Abstracts
Global climate models predict changes on the length of the dry season in the Amazon which may affect tree physiology. The aims of this work were to determine the effect of the rainfall regime and fraction of sky visible (FSV) at the forest understory on leaf traits and gas exchange of ten rainforest tree species in the Central Amazon, Brazil. We also examined the relationship between specific leaf area (SLA), leaf thickness (LT), and leaf nitrogen content on photosynthetic parameters. Data were collected in January (rainy season) and August (dry season) of 2008. A diurnal pattern was observed for light saturated photosynthesis (Amax) and stomatal conductance (g s), and irrespective of species, Amax was lower in the dry season. However, no effect of the rainfall regime was observed on g s nor on the photosynthetic capacity (Apot, measured at saturating [CO2]). Apot and leaf thickness increased with FSV, the converse was true for the FSV-SLA relationship. Also, a positive relationship was observed between Apot per unit leaf area and leaf nitrogen content, and between Apot per unit mass and SLA. Although the rainfall regime only slightly affects soil moisture, photosynthetic traits seem to be responsive to rainfall-related environmental factors, which eventually lead to an effect on Amax. Finally, we report that little variation in FSV seems to affect leaf physiology (Apot) and leaf anatomy (leaf thickness).
diurnal variation; forest understory; photosynthetic capacity; rainfall seasonality
Os modelos climáticos globais prevêem mudanças na extensão da época seca na Amazônia, o que pode afetar a fisiologia das árvores. Os objetivos deste trabalho foram determinar o efeito da sazonalidade da precipitação e fração de céu visível (FSV) no sub-bosque da floresta nas características foliares e trocas gasosas de 10 espécies florestais da Amazônia Central. Também examinou-se a relação entre área foliar específica (SLA), espessura da folha (LT) e nitrogênio foliar em parâmetros fotossintéticos. Os resultados foram coletados nos meses de janeiro (época chuvosa) e agosto (época seca) de 2008. Observou-se um padrão de variação diurna na fotossíntese saturada por luz (Amax) e na condutância estomática (g s). Independente da espécie, Amax foi menor na época seca. No entanto, não houve efeito da sazonalidade das chuvas em g s nem na capacidade fotossintética (Apot medida em [CO2] saturante). Apot e a espessura da folha (LT) aumentaram com FSV, o contrário foi observado para a relação FSV-SLA. Também, observou-se uma relação positiva entre Apot por unidade de área e conteúdo de nitrogênio foliar, e entre Apot, por unidade de massa e SLA. Embora o regime das chuvas apenas levemente influenciou a umidade do solo, características fotossintéticas parecem responderem a fatores relacionados com as chuvas, o que repercute em Amax. Finalmente, relata-se que pequenas variações em FSV parecem afetar a fisiologia da folha (Apot) e a anatomia foliar (espessura da folha).
capacidade fotossintética; sub-bosque; variação diurna; sazonalidade das chuvas
CROP SCIENCE
Leaf traits and gas exchange in saplings of native tree species in the Central Amazon
Características foliares e trocas gasosas em arvoretas de espécies nativas da Amazônia Central
Keila Rego MendesI; Ricardo Antonio MarencoII,* * Corresponding author < rmarenco@inpa.gov.br>
IINPA/Instituto Nacional de Pesquisas da Amazônia Programa de Pós-Graduação em Botânica
IIINPA Coordenação de Pesquisas em Silvicultura Tropical, Av. André Araújo, 1756 C.P. 478 69011-970 Manaus, AM Brasil
ABSTRACT
Global climate models predict changes on the length of the dry season in the Amazon which may affect tree physiology. The aims of this work were to determine the effect of the rainfall regime and fraction of sky visible (FSV) at the forest understory on leaf traits and gas exchange of ten rainforest tree species in the Central Amazon, Brazil. We also examined the relationship between specific leaf area (SLA), leaf thickness (LT), and leaf nitrogen content on photosynthetic parameters. Data were collected in January (rainy season) and August (dry season) of 2008. A diurnal pattern was observed for light saturated photosynthesis (Amax) and stomatal conductance (gs), and irrespective of species, Amax was lower in the dry season. However, no effect of the rainfall regime was observed on gs nor on the photosynthetic capacity (Apot, measured at saturating [CO2]). Apot and leaf thickness increased with FSV, the converse was true for the FSV-SLA relationship. Also, a positive relationship was observed between Apot per unit leaf area and leaf nitrogen content, and between Apot per unit mass and SLA. Although the rainfall regime only slightly affects soil moisture, photosynthetic traits seem to be responsive to rainfall-related environmental factors, which eventually lead to an effect on Amax. Finally, we report that little variation in FSV seems to affect leaf physiology (Apot) and leaf anatomy (leaf thickness).
