Abstract
In common bean (Phaseolus vulgaris L.) breeding, plant selection that associate erect plant architecture, high yield, and grains with good commercial acceptance has been the choice of breeders. Thus, this study aimed to evaluate potential parents, to obtain promising segregating populations that associate high yield, erect plant architecture and carioca grain type, as well as to obtain information on heterosis, general and specific combining ability of these parents regarding grain yield and traits related to plant architecture. Fourteen common bean lines were crossed under a partial diallel scheme. Group 1 was composed by eight erect plant lines and group 2 by six carioca grain type lines. The F1's plants from the crosses and the 14 parents were evaluated during spring (Mar. sowing) for plant architecture grade, diameter of the hipocotyl, plant mean height, and grain yield. Predominance of additive effects was observed for plant architecture grade and diameter of the hypocotyls. For grain yield and plant mean height, there was a greater contribution of the dominance effects. Thus, selection of erect plants, with a larger diameter of the hypocotyl can be carried out in early generations; while for grain yield and plant mean height, it must be delayed, preferably, to later generations.
Phaseolus vulgaris L.; general and specific combining ability; partial diallel; quantitative genetics
GENETICS AND PLANT BREEDING
Genetic potential of common bean parents for plant architecture improvement
Vanessa Maria Pereira e SilvaI,§ § Present Address: IAPAR Área de Melhoramento Genético, Rod. Celso Garc ia Cid, km 375, C.P. 301 86001-970 Londrina, PR Brasil ,* * Corresponding author < vanessa@iapar.br> ; Pedro Crescêncio Souza CarneiroI; José Ângelo Nogueira de Menezes JúniorII,§ § Present Address: IAPAR Área de Melhoramento Genético, Rod. Celso Garc ia Cid, km 375, C.P. 301 86001-970 Londrina, PR Brasil ; Vinícius Quintão CarneiroI; José Eustáquio de Souza CarneiroII; Cosme Damião CruzI; Aluizio BorémII
IUFV Depto. de Biologia Geral Av. Peter Henry Rolfs, s/n, Campus Universitário 36570-000 Viçosa, MG Brasil
IIUFV Depto. de Fitotecnia
ABSTRACT
In common bean (Phaseolus vulgaris L.) breeding, plant selection that associate erect plant architecture, high yield, and grains with good commercial acceptance has been the choice of breeders. Thus, this study aimed to evaluate potential parents, to obtain promising segregating populations that associate high yield, erect plant architecture and carioca grain type, as well as to obtain information on heterosis, general and specific combining ability of these parents regarding grain yield and traits related to plant architecture. Fourteen common bean lines were crossed under a partial diallel scheme. Group 1 was composed by eight erect plant lines and group 2 by six carioca grain type lines. The F1's plants from the crosses and the 14 parents were evaluated during spring (Mar. sowing) for plant architecture grade, diameter of the hipocotyl, plant mean height, and grain yield. Predominance of additive effects was observed for plant architecture grade and diameter of the hypocotyls. For grain yield and plant mean height, there was a greater contribution of the dominance effects. Thus, selection of erect plants, with a larger diameter of the hypocotyl can be carried out in early generations; while for grain yield and plant mean height, it must be delayed, preferably, to later generations.
Keywords:Phaseolus vulgaris L., general and specific combining ability, partial diallel, quantitative genetics
Introduction
In common bean (Phaseolus vulgaris L.) breeding, selection of individuals with erect plant architecture has been a strategy adopted of many breeders to foster high grain yield (Adams, 1982; Brothers and Kelly, 1993; Coyne, 1980; Dawo et al., 2007; Izquierdo and Hosfield, 1983; Kelly and Adams, 1987; Kelly, 2001). Such plant types ease the use of cultural practices, allow mechanized harvest and prevent pods to come in contact with the ground, ensuring better seed quality. Plants with this architecture can also reduce the incidence of some diseases, such as white mold.
Besides erect plant architecture, selection must also aim at high grain yield and commercial grains. Those traits are not commonly present in a single parent. In this case, hybridization is the most indicated breeding strategy to combine them in a single improved line. Thus, selection of parents with good complementing traits is paramount. Diallel crossing is a method to select parents based on their genetic values, and especially, on their combining ability (Allard, 1960; Griffing, 1956).
