mL of water for each kg of seeds (Henning, 2004). After treatment, seeds were sown in rows spaced at 0.45 m at a depth of 3 to 4 cm. Seedling evaluation was determined 15 days after sowing (Nakagawa, 1999).
Four procedures of the cold test (CT) were evaluated:
i) CT using plastic boxes with soil (PBS): Eight replications of 50 seeds were sown in plastic boxes (30:20:10 cm) filled with two parts of soil to one part of sand moistened at about 60% water retention capacity. Boxes were kept at 10ºC for 5 days (Barros et al., 1999). Following this period boxes were kept at room temperature 25/30ºC for an additional period of five days for seed germination and seedling emergence (Barros et al., 1999). Seedling emergence percentage was calculated in a single count on the fifth day (AOSA, 2002).
ii) CT using rolled paper towel with soil (RPT): Eight replications of 50 seeds were sown using germination paper towel that had been moistened with distilled water up to 2.5 times its dry weight and covered with 60g of a soil/sand mixture. The rolls were then wrapped and placed in upright position into polypropylene packages inside plastic containers and maintained in a cold chamber at 10ºC for five days. The rolls were then transferred to germinators set at 25ºC. Evaluation of normal seedlings was assayed in a single count on the fifth day after transferring to room temperature (AOSA, 2002).
iii) CT using rolled paper towel without soil (RPTWS): Eight replications of 50 seeds were sown on germination paper towel that had been moistened with distilled water to 2.5 times its dry weight. The rolls were then wrapped and placed in upright position into polypropylene packages inside plastic containers and kept in a cold chamber at 10ºC for five days. The rolls were then transferred to germinators at 25ºC (Loeffler et al., 1985). Evaluation of normal seedlings was assayed in a single count on the fifth day after transferring to specific room temperature (AOSA, 2002).
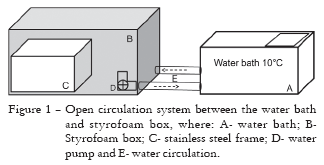
iv) Alternative CT procedure (ALTER): it was developed to create stressful conditions with high substrate moisture and low temperature. Germination paper towel without soil was used as substrate, which is considered to be a limiting factor by some authors (Hoppe, 1956; Crosier, 1957; Loeffler et al., 1985; Caseiro and Marcos-Filho, 2002). The alternative cold test was performed by using eight replications of 50 seeds per lot sown on germination paper towel that had been moistened with water in the proportion of 2.5 times its dry weight. Each replication was conditioned in sealed polypropylene bags and placed on stainless steel frames, which were inserted into a styrofoam box inside a water bath and maintained submersed in chilled water at the temperature of 10ºC for five days by using an open circulation system between the water bath and the styrofoam box (Figure 1). Following this period, the rolls were transferred to a germinator set at 25ºC (AOSA, 2002). The number of normal seedlings was evaluated in a single count on the fifth day after transferring to room temperature that ranged from 25ºC to 30ºC.
A completely randomized experimental design with eight replications was used. The data were analyzed statistically and the means were compared by the Tukey test (p = 0.05). To verify the dependence between the alternative test and others the linear single correlation coefficient was used.
Results and Discussion
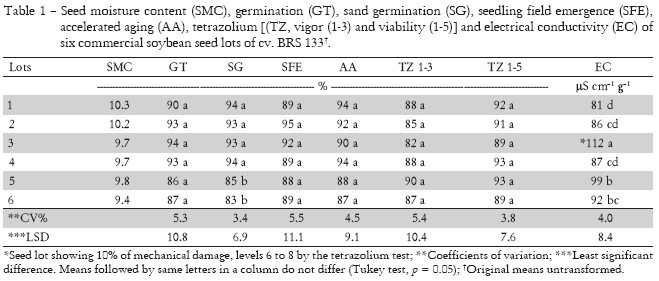
Variation in seed moisture content was less than 3.0% comparing all seed samples (Table 1), which is within the acceptable range for the accelerated aging test, as indicated by Marcos-Filho (1999). Acceptable seed moisture content, standardized evaluations and consistent results are extremely important in seed vigor tests (Loeffler et al., 1988).