Key words: diurnal variation, forest understory, photosynthetic capacity, rainfall seasonality
RESUMO
Os modelos climáticos globais prevêem mudanças na extensão da época seca na Amazônia, o que pode afetar a fisiologia das árvores. Os objetivos deste trabalho foram determinar o efeito da sazonalidade da precipitação e fração de céu visível (FSV) no sub-bosque da floresta nas características foliares e trocas gasosas de 10 espécies florestais da Amazônia Central. Também examinou-se a relação entre área foliar específica (SLA), espessura da folha (LT) e nitrogênio foliar em parâmetros fotossintéticos. Os resultados foram coletados nos meses de janeiro (época chuvosa) e agosto (época seca) de 2008. Observou-se um padrão de variação diurna na fotossíntese saturada por luz (Amax) e na condutância estomática (gs). Independente da espécie, Amax foi menor na época seca. No entanto, não houve efeito da sazonalidade das chuvas em gs nem na capacidade fotossintética (Apot medida em [CO2] saturante). Apot e a espessura da folha (LT) aumentaram com FSV, o contrário foi observado para a relação FSV-SLA. Também, observou-se uma relação positiva entre Apot por unidade de área e conteúdo de nitrogênio foliar, e entre Apot, por unidade de massa e SLA. Embora o regime das chuvas apenas levemente influenciou a umidade do solo, características fotossintéticas parecem responderem a fatores relacionados com as chuvas, o que repercute em Amax. Finalmente, relata-se que pequenas variações em FSV parecem afetar a fisiologia da folha (Apot) e a anatomia foliar (espessura da folha).
Palavras-chave: capacidade fotossintética, sub-bosque, variação diurna, sazonalidade das chuvas
Introduction
Global climate models predict that in the Amazon the length of the dry season period will be extended as a result of global warming associated to an increase of atmospheric CO2 concentration (Cox et al., 2004). Indeed, a prolonged dry period may affect plant growth and physiological processes, such as photosynthesis and respiration (Hughes, 2000). Although severe soil moisture depletion during prolonged drought may lead to stomatal closure and a decline in leaf area (Nepstad et al., 1994), there is still controversy on whether draught-induced water deficit limits tree growth in the Central Amazon. During the 2005 drought, for example, Saleska et al. (2007) reported an enhanced vegetation index of the forest based on moderate resolution imaging spectroradiometer (MODIS) satellite data. This is contrary to what should be expected, as changes in precipitation can alter growth rates (Lewis et al., 2004). In a rainfall exclusion experiment, a 60% reduction of incoming throughfall led to a drastic increase (38%) in tree mortality (Nepstad et al., 2007), much higher than commonly recorded in the Central Amazon, about 1.1% per year (Williamson et al., 2000). Although sapling photosynthetic traits of canopy trees have received some attention in tropical forest (e.g. Marenco and Vieira, 2005; Poorter and Oberbauer, 1993), how seasonality of the rainfall regime affects seedling and sapling photosynthetic traits in the Central Amazon still remains to be elucidated.
In addition to soil moisture, light availability is one of the most important factors limiting seedling and sapling growth in the forest understory (Denslow et al., 1990; Valladares and Niinemets, 2008). Through the canopy profile light varies not only in total quantity, but also in quality, as the red/far red (R/Fr) ratio declines towards the forest floor (Smith, 1982). Low irradiance often leads to a decrease in leaf thickness and light saturated photosynthesis (Amax), whereas specific leaf area (SLA) commonly increases under low light intensity (Oguchi et al., 2005). Plant growth is the result of a complex of interacting factors intrinsically related to carbon gain via photosynthesis and loss due to respiration. However, over a wide range of plants species and growth conditions there seems to be a positive relationship between plant growth and photosynthetic rates (Kruger and Volin, 2006)
In this study we hypothesized that variation in soil moisture and subtle changes in light availability in the forest understory affect leaf traits and carbon gain in saplings of canopy trees. Thus, the aims of this work were to determine the effect of the seasonal rainfall regime and understory irradiance on leaf traits and gas exchange in ten rainforest tree species. We also examined the effect of specific leaf area (SLA), leaf thickness (LT), and nitrogen content on the photosynthetic capacity.
