Abstract
Olive (Olea europaea L.) production in the world has been made by using many cultivars, and the genetic uniformity of commercial cultivars is important for standard olive oil and table olive production. The genetic variation among and within commonly cultivated olive cultivars in Turkey was analyzed using SSR markers. A total of 135 leaf samples were collected from 11 commonly cultivated olive cultivars from 11 provinces in four geographical regions of Turkey. Seven SSR primer pairs generated 46 SSR markers, and the number of SSR markers per primer pair ranged from 4 (UDO-14) to 9 (GAPU-89) with an average of 6.57. This high level of SSR polymorphism suggests that olive production in Turkey has been made using genetically diverse olive cultivars and this high level of genetic variation is probably due to the location of Turkey in the center of the origin of olive. The UPGMA dendrogram, developed to visualize the estimated genetic relationships among the 135 samples, demonstrated that the clustering of olive cultivars was not based on geographical regions of cultivation. Presence of genetic variation was detected within a nationwide grown Turkish olive cultivar, called 'Gemlik'. Olive growers successfully discriminated olive cultivars with distinct morphological and pomological characters. However, there was some confusion about the identification of cultivars with similar phenotypic traits. To prevent misidentification of olive cultivars and to minimize intra-cultivar variation, certified propagation materials which were characterized using DNA based molecular markers should be used during the establishment of new olive orchards.
Olea europaea L.; microsatellite; intra-varietal variation; synonyms; simple sequence repeats
GENETICS AND PLANT BREEDING
Assessment of inter- and intra-cultivar variations in olive using SSR markers
Ahmet Ipek* * Corresponding author < maipek@uludag.edu.tr> ; Erdogan Barut; Hatice Gulen; Meryem Ipek
Uludag University/Faculty of Agriculture Dept. of Horticulture 16059 Görükle, Bursa Turkey
ABSTRACT
Olive (Olea europaea L.) production in the world has been made by using many cultivars, and the genetic uniformity of commercial cultivars is important for standard olive oil and table olive production. The genetic variation among and within commonly cultivated olive cultivars in Turkey was analyzed using SSR markers. A total of 135 leaf samples were collected from 11 commonly cultivated olive cultivars from 11 provinces in four geographical regions of Turkey. Seven SSR primer pairs generated 46 SSR markers, and the number of SSR markers per primer pair ranged from 4 (UDO-14) to 9 (GAPU-89) with an average of 6.57. This high level of SSR polymorphism suggests that olive production in Turkey has been made using genetically diverse olive cultivars and this high level of genetic variation is probably due to the location of Turkey in the center of the origin of olive. The UPGMA dendrogram, developed to visualize the estimated genetic relationships among the 135 samples, demonstrated that the clustering of olive cultivars was not based on geographical regions of cultivation. Presence of genetic variation was detected within a nationwide grown Turkish olive cultivar, called 'Gemlik'. Olive growers successfully discriminated olive cultivars with distinct morphological and pomological characters. However, there was some confusion about the identification of cultivars with similar phenotypic traits. To prevent misidentification of olive cultivars and to minimize intra-cultivar variation, certified propagation materials which were characterized using DNA based molecular markers should be used during the establishment of new olive orchards.
Keywords: Olea europaea L., microsatellite, intra-varietal variation, synonyms, simple sequence repeats
Introduction
Olive (Olea europaea L.) is the only species of the genus, Olea cultivated in the Mediterranean basin, and its domestication goes back to 6,000 years in the area bordering the east coast of the Mediterranean Sea (Sensi et al., 2003). Olive production in the Mediterranean basin accounts for more than 95 % of world's olive production (FAO, 2008). Located on the northeastern coast of the Mediterranean Sea, Turkey is a major olive-producing country. Olives originated from the coast of Eastern Mediterranean Sea (Zohary and Spegiel-Roy, 1975) and, to date, more than 1250 cultivars have been used worldwide for olive production (Bartolini et al., 1997). Most of these cultivars are present in countries located in the Mediterranean basin (Sarri et al., 2006). The presence of 87 local olive cultivars has been documented in Turkey (http://www.zae.gov.tr; last accessed March 15th, 2011).