Although the six lots used in this study had distinct germination percentage values (Table 1), no differences were found among them. All six lots had germination percentage values above the minimum standard required for commercialization (80%) (Brazil, 2004). Lots 5 and 6 were physiologically inferior to the others (Table 1), although still within acceptable commercial standards. In relation to seed vigor as determined by the accelerated aging (AA), tetrazolium (TTC 1-3), seedling field emergence (SFE) tests, no differences in quality were detected among the seed lots. The seed lots evaluated in the present experiment presented adequate performance as measured by the seedling emergence in the field (SFE), with averages ranging from 88% to 95% (Table 1). However, the EC was able to detect differences among the lots. Seed lot 3 had electrical conductivity higher than other lots (Table 1). This may be due to the high percentage of mechanical damage detected by the TT, levels 1-8 and 6-8 (Table 2). Soybean seeds are very susceptible to mechanical damage, since vital parts of the embryonic axis (radicle, hypocotil and plumule) are located under a thin seed coat that offers little protection to the seed (França-Neto and Henning, 1984). Mechanical damage is considered by many researchers (França-Neto and Potts, 1979; Vieira et al., 1994) to be one of the most serious problems for soybean seed production, primarily during harvest and processing. Seed mechanical damage is an inevitable problem (Carvalho and Nakagawa, 2000); however, it can be minimized by using well adjusted harvest and cleaning equipments.
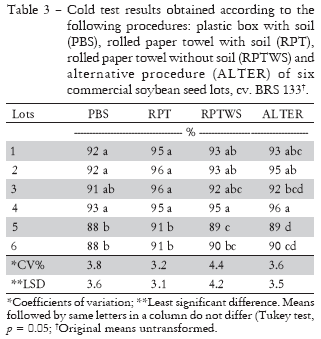
Regarding the results obtained by all evaluated procedures of the cold test (Table 3), normal seedling percentages varied from 88% to 96% for the different procedures of the cold test. Grabe (1976) reported 70% to 80% normal seedlings resulting from the cold test as being adequate levels of quality for commercial seed lots. Lots 5 and 6 had the lowest vigor levels as compared to the other lots and their results did not differ from each other (Table 3). Comparing the results obtained in the cold test by the PBS, RPT and RPTWS procedures, lots 1 through 4 had approximately the same level of vigor, since their results did not differ within each procedure. ALTER was able to better stratify the quality of these same seed lots, since lot 1, 2 and 4 were the highest in quality and lot 3 had intermediate quality. This fact made this procedure more reliable as compared to the other. The coefficients of variation obtained for all the tests performed were relatively low and acceptable for laboratory tests (Table 3).
In general, the procedure RPT had the highest percentage values of all tests (Table 3). However, Miguel and Cicero (1999a) obtained greater CT values for soybean and cotton seeds using RPTWS when compared to other procedures. According to Burris and Navratil (1979), CT performed by RPT, without the effects of soil microrganisms such as reported above by Miguel and Cicero (1999a), will tend to present a more standardized procedure, providing uniform results. This was not the case as reported in this study, since the RPT procedure resulted in the highest observed results.
The CT using plastic boxes and the soil/sand mixture (PBS) presented lower values of normal seedling than the other procedures (Table 3). Similar results were obtained with soybean (Miguel and Cicero, 1999b) and cotton (Miguel et al., 2001), in which the CT with this type of substrate produced greater germination losses regardless of time and temperature of exposure, since prolonged periods of cold promote greater sugar exudation, thereby facilitating fungus development (Wassink and Hoefman, 1992).