Material and Methods
The study was conducted 60 km north of Manaus (02º36'21" S; 60°08'11" W), state of Amazonas, Brazil, in an area of native "terra-firme" forest. The region has characteristics of a humid equatorial climate, with a short mild dry season (July-September, with a rainfall of 50-100 mm per month), and a dry-wet transition month (October). The wet season extends from November to May (200-300 mm month1). Annual precipitation is 2240 mm (Inmet, 2008, mean of 1961 to 1990). The area is covered by a dense forest and the predominant soil type is an Oxisol of low fertility, clay texture and pH of 4.2 to 4.5.
We used saplings (1.5 to 2-m tall) of 10 tree species selected taking into account their shade tolerance, relative abundance of saplings in the forest understory (at least three replications per species), and economic importance (Table 1). The gas exchange parameters were measured with an infrared gas analyzer (Li-6400, Li-Cor, NE, USA) using one or two leaves per plant and three saplings per species on each season. Light saturated photosynthesis (Amax) was measured at ambient CO2 concentration (380 mmol mol1), light saturation (1000 mmol m2 s1, and ambient temperature (28 ± 1ºC). Potential photosynthesis (Apot, hereafter termed photosynthetic capacity) was also measured at light saturation, but at a [CO2] of 2000 mmol mol1, rather than ambient [CO2]. Gas exchange data were collected after a stabilization period of about 10-15 min (total coefficient of variation < 0.7%). The effect of the time of day on stomatal conductance (gs) and Amax was assessed across species by collecting data between 06h00 and 18h00. Data were collected in January and August 2008 in mature and fully expanded leaves.
Specific leaf area (SLA, the leaf area to leaf mass ratio) was determined in both seasons. As additional information, leaf thickness was determined in the dry season and leaf nitrogen content in the wet season. We measured SLA in a sample of six circles of 240-mm2- per leaf obtained from a sample of two to eight leaves per plant, depending on leaf size. We only determined nitrogen (Kjeldahl method) in the wet season (first studied season) in order to preserve the foliage for further studies in the same area. Fresh leaf thickness (FLT) and dry leaf thickness (DLT) were measured with digital calipers in 240-mm2-leaf circles (two per leaf) punched from the widest part of the leaf blade, and between the major veins (accuracy of 10 mm). Leaves used for SLA, leaf nitrogen and leaf thickness measurements were the same or similar in appearance (when more than two leaves were required for analysis) to those used for gas exchange determinations. Leaf dry mass was obtained after leaf dehydration at 72°C until constant mass. The fraction of sky visible (FSV) beneath the canopy was measured using a canopy analyzer (LAI-2000, Li-Cor, NE, USA), under overcast sky conditions to improve the accuracy of the instrument, and calculated by integrating the gap fraction to yield the fraction of sky not blocked by foliage. For each sapling, six FSV readings, collected at a distance of about 1.5 m from the stem and forming a circle around the plant (the microsite), were recorded at each microsite and a mean value was obtained. The height of the sensor above the ground corresponded to the height of leaves used for the gas exchange measurements (1 to 2 m above the ground). Finally, we used a second LAI-2000 sensor, operating in the remote mode and installed on the top of a 40-m-tall observation tower (located in a nearby area), to log FSV values above the forest canopy.
Irradiance and rainfall data were recorded above the forest canopy at the top of the 40-m tall observation tower. Irradiance at the observation tower (Iopen) was measured using a quantum sensor (Li-190 SA, Li-Cor, NE, USA). Understory irradiance (Iund) was estimated as the product of FSV by Iopen (i.e. Iund = FSV x Iopen). We are aware that Iund is lower than the actual light availability at sapling height, as it does not take into account the background of diffuse light in the forest understory (about 5-8 mmol m2 s1 at midday (Marenco and Vieira, 2005). Temperature and air humidity data were recorded at 30-min-intervals with a sensor (Humitter 50y, Vaisala Oy, Finland) connected to a datalogger (Li-1400, Li-Cor, NE, USA) at a selected site in the understory. In addition, an external quantum sensor (Li-190 SA, Li-Cor, USA) mounted on the Li-6400's irga head was used to log irradiance data at the same time as gas exchange measurements were made. Both in the dry and rainy season, soil moisture was determined gravimetrically: 100(Sw - Sd)/Sw,where Sw and Sd represent the mass of wet and dry, undisturbed 110-cm3-soil samples. Soil samples were collected at random in the study area at the depth of 200 mm, both in the wet (26 samples) and dry season (12 samples). All data, but leaf thickness and N content (determined only in one season), were subjected to analysis of variance (ANOVA) to assess the effect of the rainfall seasonality on the parameters. The Lilliefors test was conducted to assess whether experimental errors were normally distributed. As no transformation was needed, all statistical analyses were carried out on untransformed data. When the effect of rainfall seasonality on the variables was not significant (p > 0.05), data were pooled and linear or quadratic regression analyses conducted to examine the effect of FSV and SLA on photosynthetic traits. Tukey post-hoc test was used for mean separation (p £ 0.05).