Cultivar identification of olives has been based on morphological and phenological characteristics (Fabbri et al., 1995). Traditionally, fruit characteristics have been used for the identification of olive cultivars (Besnard et al., 2001). However, it is impossible to use fruit characteristics for seedlings in nurseries or for young trees in orchards due to the juvenility. In addition, fruit characteristics can be easily affected by environmental factors and alternate bearing. Moreover, the continuous interchange of plant materials among the different olive-producing regions and the simultaneous presence of local and patchy-distributed cultivars with ambiguous naming have complicated the identification of olive cultivars in Turkey. Morphological and pomological characteristics of common Turkish olive cultivars were previously determined by Canozer (1991). Genetic variation among some Turkish olive cultivars was analyzed using DNA based molecular markers (Owen et al., 2005; Ozkaya et al., 2006; Ercisli et al., 2009; Ipek et al., 2009).
Standardized olive oil and table olive production for better marketing can be made possible by the identification of superior genotypes which were adapted to the major olive-producing regions. In this respect, analyses of intra- and inter-cultivar variations can be helpful for the determination of standard olive cultivars for each olive-producing region in Turkey. In addition, determination of genetic variation within a cultivar or among olive cultivars can be useful data for future breeding programs. Therefore, the objectives of this study were to determine the genetic uniformity of the commercially important olive cultivars grown in the Aegean, Mediterranean, Southeastern Anatolia and Southwestern Marmara Regions of Turkey and to assess genetic relationship among these olive cultivars using simple sequence repeats (SSR) DNA markers.
Materials and Methods
Plant Materials: During the germplasm acquisition, 135 leaf samples were analyzed, stemming from trees of 11 common olive cultivars in Antalya, Aydın, Balıkesir, Çanakkale, Gaziantep, Hatay, İçel, İzmir, Kilis, Manisa and Muğla provinces from the Aegean, Mediterranean, Southeastern Anatolia and Southwestern Marmara Regions of Turkey (Table 1; Figure 1). The names of the cultivars given by the growers were recorded and the morphological characteristics of the sampled trees were compared with morphological characteristics of major olive cultivars described by Canozer (1991).
Preparation of DNA samples: DNA samples were extracted from 100 mg of lyophilized and powdered leaf samples using a modified CTAB method described by Futterer et al. (1995). The concentration of each DNA sample was measured using a Qubit Fluorometer (Invitrogen, Carlsbad, CA, USA) and adjusted to 20 ng µL1 for further analysis.
SSR analysis: Seven previously developed SSR primer pairs were used for the amplification of SSR markers in this study (Table 2). Each 20 µL polymerase chain reaction (PCR) mixture for the amplification of SSR markers consisted of 1.0 U Taq DNA polymerase (Fermentas, Hanover, MD, USA) with supplied reaction buffer at 1 × concentration, 0.25 µM of each primer, dNTPs at 0.25 mM each, and 50 ng template DNA. The thermal cycling conditions were as follows: 2 min at 94 ºC; 10 cycles of 45 sec at 94 ºC, 1 min at 65 ºC (annealing temperature was reduced 1 ºC after every cycle), and 1 min and 30 sec at 72 ºC; 35 cycles of 45 s at 94 ºC, 1 min at 55 ºC, and 1 min and 30 s at 72 ºC; and a final extension step of 5 min at 72 ºC. An Applied Biosystems Thermal Cycler was used for these reactions. The PCR products were separated in 4 % high-resolution agarose in 1 × Tris-borate (TBE) buffer. The gels were stained with ethidium bromide (0.5 mg mL1) (Sigma, St Louis, MO, USA) and photographed.
Data analysis: Each SSR marker was scored as present (1) or absent (0) because the allelic constitutions of these SSR markers were not known in the studied plant materials. Genetic distance (GD) matrices were calculated using the Nei and Li (1979) coefficient. A UPGMA dendrogram was developed using TREECON for Windows software (Van de Peer and De Wachter, 1994). Data were bootstrapped 2,000 X to test the reliability and robustness of the phenogram. Heterozygosity expected (He) and heterozygosity observed (Ho) were calculated according to the method of Levene (1949) using POPGEN32 software v.1.31 (Yeh et al., 1997).
