Abstract
The freshwater phase of Atlantic salmon Salmo salar L vary between one and eight years. The reduction of the freshwater phase is desirable to reduce freshwater usage, human resources and to increase year round availability of pan-sized salmon. Three trials were conducted to investigate the possibility of supply the market in a year-round basis, with pan-sized Atlantic salmon Salmo salar L. (250-300 g) in Portugal (southern limit of the natural distribution of this species). This study primarily aimed to compare the osmoregulatory ability and growth of different fish sizes, smolts 1+ (trial 1), 1.5+ (trial 2) and 2+ (trial 3), in freshwater and seawater conditions. Additionally, effects of photoperiod were determined in smolts 1.5+ (trial 2) for both freshwater and seawater groups. The increments in the plasma osmolality and chlorine concentrations after seawater transfer suggest an identical development in the hypo-osmoregulation capacity among the different age classes. In all trials, weight gain was smaller after 30 d of saltwater transfer when compared to fish reared in freshwater. However, the growth depression was temporary. Seawater group showed a compensatory growth in the immediate months, which permitted an improvement in growth rates. At the end of trials there were minor differences on growth performance between freshwater and seawater groups. Specific growth rates varied between 0.7 and 1.0 % day-1, according to the age and /or size and transfer season.
Atlantic salmon; post-smolts development; smoltification; seawater transfer
ANIMAL SCIENCE AND PASTURES
Growth and osmoregulation in Salmo salar L. juveniles 1+, 11/2 + and 2+ reared under restrained salinity
José Fernando Magalhães GonçalvesI, II; Stela CarraçaI; Alfredo Damasceno-OliveiraII; Córalia VicenteI; Paulo Martins da CostaI, II; Manuel Lopes-LimaI; Rodrigo Otávio de Almeida OzórioII, * * Corresponding author < rodrigo.ozorio@ciimar.up.pt>
IUniversidade do Porto/ICBAS - Instituto de Ciências Biomédicas de Abel Salazar, Lg. Prof. Abel Salazar 2 - 4099-003 - Porto - Portugal
IIUniversidade do Porto/CIMAR/CIIMAR - Centro Interdisciplinar de Investigação Marinha e Ambiental, R. dos Bragas 289 - 4050-123 - Porto - Portugal
ABSTRACT
The freshwater phase of Atlantic salmon Salmo salar L vary between one and eight years. The reduction of the freshwater phase is desirable to reduce freshwater usage, human resources and to increase year round availability of pan-sized salmon. Three trials were conducted to investigate the possibility of supply the market in a year-round basis, with pan-sized Atlantic salmon Salmo salar L. (250-300 g) in Portugal (southern limit of the natural distribution of this species). This study primarily aimed to compare the osmoregulatory ability and growth of different fish sizes, smolts 1+ (trial 1), 1.5+ (trial 2) and 2+ (trial 3), in freshwater and seawater conditions. Additionally, effects of photoperiod were determined in smolts 1.5+ (trial 2) for both freshwater and seawater groups. The increments in the plasma osmolality and chlorine concentrations after seawater transfer suggest an identical development in the hypo-osmoregulation capacity among the different age classes. In all trials, weight gain was smaller after 30 d of saltwater transfer when compared to fish reared in freshwater. However, the growth depression was temporary. Seawater group showed a compensatory growth in the immediate months, which permitted an improvement in growth rates. At the end of trials there were minor differences on growth performance between freshwater and seawater groups. Specific growth rates varied between 0.7 and 1.0 % day-1, according to the age and /or size and transfer season.
Keywords: Atlantic salmon, post-smolts development, smoltification, seawater transfer
Introduction
The worldwide production of farmed Atlantic salmon Salmo salar L. has increased steadily over the past two decades. This expansion in output capacity has resulted in a great opportunity for producers to acquire new innovations and modify technologies with the aim of enhancing their productivity and reducing costs, while maintaining product safety and quality. Particular attention has been given to the possibility of reducing the period of the freshwater phase by using out-of-season smolts, to reduce water, equipment and human resources consumption and to increase year round availability (Fitzgerald et al., 2002). In addition, for obvious economic reasons, management operations have striven for minimum mortality and rapid increase in growth rate when smolts are transferred to seawater (Staurnes et al., 2001).
Fish growth usually increases proportionally to the increase of water temperature (Brett, 1979; Austreng et al., 1987), until an optimum growth performance is reached. Moreover, water temperature has a strong influence on acclimation to seawater and in subsequent fish performance (Solbakken et al., 1994; Stefansson et al., 1998; Handeland et al., 2008).