Loeffler et al. (1985), Molina et al. (1987), Medina and Marcos-Filho (1990) recommend the traditional CT procedure (box with soil), despite being more severe due to its more stressful conditions. According to these authors, this procedure is the most efficient in ranking corn seed lots into different vigour levels compared to other procedures. However, in the present study, ALTER was more precise in ranking the seed vigor level of soybean seed lots. ALTER does not use soil as substrate. CT results using soil are subject to variation over time due to the inconsistent activity of microorganisms among substrate sources from the same or different areas (Loeffler et al., 1985). Another limitation of this procedure was pointed out by Hooks and Zuber (1963) who reported variable results when soil was obtained from different sources, or collected on different days or years.
In general, research has sought to indicate convenient solutions for perfecting the CT,such as the RPT (Hoppe, 1956; Crosier, 1957) and RPTWS (Loeffler et al., 1985), reducing the quantity of substrate and space necessary, which are often limiting factors in seed laboratories (Hoppe, 1956; Crosier, 1957; Loeffler et al., 1985; Caseiro and Marcos-Filho, 2002). ALTER evaluated in this study fits to the characteristics mentioned above.
Data regarding single correlation analysis, calculated between the alternative and traditional (plastic box with soil) procedures and several seed quality tests, are shown in Table 4. The ECT had negative correlation with ALTER. The germination test in sand had positive correlation with ALTER. Unexpectedly, no significance was observed for the correlation between both methods of the cold test (ALTER X PBS - Table 4). Correlation only indicates a similar tendency of variation between two parameters, but it does not indicate a corresponding precision in estimating the physiological quality of the lot, since this procedure may provide incomplete or incorrect information (Marcos-Filho et al., 1987).
As a conclusion, ALTER,using germination paper without soil under chilled water in a temperature controlled water bath (10ºC), has satisfactory results for soybean seeds. Results are as consistent as those procedures of the cold test traditionally used in seed laboratories.
Acknowledgements
To CAPES and CNPq, for financial support and research scholarship.
Received December 03, 2008
Accepted April 16, 2010
- Association of Official Seed Analysts [AOSA] 2002. Seed Vigor Testing Handbook. AOSA, Lincoln, NE, USA. (Contribuition, 32).
- Barros, S.R.B.; Dias, M.C.L.L.; Cicero, S.M.; Krzyzanowski, F.C. 1999. Cold test. chap.5, p. 1-15. In: Krzyzanowski, F.C.; Vieira,R.D.; França Neto, J.B., eds. Seed vigor: concepts and tests. ABRATES, Londrina, PR, Brazil. (in Portuguese).
- Burris, J.S.; Navratil, R.J. 1979. Relationship between laboratory cold test methods and field emergency in maize inbreds. Agronomy Journal 71: 985-988.
- Brazil. Department of Agriculture. 2004. Seed rules for production, trading and use; Law nş 10.711, of August 5, decree nş 5.153, of July 23, 2004. MAPA/SNPC, Brasília, DF, Brazil. (in Portuguese).
- Carvalho, N.M.; Nakagawa, J. 2000. Seeds: science, technology and production. FUNEP, Jaboticabal, SP, Brazil. (in Portuguese).
- Caseiro, R.F.; Marcos-Filho, J. 2000. Alternative methods of the cold test for evaluation of corn seed vigor. Scientia Agricola 57: 459-466. (in Portuguese, with abstract in English).
- Caseiro, R.F.; Marcos-Filho, J. 2002. Procedures for cold test in maize seeds: prechilling and position of substrate inside the cold room. Revista Brasileira de Sementes 24: 6-11. (in Portuguese, with abstract in English).
- Crosier, W.F. 1957. Fungi envolved and methods of conducting cold tests. Proceedings of the Association of Official Seed Analysts 47: 185-190.
- Fiala, F. 1981. Cold test. p. 28-36. In: Perry, D.A., ed. Handbook of vigour test methods. ISTA, Zürich, Switzland.
- França-Neto, J.B.; Henning, A.A. 1984. Physiological and sanitary quality of soybean seeds. Embrapa-CNPSo. Londrina, PR, Brazil. (Circular Técnica, 9). (in Portuguese).