Results and Discussion
SSR polymorphisms among olive cultivars
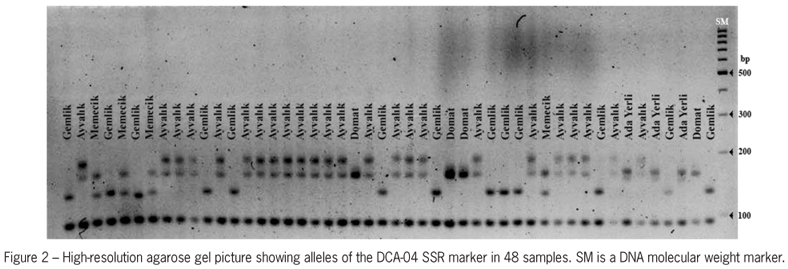
Seven SSR primer pairs generated 46 polymorphic SSR alleles among 135 leaf samples (Table 2; Figure 2). The number of SSR markers per SSR primer pair ranged from 4 (UDO-14) to 9 (GAPU-89) with an average of 6.57. The observed heterozygosity (Ho) was higher than the expected heterozygosity (He) for DCA-11, DCA-16, DCA-17 and GAPU-89, while the Ho was lower than the He for DCA-04, DCA-09 and UDO-14 (Table 2).
SSR markers have been developed for olives by several research groups (Carriero et al., 2002; Cipriani et al., 2002; Sefc et al., 2000), and this marker system was found to be the most reliable, effective and easy-to-use for cultivar identification in olives (Baldoni et al., 2009; Ipek et al., 2009; Muzzalupo et al., 2010; Sarri et al., 2006). In some studies, polyacrylamide gels and DNA analysis systems with fluorescent labeling were used for detecting polymorphic alleles of SSR markers (Baldoni et al., 2009; Carriero et al., 2002; Gomes et al., 2008; Sarri et al., 2006). Recently, Baldoni et al. (2009) selected 11 SSR primer pairs to use with DNA analysis systems with fluorescent labeling for cultivar identification.
While the use of polyacrylamide gel is a labor-intensive task, DNA analysis systems with fluorescent labeling are high-cost analysis systems and may not be available to every investigator. In this study, we selected 7 SSR primer pairs that can be used with high-resolution agarose gel electrophoresis (4 % high-resolution agarose) to detect SSR polymorphisms among olive genotypes. The level of polymorphism detected in this study is comparable to that in previous studies of Cipriani et al. (2002) and Gomes et al. (2008). High-resolution agarose gel to detect SSR polymorphisms in olive can be used in future studies without losing the discriminating power of the SSR marker system. The use of high-resolution agarose gels to detect SSR polymorphisms can decrease cost and labor significantly and it is readily applicable because agarose gel electrophoresis is available to almost all molecular biology laboratories.
Although the amount of polymorphism detected by DNA markers depends on genetic relationships among the analysed genotypes, the high level of SSR polymorphism in olives was reported by this and previous studies (Baldoni et al., 2009; Carriero et al., 2002; Gomes et al., 2008; Ipek et al., 2009; Muzzalupo et al., 2010; Sarri et al., 2006). The high level of polymorphism in the alleles of SSR markers in this study confirms that genetically diverse olive cultivars have been used for olive production in Turkey. The location of Turkey in the center of origin of the olive plant can explain the high level of genetic variation among olive cultivars in this country. This high level of genetic variation can be useful for olive-breeding programs. However, using genetically divers olive cultivars for table olive and olive oil production can pose a problem for standardization because the quality of table olive and olive oil depends largely on the genotype (Sanz-Cortés et al., 2003).
Genetic relationship among olive cultivars
A UPGMA dendrogram demonstrating the estimated genetic relationship among 135 samples was constructed using Nei and Li's (1979) distance matrix with 2,000 X bootstrapping (Figure 3). The highest genetic distance was 84 % between the cultivars 'Ayvalık' and 'Tavşan Yüreği'. According to the dendrogram, there were 22 genotypes among the 135 samples with unique SSR marker profiles because more than one sample were collected for some cultivars to assess intra-cultivar variation (Table 1). A total of seven groups were identified at the average dissimilarity level of 48 % (Figure 3).