Results and Discussion
Monthly rainfall was 353 mm in January and 105 mm in August (Table 2), which is in accordance with the historical mean (1961-1990) for the region (Inmet, 2008). In these months, soil moisture ranged between 31% in the dry season to 32% in the wet season, near the soil saturation point of 39% on a wet soil basis (Table 2). Air temperature at the forest floor ranged from 22°C at night to 29°C at noon, and for most of the day the relative humidity was above 90%, with no difference between seasons (Figure 1). Accumulated irradiance at the forest floor was 0.3 and 0.6 mol m2 day1 in the wet and dry seasons, respectively. On the other hand, mean maximum understory irradiance was about 10 and 20 mmol m2 s1 for the wet and dry seasons, respectively, or about 1.5-2% of the irradiance recorded above the forest canopy (Figure 1). Iund values reported in this study are a somewhat higher than those observed by others (Kursar and Coley, 1999; Marenco and Vieira, 2005), perhaps because our Iund values were recorded about 1-2 m above the ground rather than at the forest floor. Molion (1987) estimated that the irradiance that reaches the forest floor is 1.2% (approximately 14 mmol m2 s1 on a sunny day) of that received above the forest canopy, similar to our Iund values observed in the wet season.
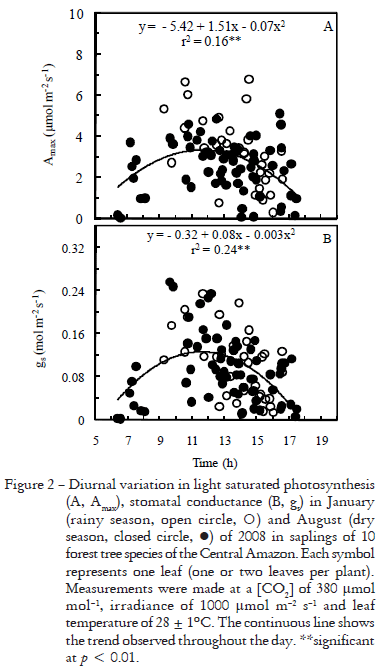
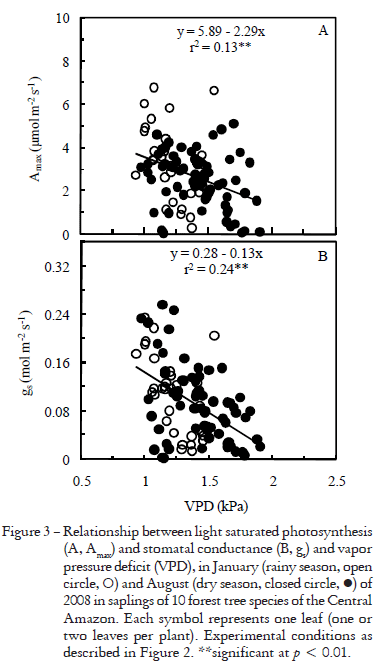
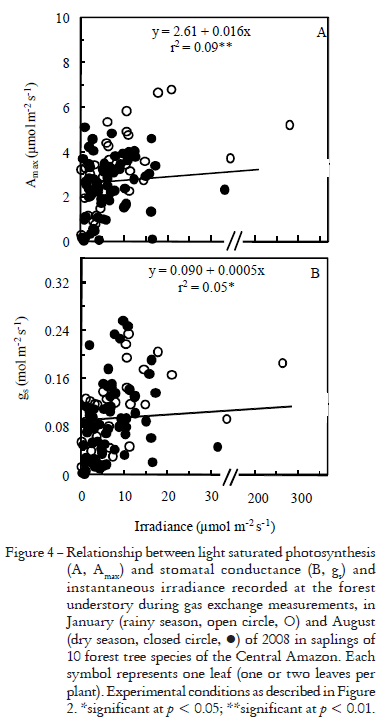
Early in the morning and late in the afternoon stomata did not respond to light stimulus, remaining closed even at saturating irradiance (1000 mmol m2 s1) for photosynthesis in the leaf chamber (Figure 2). Stomata closed and Amax declined as the vapor pressure deficit (VPD) increased (Figure 3). However, as the forest understory became brighter, Amax and gs tended to linearly increase with irradiance (Figure 4), which indicates that in this environment gs and Amax are under the influence of a diurnal cycle, perhaps affected by light and VPD (Figures 3, 4). Our results agree with those reported by Kaiser and Kappen (2000) who observed maximum gs values between 10h00 and 14h00 and a minimum stomatal aperture at sunset.