Portugal represents the southern limit of Atlantic salmon distribution in Europe, presenting high oceanic water temperatures (above 18 °C) at the warmest seasons of the year, which severely challenges the osmo-ionoregulatory mechanisms and growth performance of salmonids (Gonçalves et al., 2006). Mortality has been seen to increase in these environmental conditions and farming results are negatively affected, both biologically and economically. As a consequence, a new (and different from traditional) production system must be established in order to operate commercially in this geographical area. The choice was to decrease salinity, an effective process to maintain the osmo-ionoregulatory homeostasis in fish during long periods at adverse temperature (Handeland et al., 1998; Myoung et al., 1999; Gonçalves et al., 2006).
This study aimed to evaluate the growth and hypo-osmoregulatory ability of Atlantic salmon Salmo salar L. smolts after being transferred to seawater (28 g L-1) at different times. In addition, the results were compared to data obtained from control groups held in freshwater to investigate the possibility of supplying the market in a year-round basis, with pan-sized salmon (250-300 g). The trials were complemented with analyses of morpho-physiological parameters that have been used in previous studies, either on the parr-smolt transformation or in the subsequent periods after seawater transition. These parameters are indicators of the viability of different types of smolts herein studied and, therefore, of the capacity of assuring a continuous production.
Materials and Methods
Rearing conditions
Three successive trials were carried out using Atlantic salmon siblings, Mowi strain. In the first trial, smolts with age 1+ were used, followed by a second trial with out-of-season smolts (age 1.5+) and, finally, a third trial with smolts 2+. In all trials we used fish kept continuously in freshwater as control groups. Smolts were obtained from the same stock of eyed eggs, from a commercial culture in a common environment under conditions standardized for this species. Smolt status were evaluated by seawater challenge tests and fish used in the trials presented at least 90 % survival during 96 h (Saunders et al., 1985). Apart from the production of out-of-season smolts (pre-stage of trial 2), when light was manipulated, natural photoperiod and temperature were used in all the trials (Figure 1). The trials were carried out in a commercial fish farm on the western coast of Portugal (41°25'0" N, 8°46'0" W).
Trials were conducted in open circuit (water flow of 0.5 L s-1) and fish were fed commercial diets (4-6mm size; 42-44 % crude protein; 12-14 % crude fat; 18-20 MJ kg-1 gross energy, in quantities recommended by the manufacturer by automatic feeders. Good quality seawater and freshwater entered the fish farm, as nutrient and solids concentrations were within the values observed for unpolluted waters (nitrate levels: summer maxima of 0.54 ± 0.01 mg L-1, pH varied between 7.8 and 8.1, oxygen levels varied between 9.4 and 13.1 mg L-1). For each trial, a randomly collected sub-population of about half of the fish in each tank was individually tagged with visible implants (Northwest Marine Technology) for the calculation of growth performances. This study has been conducted in accordance with the Portuguese laws and regulations controlling experimental animals.
Trial 1 (smolts 1+)
In May, 600 smolts 1+ (70 to 115 g) were randomly selected, partially marked and equally distributed by four tanks (4 m3) in two experimental groups (two replicates per group). During the following 120 d one of the groups was subjected to a growth trial in seawater at 28 g L-1 (sample weigh = 89.5 ± 1.0 g), while the other group remained in freshwater (sample weigh = 83.1 ± 0.8 g). The transfer from freshwater to the different qualities of seawater was obtained in 48 h by changing the water supply into the tanks, which produced stable experimental conditions within 1 h with an increase rate in salinity of approximately 0.5-0.6 g L-1.
Trial 2 (smolts 1.5+)
This trial had a total duration of 90 d. It was preceded by a preliminary stage of manipulation with a total of 120 d required to produce out-of-season smolts. In Sep., 800 smolts 1.5+ were divided randomly into four tanks with 4 m3, each. All tanks were isolated with a light proof plastic cover and light was provided by a fluorescent daylight tube mounted under each tank cover (200 lux in the bottom of the tanks). During the preliminary stage, two tanks (sub sample weigh = 83.9 ± 0.7 g; n = 50) were submitted to a natural photoperiod regime (42 °N) and were designated as groups LN A (n = 193) and LN B (n = 192). The other two tanks (sub sample weigh = 75.7 ± 0.9 g; n = 50) were submitted to a compressed winter/spring photoperiod and were designated as groups LD A (n = 191) and LD B (n = 193), beginning with 8 h of light and sixteen of darkness during 60 d (L8:D16), followed by a reversion of photoperiod of 16 h of light and 8 h of darkness (L16:D8) for the remaining 60 d.