Although 'Gemlik' is the most common olive cultivar in the Southern Marmara Region (Ipek et al., 2009), this cultivar has been grown in all provinces where the leaf samples of this study were collected (Table 1; Figure 1). The samples of 'Gemlik' were placed in Group I (Figure 3). Although they were clustered in the same Group, samples, #161 and #66 had one and samples, #19 and #4 had two different SSR alleles from the cultivar 'Gemlik'. Because these samples differed from 'Gemlik' by one or two SSR alleles, the source of this type of variation could be an accumulation of somatic mutations. In another study, Muzzalopo et al. (2010) analyzed intra cultivar variability in three major Italian olive cultivars and found intra cultivar variability in 'Carolea'. Muzzalopo et al. (2010) indicated that the variation among the plants of 'Carolea' was probably due to the somatic mutations occurring in the different branches of mother plants.
Two other 'Gemlik' samples were clustered in a subgroup of the Group I with 'Erdek' and 'Mudanya Karası' (Figure 3). The names, 'Erdek' and 'Mudanya Karası', suggest that these genotypes were obtained from the towns of 'Mudanya' and 'Erdek' in the Southern Marmara Region where 'Gemlik' has been widely grown (Ipek et al., 2009). Similarly, samples #91 and #210 were also obtained from the Southern Marmara Region. The name, 'Gemlik' was probably given to these samples by growers due to the origin of repropagation materials, although they are genetically distinct. In addition, two other genotypes (#22 and #142) collected from the provinces of Izmir and İçel (Table 1) were called 'Gemlik', but the SSR analysis indicated that they were genetically different (Figure 3). This result indicates that genetically different olive genotypes may be called 'Gemlik' in different olive production regions in Turkey probably due to the nationwide popularity of this cultivar. On the other hand, samples, #165 and #211 were called 'Kilis Yağlık' and 'Basmalık', respectively by olive growers although they had an identical SSR marker profile as 'Gemlik' (Figure 3). This result shows that they were also misidentified by growers or nurseries. In a previous study, Ipek et al. (2009) analyzed the variation within the cultivars of 'Gemlik' grown in the Southern Marmara Region and reported that about 8 % of the plants analyzed had different SSR profiles from the cultivar, 'Gemlik'.
Group III of the UPGMA dendrogram was composed of three subgroups (Figure 3). One of the subgroups contained common cultivars, 'Kilis Yağlık', 'Nizip Yağlık' and 'Yağlık' from the Southeastern Anatolia Region (Table 1, Figure 1). In this study, the samples of 'Kilis Yağlık' and 'Nizip Yağlık' were clustered together by sharing 100 % of their SSR markers (Figure 3). These cultivars were misidentified by olive growers in the region because they are morphologically similar in terms of tree and fruit characteristics. Both cultivars form trees with medium vigor and their leaves have same shape and color (000761; lavender green) (Canözer, 1991). They have small fruits with similar shapes. Due to high olive oil content of these cultivars, they are mainly grown for olive oil production in the region and therefore, these cultivars are locally called 'Yağlık' by the growers, because 'Yağlık' means 'for olive oil production' in Turkish. In another study, genetic variation among Turkish olive cultivars at Olive Research Institute in Izmir was analyzed using AFLP markers (Owen et al., 2005). In that study, however, 'Kilis Yağlık' and 'Nizip Yağlık' were found to be different cultivars and the genetic relationship was quite low (< 0.75) although these cultivars have similar phenotypic traits.
The second subgroup of the Group III contained another popular olive cultivar, 'Sarı Haşebi', grown in the province of Hatay in the Eastern Mediterranean Region of Turkey (Canozer, 1991; Table 1; Figures 1; 3). This cultivar is also called 'Haşebi' locally. Although another sample was identified as 'Sarı Haşebi' in the region, it had an identical SSR marker profile to 'Nizip Yağlık', 'Kilis Yağlık' and 'Yağlık' cultivars in another cluster. This genotype was also misidentified by olive growers as 'Sarı Haşebi'. 'Karamani' is another common cultivar grown in the same region, and two samples of this cultivar were placed in another cluster in the same Group (Figure 3).