- França-Neto, J.B.; Potts, H.C. 1979. Effects of mechanical harvest and artificial drying on the quality of soybean hard seed. Revista Brasileira de Sementes 1: 64-77.
- França-Neto, J.B.; Krzyzanowski, F.C.; Costa, N.P. 1999. The tetrazolium test for soybean seeds. chap.8, p. 5-28. In: Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B., eds. Seed vigor: concepts and tests. ABRATES, Londrina, PR, Brazil. (in Portuguese).
- Grabe, D.F. 1976. Measurement of seed vigor. Journal of Seed Technology 1: 18-31.
- Henning, A.A. 2004. Pathology and Seed Treatment: An Overview. Londrina: Embrapa Soja. 51 p. (Documentos, 235). (in Portuguese).
- Hooks, J.A.; Zuber, M.S. 1963. Effects of soil and soil moisture levels on cold test germination of corn. Agronomy Journal 55: 453-455.
- Hoppe, P.E. 1956. Correlation between corn germination in laboratory cold tests and stands in the field. Plant Disease Reporter 40: 887-889.
- Loeffler, N.L.; Meier, J.L.; Burris, J.S. 1985. Comparison of two cold test procedures for use in maize-drying studies. Seed Science and Technology 13: 653-658.
- Loeffler, T.M.; TeKrony, D.M.; Egli, D.B. 1988. The bulk conductivity test as an indicator of soybean seed quality. Journal of Seed Technology 12: 37-53.
- Marcos-Filho, J. 1999. Accelerated aging test. chap.3, p. 1-21. In: Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B., eds. Seed vigor: concepts and tests. ABRATES, Londrina, PR, Brazil. (in Portuguese).
- Marcos-Filho, J.; Cicero, S.M.; Silva, W.R. 1987. Seed quality evaluation. FEALQ, Piracicaba, SP, Brazil. (in Portuguese)
- Medina, P.F.; Marcos-Filho, J. 1990. Physiological quality of corn seeds evaluation (Zea mays L.). Anais da ESALQ 47: 47-70. (in Portuguese).
- Miguel, M.H.; Carvalho, M.V.; Beckert, O.P.; Marcos-Filho, J. 2001. Cold test evaluation of the physiological potential of cotton seeds. Scientia Agricola 58: 741-746. (in Portuguese, with abstract in English).
- Miguel, M.H.; Cicero, S.M. 1999a. Cold test for bean seed vigor evaluation. Scientia Agricola 56: 233-243. Supl. (in Portuguese, with abstract in English).
- Miguel, M.H.; Cicero, S.M. 1999b. Cold test for soybean seed vigor evaluation. Revista Brasileira de Sementes 21: 35-42. (in Portuguese, with abstract in English).
- Molina, J.C.; Irigon, D.L.; Zonta, E.P. 1987. Cold test methodology comparations on physiological evaluation of corn (zea mays L.) seed. Revista Brasileira de Sementes 9: 77-85. (in Portuguese, with abstract in English).
- Nakagawa, J. 1999. Vigor tests based on seedling development. chap.2, p. 1-24. In: Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B., eds. Seed vigor: concepts and tests. ABRATES, Londrina, PR, Brazil. (in Portuguese).
- Vieira, R.D.; Krzyzanowski, F.C. 1999. Electrical conductivity test. chap.4, p. 1-26. In: Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B., eds. Seed vigor: concepts and tests. ABRATES, Londrina, PR, Brazil. (in Portuguese).
- Vieira, C.P.; Vieira, R.D.; Paschoalick, J.H.N. 1994. Effects of mechanical damage during soybean seed processing on physiological seed quality and storage. Seed Science and Technology 22: 581-589.
- Wassink, H.; Hoefman, R. 1992. De factor van de ligging. Boerdery 77: 27.
Publication Dates
-
Publication in this collection
20 Sept 2010 -
Date of issue
Oct 2010
History
-
Accepted
16 Apr 2010 -
Received
03 Dec 2008