In Jan., 120 days after photoperiod manipulation, growth was evaluated and half of the fish of each test were tagged and redistributed randomly in four 4 m3 tanks, two with freshwater and two with seawater at 28 g L-1. Fish in the upper and lower limits of the weight distribution were removed in order to eliminate the differences between tanks in the mean weight of fish. The fish group exposed to compressed winter/spring photoperiod was then submitted to a gradual increase in salinity through 48 h (transition at approximately 0.5-0.6 g L-1 per hour) until a final salinity of 28 g L-1, in order to start the trial 2.
Trial 3 (smolts 2+)
In May, 600 smolts 2+ (90 to 160 g) were randomly selected, partially marked and equally distributed in two experimental groups (132.4 ± 1.7 g for freshwater and 129.2 ± 1.2 g for seawater) according to the same procedures as in trial 1.
Sampling procedure and statistical analysis
The condition factor (CF) was calculated as 100 × W / L3, where W is the weight of each fish (30 fish tank-1, g) and L is the corresponding fork length (cm). The specific growth rate (SGR; % Wt day-1) was calculated as [(ln W2 - ln W1) / Δt] × 100, where W1 and W2 are the initial and final weights between sampling and Δt is the trial duration (days) between samplings. At the end of the trials, all the tagged fish were identified and included in the final sampling.
Blood samples were randomly collected from 8 to 12 selected fish for each group at monthly intervals. All fish were starved 24 h prior to sampling and were lightly anesthetised after capture with ethylene-glycol-monophenil-ether, 0.4 mL L-1. Blood was collected by caudal puncture with lithium-heparin coated syringes. Plasma was immediately separated by centrifugation and stored at -80 ºC until subsequent analysis. Plasma osmotic pressure (mOsm kg-1) was determined with an osmometer and plasma chloride concentration (mmol L-1) was assayed with a chloride titrator.
In trial 2, smoltification was monitored in the most important phases of the photoperiod manipulation, by the measurement of gill Na+, K+-ATPase activity. Gill filaments were isolated from branchial arches and washed in sucrose (0.25 M, pH 7.4) to remove the excess of blood, weighted and quickly frozen in liquid nitrogen and maintained at -80 ºC until further analysis according to the method of Lasserre et al. (1978).
Values in figures and tables are means ± S.E. Differences were significant if p < 0.05. One-way analysis of variance (ANOVA) was performed, followed by Scheffé's multiple comparison test where assumptions of normality and homogeneity were satisfied (Zar, 1996). Mortality was analysed by the method of Kaplan and Meier (1958) using 1 or 0 as the fish survived or died, respectively, between the time intervals, followed by the generic test of Wi1coxon (Gehan, 1965).
Results
In all trials and for all groups the highest mortality arises during the first month (Table 1). In fish transferred to seawater, the mortality increased and reached maximum values of 12.1 and 14.2 % for trials 1 and 3.
Trial 1 (smolts 1+)
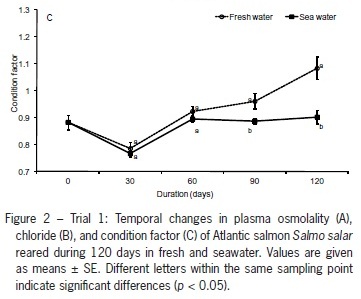
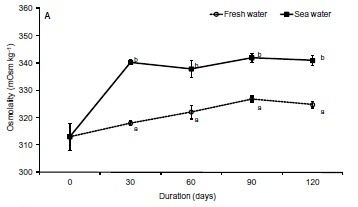
Plasma osmolality values were similar both in freshwater and seawater (p > 0.05) over the first 60 d. In contrast, there were differences (p < 0.001) in the last two sampling points (days 90 and 120), with the maximum values being registered in the seawater group (Figure 2A). Except for day 30, where plasma chloride levels were identical in both experimental groups, this parameter was affected by seawater exposure, with values 3 to 8 % higher and (p < 0.001) comparatively to the freshwater group (Figure 2B).
The condition factor (CF) presents an initial value of 0.883 ± 0.037 and changes similarly in both groups, with a decrease in the first 30 d and a consistent increase from this point onwards. At the end of trial 1, the group maintained in freshwater presents a value 20 % higher (p < 0.001) than the group transferred to seawater (Figure 2C). Both groups (freshwater and seawater) consistently increase the individual weight during the 120 days of the study. Differences (p < 0.001) in growth were only observed at day 30, with fish kept in freshwater being 8.5 % heavier. After this sampling point, fish in seawater presented higher SGR (p < 0.05) than the group in freshwater (Table 2). The weight increase for both freshwater and seawater groups was always higher (p < 0.05) in trial 1, comparatively to trials 2 and 3 (Figure 3A and B ).