The third subgroup in Group III contained a common olive cultivar called 'Memecik', grown for both olive oil and table olive production in the Aegean Region of Turkey (Canozer 1991; Table 1; Figure 1). Samples of 'Memecik' were clustered together by sharing identical SSR marker profiles (Figure 3). Three other genotypes were collected as 'Memecik' in the same region, but they had different SSR marker profiles. Another genotype, called 'Çöpaşı', shared the same SSR marker profile with 'Memecik', suggesting that this genotype was probably 'Memecik'.
'Sarı Ulak' and 'Domat' are two other standard Turkish olive cultivars grown for table olive production (Canozer, 1991). While 'Sarı Ulak' is a common cultivar of the provinces in the Eastern Mediterranean Region, 'Domat' is largely grown in the Aegean Region of Turkey (Figure 1). In this study, samples of 'Domat' were collected from different locations in the Manisa province in the Aegean Region, and samples of 'Sarı Ulak' were obtained from the İçel province in the Eastern Mediterranean Region (Table 1). Samples of both 'Sarı Ulak' and 'Domat' were clustered together in Group IV and shared identical SSR marker profiles in this study (Figure 3). In a previous study, Owen et al. (2005) analyzed genetic relationship among Turkish olive cultivars kept in Olive Research Institute in İzmir using AFLP markers and they found that 'Sarı Ulak' and 'Domat' were distinctly related with low genetic similarity. On the other hand, they shared identical SSR markers in this study suggesting that there is confusion about the identification of these cultivars by olive growers.
'Ayvalık' (Edremit Yağlık) is one of the most common olive cultivars grown for olive oil production in the Aegean Region of Turkey (Canozer, 1991). In this study, 31 samples from this cultivar were collected from Manisa, İzmir, Balıkesir and Çanakkale provinces (Table 1). All samples of 'Ayvalık' were clustered together in Group V by sharing 100 % of SSR makers, which suggested that there was no variation within this cultivar. Three other samples were given different names (Trilye, Gemlik), but these samples had identical SSR marker profiles as 'Ayvalık', indicating that some plants of this cultivar were also misidentified by olive growers (Figure 3).
'Tavşan Yüreği' is another local olive cultivar in the Western Mediterranean Region of Turkey. Two samples of this cultivar were collected from the province of Antalya (Table 1; Figure 1), and these samples were clustered in Group VI and had identical SSR profiles (Figure 3). In another study, Ozkaya et al. (2009) found the cultivars, 'Tavşan Yüreği' and 'Memecik' as being genetically the same, by sharing 100 % of their RAPD markers. 'Tavşan Yüreği' and 'Memecik' were found by Owen et al. (2005) to be genetically distinct cultivars with low genetic similarity as in our current study (Figure 3). However, Ozkaya et al. (2009) did not comment about these genetically and phenotypically distinct olive cultivars that had identical RAPD profiles in their study.
'Uslu' is an important olive cultivar grown for table olive production in the Aegean Region of Turkey. Four leaf samples of this cultivar were collected in different locations in the province of Manisa, and for this region all samples were placed in a subgroup of Group VII and shared 100 % of their SSR markers (Table 1; Figure 3). 'Ada Yerli' is a local olive cultivar grown on Gökçeada Island in the Aegean Sea, and three samples of this cultivar were collected, which were clustered together in the other subgroup of Group VII by sharing identical SSR marker profile.
Turkish olive cultivars were not clustered based on their locations of cultivation. For example, 'Memecik' from the Aegean Region was closely clustered with the olive cultivars from the Southeastern Anatolia Region in Group III (Figure 3). Similar results have been reported in other studies where olive genotypes from different countries were clustered closely in a group, suggesting that the grouping of olive genotypes was not based on geographical origin (Besnard et al., 2001; Ipek et al., 2009). Owen et al. (2005) were able to place olive cultivars from Middle East and Turkey to one broad group and olive cultivars from Greece to another broad group although there was no clear separation between Turkish and Greek cultivars. On the other hand, Sarri et al. (2006) found limited grouping on the basis of geographic origin and grouped olive cultivars as eastern, central and western Mediterranean populations. These results suggest that olive genotypes have been freely exchanged among growers within a country or among collectors in different countries for centuries without proper passport information.