Its use originates from the development of concepts of general and specific combining ability established by Sprague and Tatum (1942). General combining ability (GCA) refers to the mean behavior of each parent in crosses with all other parents and, it is associated with the additive genetic effects and the frequency of favorable alleles. The specific combining ability (SCA) is interpreted as the deviation of the hybrid performance in relation to what would be expected based on the GCA of their parents, and it provides information on the non-additive effects (Falconer, 1981; Hallauer and Miranda Filho, 1988). These estimates help to learn about potential good parents and whether selection must be delayed in function of the dominance deviations and epistasis interactions.
The use of diallel crosses is often limited due to the large number of crosses required to evaluate a particular group of parents. Besides, it is not always necessary to evaluate all possible combinations through a complete diallel. Therefore, the use of partial diallels is more desirable (Kempthorne and Curnow, 1961). Within this context, the this study aimed to evaluate the potential of 14 bean parents in a partial diallel, aiming to obtain promising segregating populations for high grain yield, good plant architecture, and carioca grain type, as well as to obtain information on heterosis, general and specific combining ability of those parents, related to plant architecture.
Materials and Methods
Fourteen common bean lines were crossed in a partial diallel scheme (Table 1). The parents were divided into two contrasting groups, based on their plant architecture, yield and grain type. The first group was composed by eight parents, three black grain and erect plants (BRS Valente, BRS Supremo and IPR Uirapuru), three of carioca grain type and also erect plants (BRS Horizonte, CNFC 9466 and A805), but presenting poor yield and/or grain type, and two erect plant lines of mulatinho grain type (A170 and A525). Group 2 was composed by six carioca grain beans, with three originated from crosses with isoline Rudá-R, a source of different genes resistant to anthracnose, angular spot and rust (Ragagnin et al., 2009) (UTF 0013 × Rudá-R, GEN 12-2 × Rudá-R and CNFC 9437 × Rudá-R), denominated in this work as L1, L2, L3, and lines VC6, BRSMG Majestoso and BRSMG Madrepérola. The group 2 lines present good yield and grains with good commercial aspects, but poor plant architecture (Table 1).
The F1's seeds were later sown in the field with the parents, in an experiment with 62 treatments (48 hybrids + 14 parents). Evaluation was carried out during spring (Mar. sowing) in a randomized complete block design with three replications. The plots were three lines of 1.4 m, with planting density of 12 seeds m1 and 0.50 m between rows. The experiment was conducted in Viçosa, State of Minas Gerais, Brazil (690 m a.s.l., 20o45' S, 42o51' W). The cultural practices adopted were those recommended for the bean crop in the region.
In the field, plant mean height (cm) was evaluated, and plant architecture was rated by a grade scale ranging from 1 to 5, according to Collicchio et al. (1997). In this range, grade 1 refers to type II plant, erect, with a single stem, and high first pod insertion; grade 2 refers to type II plant, erect, and with some ramifications; grade 3, to type II or III plant, erect, with many ramifications and tendency to prostrate growth; grade 4, to type III plant, semi-erect, partially prostrated; and grade 5, to type III plant, with long internodes and very prostrated.
After harvest, other traits related to plant architecture were also evaluated: diameter of the hypocotyls, height of first pod insertion, total number of pods, number of pods in the branches and number of branches. These traits were measured in ten plants removed from the central line from each plot, using their means for the statistical analysis.
The pair-wise genotypic correlations between plant architecture grade, plant mean height, diameter of the hypocotyl, height of insertion of first pod, number of total pods, number of pods in the branches, number of branches and grain yield were estimated to explore the possibility of using them in the selection of more erect plants. The significance of genotypic correlations was tested using the bootstrap a 5 % probability, with 5000 simulations (Davison and Hinkley, 1997).
The mean of the parents and F1's plants were analyzed according to the partial diallel model proposed by Geraldi and Miranda Filho (1988) and Miranda Filho and Geraldi (1984), adapted from the models proposed by Griffing (1956) and Gardner and Eberhart (1966), respectively. The statistical analyses were carried out using the software GENES (Cruz, 2006).