Trial 2 (smolts 1.5+)
During the production of out-of-season smolts, differences (p < 0.05) were observed in plasma osmolality, chloride levels and CF within each photoperiod regime, LN or LD (Figure 4A, B and C ). These parameters differ (p < 0.001) between both groups, either in the freshwater phase or after seawater transfer of the LD group. During the freshwater phase, differences in plasma osmolality were registered (p < 0.01) between the two groups (LN and LD) at the time of photoperiod reversal (day 60), whereas prior and after that reversal, this parameter was similar for both groups. After seawater transfer (beginning of trial 2) this parameter and the plasma chloride concentration were always higher (p < 0.001) in fish kept in seawater.
The CF increases similarly for both groups during the first 60 days of the preliminary stage. After the reversal of photoperiod, the CF of the LD group decreases consistently, presenting lower values in the beginning of trial 2 (p < 0.001) compared to the LN group (Figure 4C ). The group that was transferred to seawater presents no further changes in the CF, whereas in the freshwater control group the CF decreased sharply, presenting a lower value at the end of the trial (p < 0.001) compared to the observed in the seawater fish.
After photoperiod reversal, in the production of out-of-season smolts, the gill Na+, K+-ATPase activity in the LD groups increased progressively, with a peak in Jan. (14.6 ± 0.7 µmol Pi mg-1 protein h-1) and just before the seawater transfer. In contrast, the LN group remains with a constant and lower (p < 0.001) activity value (Figure 5). Also, in this preliminary stage (first 60 d), LN fish presented higher mean weight (p < 0.001) comparing to fish on short-day photoperiod. Two months after the photoperiod reversal, fish on the LD group presented a higher (p < 0.001) mean weight when compared with the LN fish (Table 3). Growth during the second phase of the trial differs between groups (p < 0.001). However, at the end of the trial period, the group kept in freshwater had greater weight gain although the final average weights were similar for both salinity regimes (Table 2).
Trial 3 (smolts 2+)
Throughout trial 3, differences in plasmatic osmolality, chloride and CF were observed for both freshwater and seawater groups (Figure 6A, B and C ). In all sampling points the levels of the plasmatic osmolality and chloride were always higher (p < 0.05) in the group transferred to seawater. At the end of trial 3, fish kept in freshwater showed a CF value (0.98 ± 0.02) about 10 % higher (p < 0.001) compared to fish transferred to seawater, as observed in trial 1. Both experimental groups presented an overall increase in growth during the 120 days of the study. However, the weight gain increment was lower (p < 0.05) when compared with trials 1 and 2 (Figure 3A and B ). Fish maintained in freshwater showed enhanced growth performance compared to those kept in seawater in all the sampling points (Table 2), although the differences were not always significant.
Discussion
Hydro-mineral balance
Many salmonid species have disturbances in the hidro-mineral balance during the smoltification period when kept in freshwater (Wedemeyer et al., 1980; McLeese et al., 1994; Björnsson et al., 2011). Those alterations include decreases in the plasma osmolality (Kubo, 1955) and in the plasma and tissue chloride levels (Fontaine, 1951; Houston and Threadgold, 1963). The increase in plasma osmolality and chlorine concentration after seawater transfer suggests an identical development of the osmoregulatory capacity in all the age classes used in this study and has been described before (Blackburn and Clarke, 1987; Stagg et al., 1989; Handeland et al., 2003). However, if the osmoregulatory capacity is well developed, in a few days those parameters strongly decline, stabilizing at levels slightly higher than those found in fish kept in freshwater (Stagg et al., 1989; Usher et al., 1991). Handeland et al. (1998) demonstrated that chlorine plasmatic concentration of fish maintained in seawater is approximately 8 % to 12 % higher than in fish kept in freshwater. The overall results in the present study are in agreement with this observation.
During the first 30 d of trials 1 and 3 all the groups showed a reduction in the condition factor (CF). This was an effect of smoltification in trial 1 and of seawater transfer in trial 3, with the CF values being in agreement with the values previously cited (Stefansson et al., 1991; Solbakken et al., 1994; Duncan et al., 1998). During the preliminaty stage of trial 2, CF in fish submitted to compressed (winter/spring) photoperiod showed an initial increase similar to fish submitted to natural photoperiod. However, when the amount of daily light started to increase (photoperiod reversal), CF decreased constantly until the end of the preliminary stage. During trial 2, the fish showed a similar profile of CF evolution as fish in trial 1 and 3. These results are in agreement with those of previous studies in which similar photoperiod regimes were used (Stefansson et al., 1991; Solbakken et al., 1994).