In conclusion, genetic variation among the cultivated olive cultivars in Turkey is high. This high genetic variation can be useful for clonal selection of superior olive genotypes or for olive cross-breeding programs. Intra-cultivar variation was observed in 'Gemlik' but there was no SSR polymorphism within other cultivars, suggesting that they are of monoclonal origin. Olive production in Turkey has been made using genetically diverse olive cultivars, which can be a problem for standardized table olive and olive oil production because the quality of table olives and olive oil is significantly affected by genotype. Olive genotypes could have different names in different production regions and that the name of a popular olive cultivar is given to genetically different olive genotypes in different geographical regions of Turkey. Although cultivar identification by growers or nurseries in Turkey was correct for most of the samples, there was some confusion about the discrimination of olive cultivars with similar phenotypic traits. Therefore, certified repropagation materials characterized with DNA-based molecular markers should be used during the establishment of new olive orchards.
Acknowledgements
To MARMARABIRLIK (Agricultural Sales Cooperatives and Associations) for their financial support.
Received June 17, 2011
Accepted January 10, 2012
Edited by: Antonio Costa de Oliveira
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G.; Buonamici, A.; Porceddu, A.; Sarri, V.; Ojeda, M.A.; Trujillo, I.; Rallo, L.; Belaj, A.; Perri, E.; Salimonti, A.; Muzzalupo, I.; Casagrande, A.; Lain, O.; Messina, R.; Testolin, R. 2009. A consensus list of microsatellite markers for olive genotyping. Molecular Breeding 24: 213231.
- Bartolini, G.; Prevost, G.; Messeri, C.; Carignani, G. 1997. Olive germplasm: cultivars and world-wide collections. FAO. Available at: http://apps3.fao.org/wiews/olive/oliv.jsp [Accessed Apr. 27, 2010]
- Besnard, G.; Breton, C.; Baradat, P.; Khadari, B.; Bervillé, A. 2001. Cultivar identification in olive based on RAPD markers. Journal of the American Society for Horticultural Science 126: 668675.
- Canözer, Ö. 1991. Catalog of Standard Olive Cultivars. Ministry of Turkish Agriculture and Rural Affairs, Ankara, Turkey. (Publication of the Ministry of Turkish Agriculture and Rural Affairs, 16).
- Carriero, F.; Fontanazza, G.; Cellini, F.; Giorio, G. 2002. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theoretical and Applied Genetics 104: 301307.
- Cipriani, G.; Marrazzo, M.T.; Marconi, R.; Cimato, A.; Testolin, R. 2002. Microsatellite markers isolated in olive (Olea europaea L.) are suitable for individual fingerprinting and reveal polymorphism within ancient cultivars. Theoretical and Applied Genetics 104: 223228.
- Ercisli, S.; Barut, E.; Ipek, A. 2009. Molecular characterization of olive cultivars using amplified fragment length polymorphism markers. Genetics and Molecular Research 8: 414419.
- Fabbri, A.; Hormaza, J.I.; Polito, V.S. 1995. Random amplified polymorphic DNA analysis of olive (Olea europaea L) cultivars. Journal of the American Society for Horticultural Science 120: 538542.
- Food and Agriculture Organization [FAO]. 2008. Agricultural Statistics of the Food and Agriculture Organization of the United Nations. Available at: http://www.FAO.org [Accessed Feb. 07, 2011]
- Futterer, J.; Gisel, A.; Iglesias, V.; Kloti, A.; Kost, B.; Mittelsten-Scheid, O.; Neuhaus, G.; Neuhaus-Url, G.; Schrott, M.; Shillito, R.; Spangenberg, G.; Wang, Z.Y. 1995. Standard molecular techniques for the analysis of transgenic plants. p. 215218. In: Potrykus, I.; Spangenberg, G., eds. Gene transfer to plants. Springer-Verlag, New York, USA.