Light induces stomatal opening (Shimazaki et al., 2007) and thus stomata may open at irradiances above 2-8 mmol m2 s1 (Habermann, 1973). However, soon after dawn light was ineffective in triggering stomatal opening. Since irradiance, relative humidity and temperature changed during daytime on the forest floor (Figure 1), it is possible that somehow these environmental factors affected stomatal functioning during the day. Although the light environment had an effect on gs (p < 0.05, Figure 4B), the correlation between gs and irradiance at measuring time was tenuous (r2 = 0.05*). VPD has an important effect on gs, but it only explains 24% of variation (Figure 3B). Thus, we cannot rule out the effect of endogenous factors in modulating stomatal functioning, as reported by others, both in herbaceous plants (Gorton et al., 1993; Holmes and Klein, 1986) and forest trees (Doughty et al., 2006).
Although there was no difference in soil moisture between the dry and rainy season, Amax was lower in the dry season (Table 2), which suggests that even a slight decline in soil moisture, or perhaps in leaf water potential associated to a higher irradiance in the dry period, may affect some photosynthetic traits of understory saplings, perhaps mesophyll conductance (gm). Under progressive drought gm may decline (Flexas et al., 2002). This hypothesis is consistent with the fact that both Apot and gs were unaffected by rainfall seasonality. As gs was not influenced by rainfall seasonality (Table 2), differences in Amax between seasons may be ascribed to a limitation of carbon uptake imposed by non-stomatal factors. Had the dry season had any detrimental effect on Rubisco activity or ATP synthesis (Flexas and Medrano, 2002) Apot should have declined, but it did not. This allows us to conclude that the seasonality may have an effect on gm. In relation to the seasonal effect on Amax is important to take into account that climate models predict changes in the total rainfall in the Amazon as a result of global warming (Cox et al. 2004; Oyama and Nobre, 2003). Besides, differences among species were not observed in Amax nor in Apot, so data from both rainfall seasons were pooled to obtain a mean value for each species (Table 3).
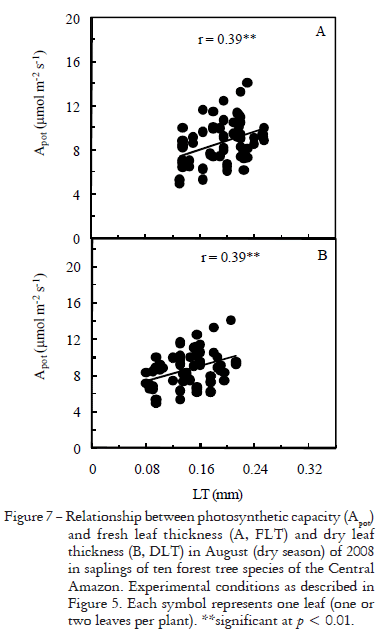
At ambient CO2 concentration (380 mmol mol1), Amax was closely related with gs (r2 = 0.60**, Figure 5A), which is consistent with results reported by others (Machado et al., 2002; Marenco et al., 2006; Park and Furukawa, 1999). Nonetheless, the correlation between Apot and gs was very low (r2 = 0.01ns) at saturating CO2 concentration (Figure 5B), indicating that Apot is little influenced by stomatal opening in the gs range observed for most of the day (08h00 to 16h00). Except very early in the morning, when most stomata were closed, their resistance to CO2 diffusion into intercellular spaces was offset by an elevated CO2 concentration in the leaf chamber. However, when gs was very low (less than 0.015 mol m2 s1, denoted by diamonds in Figure 5B) the resistance imposed by stomatal closure was not compensated by a high CO2 concentration in the leaf chamber, which led to a reduction in photosynthetic capacity. Thus, a gs of 0.015 mol m2 s1 most likely reflects a threshold below which leaf conductance is mainly due to cuticular conductance (gc). Because Apot remained quite constant for most of the day, the effect of FSV, SLA, LT and leaf nitrogen on photosynthetic rates were examined with respect to Apot rather that Amax, which was strongly dependent on gs. FSV was positively related to leaf thickness (Figures 6A, B) and Apot (Figure 6C), whereas its relationship with SLA was negative (Figure 6D). DLT ranged from 0.11 mm in P. guianensis and T. unifoliolata to 0.18 mm in C. duckeana (Table 3), with an increase of about 35% in fresh leaves (Table 3), in both cases, a positive correlation between leaf thickness and Apot was found (Figure 7).