The Na+, K+-ATPase results (14.6 µmol Pi mg-1 protein h-1) obtained in the preliminary stage of trial 2, agrees with previous studies (Gaignon and Quémener, 1992; Sigholt et al., 1998). It is coherent when compared to values observed in smolts of the upper modal group, which present a good acclimation capacity to high salinity in the normal transfer season (Gaignon and Quémener, 1992; Reis-Henriques et al., 1996).
Growth
In trial 2 (age 1.5+) growth differences were observed during the preliminary stage. These results are supported by previous studies on the seasonal modifications related to the photoperiod cycle. Light regimes with two months of short days reduced fish growth (Skilbrei et al., 1997; Duncan and Bromage, 1998), while continuous illumination or extensive day-length increased growth (Saunders et al., 1985; McCormick et al., 1987; Solbakken et al., 1994; Sigholt et al., 1998). The overall decline in growth performance of fish kept under reduced photoperiod is however compensated in the following months after the photoperiod were reversed. Moreover, Hansen et al. (1992) demonstrated that fish submitted to continuous light has double growth indexes comparing to fish maintained under natural photoperiod. Although continuous light was not used during trial 2, a high growth increment was observed in fish submitted to long days after photoperiod reversion, with growth rates 30 to 70 % higher than fish on natural photoperiod (LN). Studies carried out in cages (Krakenes et al., 1991; Noble et al., 2008) and in tanks (Bergheim and Forsberg, 1993) showed that prolonging the feeding hours from a natural photoperiod to a continuous light did not affect the daily ingestion rates in Atlantic salmon. Since in the present study, fish were always fed in a regime corresponding to the same interval as in the trial with less illumination, and water temperature was continuously decreasing until Jan. (Figure 1), we may conclude that the photoperiod manipulation is a simple and effective method to increase growth even at southern latitudes with relative high temperatures.
Except for trial 3 with smolts 2+, differences in growth between freshwater and seawater groups were not detected, which is in accordance with results from previous studies (Duston, 1994; Jorgensen and Jobling, 1994; Damsgard and Arnesen, 1998). Comparing the results for all trials, it was observed that for both freshwater and seawater groups, the smolts 1+ had higher weight increase, followed by smolts 1.5+ and 2+. Smolts 1+ were from an upper modal class and in Atlantic salmon, fish that belong to this class usually present a higher growth and metabolism (Thorpe et al., 1980; Skilbrei and Hansen, 2004). Furthermore, by comparing the growth in freshwater between all experimental groups it is clear that fish in trial 2 took advantage of a thermal positive differential (5-6 °C).
The length of initial growth depression observed in the present study agrees with Jorgensen and Jobling (1994), that described a reduction in food ingestion and growth in fish during the first 14 d after seawater transfer, but after one month these parameters resumed the levels observed in freshwater. Other authors have observed growth depression during one week (Damsgard and Arnesen, 1998) to over a month (Usher et al., 1991; Handeland et al., 2003; Alne et al., 2011), largely exceeding the critical osmo-regulation period. All these differences reflect the specific characteristics of each trial and their multiple interactive variables. Arnesen et al. (1998) demonstrated in Atlantic salmon a strong correlation between seawater transition and temperature, on food ingestion and growth performance. The salinity reduction imposed in the present study clearly contributed for the positive performance of the fish, otherwise average seawater temperatures nearly 18 °C, in the first month after transfer, would certainly induced osmotic disturbances with negative reflexes in the growth and mortality, as demonstrated by Handeland et al. (2000) and Gonçalves et al. (2006).
Galbreath and Thorgaard (1995) working with S. salar of 112 g, obtained a specific growth rate of 0.52 % day-1 after 376 d. Duncan et al. (1998) working with 0+, 1+ and natural smolts transferred to seawater in Nov., Dec. and Apr., respectively, obtained specific growth rate between 0.9 % and 1 % day-1 after 12 months of transfer in fish held at the highest temperatures (up to 14 °C). Imsland et al. (2011) also reported similar values. Handeland et al. (1998) working with 65 g salmons, showed that the biomass was duplicated in three months at 8 °C. It was also reported by these authors that the specific growth rate during the first 23 days was reduced comparing to the values observed at the end of the second and third month, similarly to the present study. Therefore, if we exclude the poor weight gain during the first month in both salinity conditions, we can conclude that the growth performances obtained herein may be considered as satisfactory. Indeed, Handeland et al. (2000) demonstrated the specific growth rate is poor (0.18 % day-1) in salmon reared at above 18 ºC and at full salinity. The growth rates reported by Austreng et al. (1987) grossly exceeds the ones reported in the present study, but they used the best results from multiple trials, whereas Handeland et al. (2008) reported at 6, 10, 14 and 18 °C growth rates of 0.78, 1.35, 1.53 and 1.29 % day-1, with a different composition of recruited fish (smolts 0+). Although our trials were performed at lower temperatures, it was concluded that the reduction of salinity was central for the outcome results. The fact that all fish were submitted to frequent manipulations which could have had an acute or chronic stress effect (Pickering, 1993) only reinforces this conclusion.