The model proposed by Geraldi and Miranda Filho (1988), adapted from the model of Griffing (1956) is as follows:
where: Yij : is the mean of the cross involving the i-th parent of group1 and the j-th parent of group 2; Yi : is the mean of the i-th parent of group 1 (i = 0, 1, 2 ...8); Y.j : is the mean of the j-th parent of group 2 (j = 0, 1, 2 ...6); : general mean of the diallel; d1,d2 contrast involving means of groups 1 and 2 and the general mean; gi : effect of general combining ability of the i-th parent of group 1; gj : effect of general combining ability of the j-th parent of group 2; sij : effect of specific combining ability; and  : experimental error average.
: experimental error average.
The adaptation of the model of Gardner and Eberhart (1966), proposed by Miranda Filho and Geraldi (1984), is used for detailed study of heterosis in partial diallel, according model described follows:
where: Yij : is the mean of the cross involving the i-th parent of group1 and the j-th parent of group 2; Yi0 : is the mean of the i-th parent of group 1 (i = 0, 1, 2 ...8), with α = 1 and θ = 0; Y0j : is the mean of the j-th parent of group 2 (j = 0, 1, 2 ...6), with α = -1 and θ = 0; u: constant associated with the model; d: measure of the difference between mean of the two groups; vi : effect of the i-th parent of group 1 vj : effect of the j-th parent of group 2; h: effect of average heterosis; hi : effect of heterosis assigned to the i-th parent of group 1; hj : effect of heterosis assigned to the j-th parent of group 2; sij : effect of specific heterosis resulting from crosses between parents of order i and j, groups 1 and 2, respectively;  : experimental error average.
: experimental error average.
Results and Discussion
Correlations among characteristics related to plant architecture
Diameter of the hypocotyl and plant mean height were correlated with plant architecture grade (Table 2), indicating that those traits are promising for selection of plants with better architecture. The traits height of the first pod insertion, total number of pods, number of pods in the branches and number of branches presented low correlation with plant architecture grade. Acquaah et al. (1991) identified not only the diameter of the hypocotyl but also plant height as the major indicators of bean plant architecture.
General and Specific Combining Ability
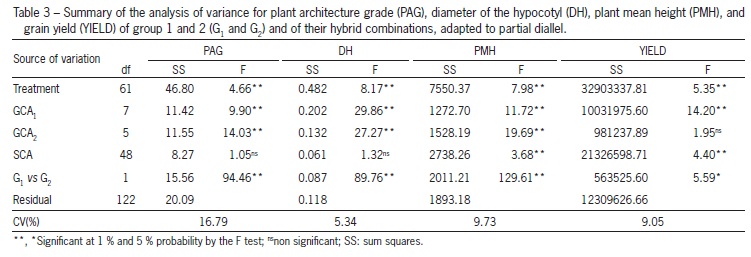
Analysis of variance of the characteristics plant architecture, diameter of the hypocotyl, plant mean height and grain yield indicated the existence of variability among the 14 parents (p ≤ 0.01) (Table 3). The source of variation treatments was decomposed into effects of general and specific combining ability and contrast for the two groups of parents (G1vs G2) were performed. Difference (p ≤ 0.05) was found between the means of the groups (G1vs G2) and between the GCA of group 1 (GCA1) for all the traits. Difference (p ≤ 0.01) for the GCA of group 2 (GCA2) was detected for plant architecture grade, diameter of the hypocotyl and plant mean height. For SCA, difference (p ≤ 0.01) was observed for plant mean height and grain yield (Table 3).
GCA predominated over SCA for plant architecture grade and diameter of the hypocotyl, suggesting predominance of the additive effects, expressed by the superiority of the sum of the GCA squares. The opposite can also be observed for plant mean height and grain yield, indicating a greater contribution of the effects of dominance for these traits. In this case, the superiority of the sum of squares of SCA was observed for GCA (Table 3). Predominance of non-additive effects associated with grain yield and plant height in common bean was also found by Gonçalves-Vidigal et al. (2008). Also studying GCA and SCA in segregating F2 populations derived from a complete diallel, Machado et al. (2002) observed predominance of SCA for grain yield. However, some researchers report that additive genetic effects for grain yield and its primary components are predominant in common bean (Kurek et al., 2001; Nienhuis and Singh, 1988). Similar results were found in other species as rice (Oryza sativa L.), in which the additive genetic effects prevailed over those of dominance for yield related traits (Torres and Geraldi, 2007).
The prevalence of dominance effects for grain yield, resulting in hybrids with higher yields than that of the best parent, can be due to a divergent selection carried out for grain yield and erect plant architecture between the parents of the two groups of this study. Group 1 was obtained with selection prioritizing plants with better architecture, which may have fixed alleles for grain yield, different from those fixed in group 2, in which selection emphasized high yielding individuals.