The effect of FSV on Apot, LT and SLA shows that even small changes in intensity of light in the forest floor can alter the performance of the photosynthetic apparatus. This is in agreement with results reported by others (Ellsworth and Reich, 1993; Oguchi et al., 2005; Weston et al., 2000), who observed increases in LT in leaves exposed to brighter environments. The relationship between LT and Apot concurs with previous findings (McMillen and McClendon, 1983; Niinemets, 1999; Reich et al., 1998). Even when Apot and LT and SLA were strongly related (Figures 7, 8), we cannot attribute increases in Apot only to variations in LT or SLA, as photosynthetic compounds are less effectively used in thicker leaves, perhaps because of a lower leaf conductance in these leaves (Niinemets, 1999).
FSV values increased from 0.014 in January (rainy season) to 0.020 in August (dry season) (Table 2), confirming results obtained previously by Marenco and Vieira (2005). We attributed the difference in FSV values between the evaluated rainfall seasons to differences in leaf area index between the rainy and dry season (5.1 versus 4.7, Table 2) or to the higher solar radiation recorded in the dry season (Table 2). High irradiance in the dry season can result in greater carbon assimilation during this part of the year as suggested by Huete et al. (2006). However, a higher light intensity in the forest canopy in the dry season apparently does not contribute to increase the photosynthetic capacity of saplings at the forest floor, although across species, we found an effect of FSV on Apot irrespective of the seasonal rainfall regime, perhaps due to an effect of the R/Fr ratio on photosynthetic rates. For Acmena ingens, for example, under low irradiance (£ 20 % of full sunlight) gs and Amax where lower in plants grown under a reduced R/Fr ratio (0.2) than in control plants (R/Fr of sunlight, 1.2) (Turnbull, 1991).
SLA ranged from 13.5 m2 kg1 in S. guilleminiana to 20.8 m2 kg1 in R. racemosa, whereas the leaf nitrogen content varied between 0.9 g m2 for T. unifoliolata and R. racemosa to 1.7 g m2 for S. guilleminiana (Table 3). SLA values found in this study are within the range (15 and 24 m2 kg1) observed by Marenco and Vieira (2005) for saplings of canopy tree species. FSV had a positive effect on LT, and a negative one on SLA (p < 0.05). The positive effect of leaf nitrogen on Apot (Figure 9) is consistent with the results reported by Hikosaka (2004; 2005). However, although significant, the relationship between Apot and leaf N was weak (r2 = 0.14, p < 0.05) (Figure 9), indicating that a substantial fraction of the leaf nitrogen is partitioned into non-photosynthetic structures. On the other hand, differences in determination coefficients (r2) between DLT and SLA against FSV (0.11** versus 0.05* for SLA, Figure 6B, D) occur because SLA depends not only on LT, but also on leaf density (Niinemets, 1999), which suggests that FSV has a lower effect on leaf density. Although LT is affected by growth irradiance, we can not explain the wide variations in SLA among species only by differences in microsite brightness (inferred by FSV values) at the forest floor. This suggests that the genetic background of each species plays a major role in determining adaptive strategies to the physical and ecological environment (soil fertility and acidity, herbivory, etc.), which thereby leads to changes in LT and SLA under a given growth conditions (Lee et al., 2000; Peeters, 2002). Increase in LT (decline in SLA) is often related to higher photosynthetic rates per unit leaf area (McMillen and McClendon, 1983; Yun and Taylor, 1986), because of a greater accumulation of photosynthetic proteins. However, it may also involve an increase in the amount of molecules and compounds not directly related to carbon assimilation but with a key role for plant defense against herbivory and for increasing resistance against other physical hazards (Coley, 1988; Wright and Cannon, 2001).