The high mortality during the first month after transfer to seawater observed in the present study reflects the difficulty of fish in maintaining the osmotic balance, but may also be an effect of excessive manipulation during the tagging procedure. In fact, the fish lots kept in freshwater also presented the highest mortality during the same period. The highest mortalities observed in trial 1 and 3 in the groups transferred to seawater are the consequence of high water temperature during the transition period. Furthermore, previous studies also reported higher mortality in S. salar juveniles associated with off-season transfers (Thrush et al., 1994; Sigholt et al., 1995). In the present study, and similarly to the results of Duncan et al. (1998), it was observed that the 1.5+ group transferred in Jan. had better survival rate. This suggests that the compressed photoperiod was effective in the parr-smolt transformation and that oceanic water temperatures in the north Iberian coastal zone did not limit performance.
In conclusion, it can be estimated a rearing period of three to four months after seawater transfer to produce smolts 1.5+ or 1+ of commercial pan-sized salmon (about 300 g). If smolts 2+ are used, the growth rates are always lower than 0.7 % and this implicates that four months are necessary to reach the commercial size, although smolts 2+ generally have twofold the mean weight of smolts 1+. This period could be very important for marketing reasons as three months is the minimum residence time in seawater for fish to develop the typical smolt pigmentation (Hansen, 1997).
Received January 18, 2012
Accepted July 26, 2012
Edited by: Concepta Margaret McManus Pimentel
- Alne, H.; Oehme, M.; Thomassen, M.; Terjesen, B.; Rørvik, K. 2011. Reduced growth, condition factor and body energy levels in Atlantic salmon Salmo salar L. during their first spring in the sea. Aquaculture Research 42: 248-259.
- Arnesen, A.M.; Johnsen, H.K.; Mortensen, A.; Jobling, M. 1998. Acclimation of Atlantic salmon (Salmo salar L.) smolts to "cold" sea water following direct transfer from freshwater. Aquaculture 168: 351-367.
- Austreng, E.; Storebakken, T.; Asgard, T. 1987. Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture 60: 157-160.
- Bergheim, A.; Forsberg, O.I. 1993. Attempts to reduce effluent loadings from salmon farms by varying feeding frequencies and mechanical effluent treatment. p. 115-124. In: Barnabe, G.; Kestemont, P., eds. Production, environment and quality. European Aquaculture Society, Ghent, Belgium.
- Björnsson, B.Th.; Stefansson, S.O.; McCormick, S.D. 2011. Environmental endocrinology of salmon smoltification. General and Comparative Endocrinology 170: 290-298.
- Blackburn, J.; Clarke, W.C. 1987. Revised procedure for the 24 h seawater challenge test to measure seawater adaptability in juvenile salmonids. Canadian Technology Reports on Fisheries and Aquatic Science 1515: 1-35.
- Brett, J.R. 1979. Environmental factors and growth. p. 599-675. In: Hoar, W.S.; Randall, D.J.; Brett, J.R., eds. Fish physiology. Academic Press, New York, USA.
- Damsgard, B.; Arnesen, A. 1998. Feeding, growth and social interactions during smolting and seawater acclimation in Atlantic salmon, Salmo salar L. Aquaculture 168: 7-16.
- Duncan, N.J.; Auchinachie, N.; Robertson, D.; Murray, R.; Bromage, N. 1998. Growth, maturation and survival of out-of-season 0 + and 1 + Atlantic salmon (Salmo salar) smolts. Aquaculture 168: 325-339.
- Duncan, N.J.; Bromage, N. 1998. The effect of different periods of constant short days on smoltification in juvenile Atlantic salmon (Salmo salar). Aquaculture 168: 369-386.
- Duston, J. 1994. Effect of salinity on survival and growth of Atlantic salmon (Salmo salar) parr and smolts. Aquaculture 121: 115-124.
- Fitzgerald, R.; Stefansson, S.O.; Garforth, D.; Irwin, S. 2002. Production II: From egg to market size: onrearing in freshwater and marine environments. p. 65-104. In: Stead, S.M.; Laird, L., eds. Handbook of salmon farming. Praxis, Berlin, Germany.