- Gomes, S.; Martins-Lopes, P.; Lima-Brito, J.; Meirinhos, J.; Lopes, J.; Martins, A.; Guedes-Pinto, H. 2008. Evidence for clonal variation in 'Verdeal-Transmontana' olive using RAPD, ISSR and SSR markers. Journal of Horticultural Science and Biotechnology 83: 395400.
- Ipek, A.; Barut, E.; Gulen, H.; Oz, A.T.; Tangu, N.A.; Ipek, M. 2009. SSR analysis demonstrates that olive production in the southern Marmara region in Turkey uses a single genotype. Genetics and Molecular Research 8: 12641272.
- Levene, H. 1949. On a matching problem arising in genetics. Annals of Mathematical Statatistics 20: 9194.
- Muzzalupo, I.; Chiappettac, A.; Benincasaa, C.; Perri, E. 2010. Intra-cultivar variability of three major olive cultivars grown in different areas of central-southern Italy and studied using microsatellite markers. Scientia Horticulturae 126: 324329.
- Nei, M.; Li, W.H. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences of the United States of America 76: 52695273.
- Owen, C.A.; Bita, E-C.; Banilas, G.; Hajjar, S.E.; Sellianakis, V.; Aksoy, U.; Hepaksoy, S.; Chamoun, R.; Talhook, S.N.; Metzidakis, I.; Hatzopoulos, P.; Kalaitzis, P. 2005. AFLP reveals structural details of genetic diversity within cultivated olive germplasm from the Eastern Mediterranean. Theoretical and Applied Genetics 110: 11691176.
- Ozkaya, M.T.; Cakir, E.; Gokbayrak, Z.; Ercan, H.; Taksin, N. 2006. Morphological and molecular characterization of Derik Halhali olive (Olea europaea L.) accessions grown in DerikMardin province of Turkey. Scientia Horticulturae 108: 205209.
- Ozkaya, M.T.; Ergulen, E.; Ulger, S.; Ozilbey, N. 2009. Molecular Characterization of Some Selected Wild Olive (Olea oleaster L.) Ecotypes Grown in Turkey. Journal of Agricultural Sciences 15: 1419.
- Sanz-Corte's, F.; Parfitt, D.E.; Romero, C.; Struss, D.; Lla'cer, G.; Badenes, M.L. 2003. Intraspecific olive diversity assessed with AFLP. Plant Breeding 122: 173177.
- Sarri, V.; Baldoni, L.; Porceddu, A.; Cultrera, N.G.M.; Contento, A.; Frediani, M.; Belaj, A.; Trujillo, I.; Cionini, P.G. 2006. Microsatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically defined populations. Genome 49: 16061615.
- Sefc, K.M.; Lopes, S.; Mendonça, D.; Dos Santos, M.R.; Machado, M.L.D.; Machado, A.D. 2000. Identification of microsatellite loci in olive (Olea europaea) and their characterization in Italian and Iberian olive trees. Molecular Ecology 9: 11711173.
- Sensi, E.; Vignani, R.; Scali, M.; Masi, E.; Cresti, M. 2003. DNA fingerprinting and genetic relatedness among cultivated varieties of Olea europaea L estimated by AFLP analysis. Scientia Horticulturae 97: 379388.
- Van de Peer, Y.; De Wachter, R. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Computer Applications in the Biosciences 10: 569570
- Yeh, F.C.; Yang, R.C.; Boyle, T.B.J.; Ye, Z.H.; Mao, J.X. 1997. POPGENE the user-friendly shareware for population genetic analysis molecular biology and biotechnology Available at: http://wwwualbertaca/~fyeh/ [Accessed Apr. 27, 2010]
- Zohary, M.; Spegiel-Roy, P. 1975. Beginnings of fruit growing in the old world. Science 187: 319327.
Publication Dates
-
Publication in this collection
28 Sept 2012 -
Date of issue
Oct 2012
History
-
Received
17 June 2011 -
Accepted
10 Jan 2012