Although the rainfall regime only slightly affected soil moisture, some photosynthetic traits (perhaps gm) seem to be responsive to rainfall-related environmental attributes, which eventually lead to an effect on Amax. In the forest understory, Amax and gs of saplings appear to be highly sensitive to diurnal variation, and even when stomatal functioning is affected by environment factors (e.g., light and VPD), somehow endogenous factors also seem to have a role in stomatal movements. However, the dry season of 2008 was not strong enough to unambiguously negate any potential effect of rainfall seasonality on the photosynthetic capacity of saplings. Further studies are needed to elucidate how a prolonged dry season may affect the diurnal pattern of photosynthesis, Rubisco activity, and electron transport rates, which may ultimately affect tree growth in the Central Amazon. Finally, even though irradiance in the forest floor is usually very low, it remarkably affects leaf physiology and leaf anatomy, as photosynthetic capacity, LT and SLA responded to variations in the fraction of sky visible in the forest understory.
Acknowledgements
To the Ministry of Science and Technology MCT/INPA, FAPEAM (project PIPT-1746-08), CAPES and CNPq.
Received March 05, 2009
Accepted June 14, 2010
- Coley, P.D. 1988. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74: 531-536.
- Cox, P.M.; Betts, R.A.; Collins, M.; Harris, P.P.; Huntingford, C.; Jones, C.D. 2004. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theoretical and Applied Climatology 78: 137-156.
- Denslow, J.S.; Schultz, J.C.; Vitousek, P.M.; Strain, B.R. 1990. Growth responses of tropical shrubs to treefall gap environments. Ecology 71: 165-179.
- Doughty, C.E.; Goulden, M.L.; Miller, S.D.; Rocha, H.R. 2006. Circadian rhythms constrain leaf and canopy gas exchange in an Amazonian forest. Geophysical Research Letters 33: 1-5 (L15404, DOI 10.1029/2006GL026750).
- Ellsworth, D.S.; Reich, P.B. 1993. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96: 169-178.
- Flexas, J.; Medrano, H. 2002. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany 89: 183-189.
- Flexas, J; Bota, J., Escalona, J.M.; Sampol, B.; Medrano, H. 2002. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29: 461-471
- Gorton, H.L.; Williams, W.E.; Assmann, S.M. 1993. Circadian rhythms in stomatal responsiveness to red and blue-light. Plant Physiology 103: 399-406.
- Habermann, H.M. 1973. Evidence for two photoreactions and possible involvement of phytochrome in light-dependent stomatal opening. Plant Physiology 51: 543-548.
- Hikosaka, K. 2004. Interspecific difference in the photosynthesisnitrogen relationship: patterns, physiological causes, and ecological importance. Journal of Plant Research 117: 481-494.
- Hikosaka, K. 2005. Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown under different temperature and light conditions: similarities and differences between temperature and light acclimation. Plant and Cell Physiology 46: 1283-1290.
- Holmes, M.G.; Klein, W.H. 1986. Photocontrol of dark circadian rhythms in stomata of Phaseolus vulgaris L. Plant Physiology 82: 28-33.
- Huete, A.R.; Didan, K.; Shimabukuro, Y.E.; Ratana, P.; Saleska, S.R.; Hutyra, L.R.; Yang, W; Nemani, R.R.; Myneni, R. 2006. Amazon rainforests green-up with sunlight in dry season. Geophysical Research Letters 33: 1-4 (L06405. DOI:10.1029/2005GL025583).
- Hughes, L. 2000. Biological consequences of global warming: is the signal already apparent? Trends in Ecology and Evolution 15: 56-61.
- Instituto Nacional de Meteorologia [INMET]. 2008. Climate. Available at: http://www.inmet.gov.br/clima [Accessed Nov. 15, 2008]
- Kaiser, H.; Kappen, L. 2000. In situ observation of stomatal movements and gas exchange of Aegopodium podagraria L. in the understorey. Journal of Experimental Botany 51: 1741-1749.
- Kruger, E.L.; Volin, J.C. 2006. Reexamining the empirical relation between plant growth and leaf photosynthesis. Functional Plant Biology 33: 421-429.
- Kursar, T.A.; Coley, P.D. 1999. Contrasting modes of light acclimation in two species of the rainforest understory. Oecologia 121: 489-498.
- Lee, D.W.; Oberbauer, S.F.; Johnson, P.; Krishnapilay, B.; Mansor, M.; Mohamad, H.; Yap, S.K. 2000. Effects of irradiance and spectral quality on leaf structure and function in seedlings of two Southeast Asian Hopea (Dipterocarpaceae) species. American Journal of Botany 87: 447-455.