- Fontaine, M. 1951. Decrease of the chlorine content of muscle of young salmon (smolt) at the time of downstream migration. Comptes Rendus de l'Académie des Sciences 232: 2477-2492 (in French).
- Gaignon, J.L.; Quémener, L. 1992. Influence of early thermic and photoperiod control on smoltification in Atlantic salmon (Salmo salar). Aquatic Living Resources 5: 185-195.
- Galbreath, P.F.; Thorgaard, G.H. 1995. Saltwater performance of all-female triploid Atlantic salmon. Aquaculture 138: 77-85.
- Gehan, E.A. 1965. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52: 203-213.
- Gonçalves, J.; Carraça, S.; Damasceno-Oliveira, A.; Fernandez-Duran, B.; Diaz, J.; Wilson, J.; Coimbra, J. 2006. Effect of reduction in water salinity on osmoregulation and survival of large Atlantic salmon held at high water temperature. North American Journal of Aquaculture 68: 324-329.
- Handeland, S.O.; Berge, A.; Bjornsson, B.T.; Lie, O.; Stefansson, S.O. 2000. Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 181: 377-396.
- Handeland, S.O.; Berge, A.; Björnsson, B.T.; Stefansson, S.O. 1998. Effects of temperature and salinity on regulation and growth of Atlantic salmon (Salmo salar L.) smolts in seawater. Aquaculture 168: 289-302.
- Handeland, S.O.; Bjornsson, B.T.; Arnesen, A.M.; Stefansson, S.O. 2003. Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture 220: 367-384.
- Handeland, S.O.; Imsland, A.K.; Stefansson; S.O. 2008. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283: 36-42.
- Hansen, T. 1997. Management of smolts. Scotish Fish Farmer 101: 19-20.
- Hansen, T.; Stefansson, S.; Taranger, G.L. 1992. Growth and sexual maturation in Atlantic salmon, Salmo salar L., reared in sea cages at two different light regimes. Aquaculture and Fisheries Management 23: 275-280.
- Houston, A.H.; Threadgold, L.T. 1963. Body fluid regulation in smolting Atlantic salmon. Journal of the Fisheries Research Board of Canada 20: 1355-1369.
- Imsland, A.K.; Väge, K.A.; Handeland, S.O.; Stefansson, S.O. 2011. Growth and osmoregulation in Atlantic salmon (Salmo salar) smolts in response to different feeding frequencies and salinities. Aquaculture Research 42: 469-479.
- Jorgensen, E.H.; Jobling, M. 1994. Feeding and growth of exercised and unexercised juvenile Atlantic salmon in freshwater, and performance after transfer to seawater. Aquaculture International 2: 154-164.
- Kaplan, E.L.; Meier, P. 1958. Nonparametric estimation from incomplete observations. Journal of the American Statistics Association 58: 457-481.
- Krakenes, R.; Hansen, T.; Stefansson, S.O.; Taranger, G.L. 1991. Continuous light increases growth rate of Atlantic salmon (Salmo salar L.) postsmolts in sea cages. Aquaculture 95: 281-287.
- Kubo, T. 1955. Changes of some characteristics of blood in smolts of Oncorhynchus masou during seawater migration. Bulletin of the Faculty of Fisheries Hokkaido University 6: 201-207.
- Lasserre, P.; Boeuf, G.; Harache, Y. 1978. Osmotic adaptation of Oncorhynchus Kistutch Walbaum. I: Seasonal variations of gill Na+/K+-ATPase activity in coho salmon, 0+ age and yearling, reared in freshwater. Aquaculture 14: 365-382.
- McCormick, S.D.; Saunders, R.L.; Henderson, E.B.; Harmon, P.R. 1987. Photoperiod control of parr-smolt transformation in Atlantic salmon (Salmo salar): changes in salinity tolerance, gill Na+, K +-ATPase activity, and plasma thyroid hormones. Canadian Journal of Fisheries and Aquatic Sciences 44: 1462-1468.
- McLeese, J.M.; Johnsson, J.; Huntley, F.M.; Clarke, W.C.; Weisbart, M. 1994. Seasonal changes in osmoregulation, cortisol and cortisol receptor activity in the gills of parr/smolt of steelhead trout and steelhead-rainbow trout hybrids, Oncorhynchus mykiss General and Comparative Endocrinology 93: 103-113.
- Myoung, J.G.; Arnesen, A.M.; Mortensen, A. 1999. Effect of freshwater supply on the survival and growth of Atlantic salmon, Salmo salar L., "0+ autumn smolts" reared in sea cages during winter. Aquaculture Research 30: 65-67.