- Lewis, S.L.; Malhi, Y.; Phillips, O.L. 2004. Fingerprinting the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society of London 359: 437-462.
- Machado, E.C.; Medina, C.L.; Gomes, M.M.A.; Habermann, G. 2002. Seasonal variation of photosynthetic rates, stomatal conductance and leaf water potential in `Valencia' orange trees. Scientia Agricola 59: 53-58 (in Portuguese, with abstract in English).
- Marenco, R.A.; Siebke, K.; Farquhar, G.D.; Ball, M.C. 2006. Hydraulically based stomatal oscillations and stomatal patchiness in Gossypium hirsutum Functional Plant Biology 33: 1103-1113.
- Marenco, R.A.; Vieira, G. 2005. Specific leaf area and photosynthetic parameters of tree species in the forest understorey as a function of the microsite light environment in Central Amazonia. Journal of Tropical Forest Science 17: 265-278.
- McMillen, G.G.; McClendon, J.H. 1983. Dependence of photosynthetic rates on leaf density thickness in deciduous woody plants grown in sun and shade. Plant Physiology 72: 674-678.
- Molion, L.C.B. 1987. Micrometeorology of an Amazonian rain forest. p. 255-272. In: Dickinson, R.E, ed. The geophysiology of Amazonia: vegetation and climate interactions. John Wiley, New York, NY, USA.
- Nepstad, D.C.; Carvalho, C.R.; Davidson, E.A.; Jipp, P.H.; Lefebvre, P.A.; Negreiros, G.H.; Silva, E.D.; Stone, T.A.; Trumbore, S.E.; Vieira, S. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372: 666-669.
- Nepstad, D.C.; Tohver, I.M.; Ray, D.; Moutinho, P.; Cardinot, G. 2007. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88: 2259-2269.
- Niinemets, U. 1999. Research review components of leaf dry mass per area: thickness and density; alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist 144: 35-47.
- Oguchi, R.; Hikosaka, K.; Hirose, T. 2005. Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant, Cell and Environment 28: 916-927.
- Oyama, M.D.; Nobre, C.A. 2003. A new climate-vegetation equilibrium state for Tropical South America. Geophysical Research Letters 30: 1-4 (DOI:10.1029/2003GL018600).
- Park, S.Y.; Furukawa, A. 1999. Photosynthetic and stomatal responses of two tropical and two temperate trees to atmospheric humidity. Photosynthetica 36: 181-186.
- Peeters, P.J. 2002. Correlations between leaf structural traits and the densities of herbivorous insect guilds. Biological Journal of the Linnean Society 77: 43-65.
- Poorter, L.; Oberbauer, S.F. 1993. Photosynthetic induction responses of two rain-forest tree species in relation to light environment. Oecologia 96: 193-199.
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B. 1998. Leaf structure (specific leaf area) modulates photosynthesis-nitrogen relations: evidence from within and across species and functional groups. Functional Ecology 12: 948-958.
- Saleska, S.R.; Didan, K.; Huete, A.R.; Da Rocha, H.R. 2007. Amazon forests green-up during 2005 drought. Science 318: 612-612.
- Shimazaki, K.I.; Doi, M.; Assmann, S.M., Kinoshita, T. 2007. Light regulation of stomatal movement. Annual Review of Plant Biology 58: 219-247.
- Smith, H. 1982. Light quality, photoperception, and plant strategy. Annual Review of Plant Physiology 33: 481-518.
- Turnbull, M.H. 1991. The effect of light quantity and quality during development on the photosynthetic characteristics of six Australian rainforest tree species. Oecologia 87: 110-117.
- Valladares, F.; Niinemets, U. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology Evolution and Systematics 39: 237-257.
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. 2000. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 211: 807-815.
- Williamson, G.B.; Laurance, W.F.; Oliveira, A.A.; Delamônica, P.; Gascon, C.; Lovejoy, T.E.; Pohl, L. 2000. Amazonian tree mortality during the 1997 El Niño drought. Conservation Biology 14: 1538-1542.
- Wright, I.J.; Cannon, K. 2001. Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Functional Ecology 15: 351-359.
- Yun, J.I.; Taylor, S.E. 1986. Adaptive implications of leaf thickness for sun- and shade-grown Abutilon theophrasti Ecology 67: 1314-1318.
Publication Dates
-
Publication in this collection
23 May 2011 -
Date of issue
Dec 2010
History
-
Received
05 May 2009 -
Accepted
14 June 2010