- Noble, C.; Kadri, S.; Mitchell, D.F.; Huntingford, F.A. 2008. Growth, production and fin damage in cage-held 0+ Atlantic salmon pre-smolts (Salmo salar L.) fed either a) on-demand, or b) to a fixed satiation-restriction regime: data from a commercial farm. Aquaculture 275: 163-168.
- Pickering, A.D. 1993. Endocrine-induced pathology in stressed salmonid fish. Fisheries Research 17: 35-40.
- Reis-Henriques, M.A.; Silva, L.; Coimbra, J. 1996. Gill Na+-K+ ATPase carbonic anhydrase activities and plasma osmotic and ionic variations during smoltification of Atlantic salmon in the north of Portugal. Aquaculture International 4: 117-128.
- Saunders, R.L.; Henderson, E.B.; Harmon, P.R. 1985. Effects of photoperiod on juvenile growth and smolting of Atlantic salmon and subsequent survival and growth in sea cages. Aquaculture 45: 55-66.
- Sigholt, T.; Asgard, T.; Staurnes, M. 1998. Timing of parr-smolt transformation in Atlantic salmon (Salmo salar): effects of changes in temperature and photoperiod. Aquaculture 160: 129-144.
- Sigholt, T.; Staurnes, M.; Jakobsen, H.J.; Asgard, T. 1995. Effects of continuous light and short-day photoperiod on smolting, seawater survival and growth in Atlantic salmon (Salmo salar). Aquaculture 130: 373-388.
- Skilbrei, O.T.; Hansen, T. 2004. Effects of pre-smolt photoperiod regimes on post-smolt growth rates of different genetic groups of Atlantic salmon (Salmo salar). Aquaculture 242: 671-688.
- Skilbrei, O.T.; Hansen, T.; Stefansson, S.O. 1997. Effects of decreases in photoperiod on growth and bimodality in Atlantic salmon (Salmo salar L.). Aquaculture Research 28: 43-49.
- Solbakken, V.A.; Hansen, T.; Stefansson, S.O. 1994. Effects of photoperiod and temperature on growth and parr-smolt transformation in Atlantic salmon (Salmo salar L.) and subsequent performance in seawater. Aquaculture 121: 13-27.
- Stagg, R.M.; Talbot, C.; Eddy, F.B.; Williams, M. 1989. Seasonal variations in osmoregulatory and respiratory responses to seawater exposure of juvenile Atlantic salmon (Salmo salar) maintained in freshwater. Aquaculture 82: 219-228.
- Staurnes, M.; Sigholt, T.; Asgard, T.; Baeverfjord, G. 2001. Effects of a temperature shift on seawater challenge test performance in Atlantic salmon (Salmo salar) smolt. Aquaculture 201: 153-159.
- Stefansson, S.O.; Berge, A.I.; Gunnarsson, G.S. 1998. Changes in seawater tolerance and gill Na+, K+-ATPase activity during desmoltification in Atlantic salmon kept in freshwater at different temperatures. Aquaculture 168: 271-277.
- Stefansson, S.O.; Bjornsson, B.T.; Hansen, T.; Haux, C.; Taranger, G.L.; Saunders, R.L. 1991. Growth, parr-smolt transformation, and changes in growth hormone of Atlantic salmon (Salmo salar) reared under different photoperiods. Canadian Journal of Fisheries and Aquatic Sciences 48: 2100-2108.
- Thorpe, J.E.; Morgan, R.I.G.; Ottaway, E.M.; Miles, M.S. 1980. Time of divergence of growth groups between potential 1+ and 2+ smolts among sibling Atlantic salmon. Journal of Fish Biology 17: 13-21.
- Thrush, M.A.; Duncan, N.J.; Bromage, N.R. 1994. The use of photoperiod in the production of out-of-season Atlantic salmon (Salmo salar) smolts. Aquaculture 121: 29-44.
- Usher, M.L.; Talbot, C.; Eddy, F.B. 1991. Effects of transfer to seawater on growth and feeding in Atlantic salmon smolts (Salmo salar L.). Aquaculture 94: 309-326.
- Wedemeyer, G.A.; Saunders, R.L.; Clarke, W.C. 1980. Environmental factors affecting smoltification and early marine survival of anadromous salmonids. Marine Fisheries Reviews 42: 1-14
- Zar, J.H. 1996. Biostatistical Analysis. Prentice-Hall, Upper Saddle River, NJ, USA.
Publication Dates
-
Publication in this collection
07 Jan 2013 -
Date of issue
Feb 2013
History
-
Received
18 Jan 2012 -
Accepted
26 July 2012