The significance of the contrast between mean of the groups (G1vs G2) for all the traits (Table 3), confirms that the two groups of parents differ from each another. Group 1 has erect lines while group 2 possesses elite cultivars as far as yield and carioca grain type.
GCA estimates depend on the genetic difference of the parents and on the mean effect of allelic substitution in the other group and are associated with additive effects. SCA, in partial diallel, is a function of the dominance effects and of the product of the differences of allelic frequencies of the parents of the opposite group, making the SCA related to the dominance and epistasis effects (Hallauer and Miranda Filho, 1988).
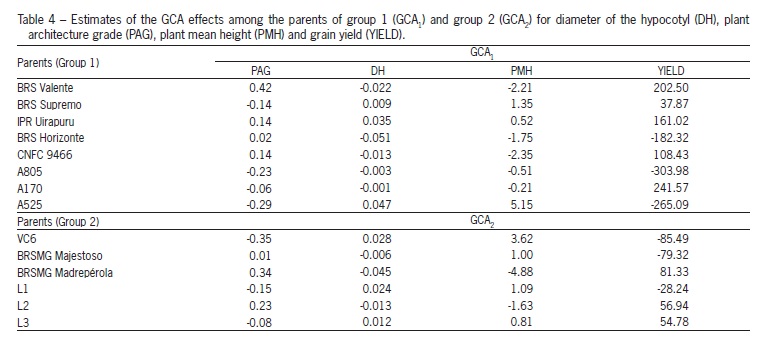
Line A525, of group 1, and line VC6, of group 2, stand out in relation to the GCA estimates for diameter of the hypocotyl, plant mean height and plant architecture grade (Table 4). For the latter variable, the lowest value is the most desirable, since lower grades indicate plants with more erect architecture. As for grain yield, group 1 A170 line and group 2 BRSMG Madrepérola were outstanding for GCA. Those parents (A525, VC6, A170 and BRSMG Madrepérola) have a higher frequency of favorable alleles for these traits, considering that the allelic frequencies in the parents of one group are relative to that of the parents of the other group.
According to the SCA estimates for grain yield, the most outstanding hybrids were A170 × VC6 and A525 × BRSMG Majestoso (Table 5). For breeding purposes, hybrid combinations with high SCA estimates and involving at least one parent with high GCA are of great importance. Hence, cross A170 × VC6 tends to be more promising because of the high GCA presented by the A170 parent.
For plant architecture grade SCA estimates with negative and high values indicate more erect plants. This was observed for the crosses A170 × L2, A805 × BRSMG Majestoso and BRS Horizonte × L3, with emphasis for the cross A805 × BRSMG Majestoso, in which A805 showed high GCA. On the other hand, for plant mean height, the highest SCA were for BRS Supremo × BRSMG Majestoso, followed by BRS Horizonte x VC6 and BRS Valente × L2, with parent VC6 also presenting a high GCA. For diameter of the hypocotyl, the highest SCA estimate was found for A170 × VC6, with VC6 having high GCA (Table 5).
Lines A170, A805 and A525 presented high SCA values, indicating larger genetic distance among those lines in relation to the other lines of their group. That those lines are from the International Center for Tropical Agriculture (CIAT), showing the importance of using lines from different origins in breeding programs.
Considering grain yield and plant architecture simultaneously, the populations from crosses CNFC 9466 × VC6, BRS Valente × BRSMG Madrepérola and A525 × L1 showed the most promising results. They are promising for the extraction of lines combining these two traits. Those crosses were not the ones showing the highest potential, considering each trait individually. Therefore, alleles important for yield could not be present in populations developed for plant architecture, such as A170 × VC6. Thus, an alternative to maximize the potential of the segregating populations as a source of promising lines aiming at both yield and plant architecture would be a double cross between the F1's single crosses [(A170 × VC6) × (A805 × BRSMG Majestoso)].
Decomposition of the heterosis effect
The decomposition of the effect of SCA into medium heterosis, varietal heterosis, (attributed to the various genotypes within each group) and specific heterosis is justified only when it presents a statistical significant effect. Thus, for plant mean height and grain yield, heterosis decomposition was done and they are shown in Table 6.
Considering plant mean height, there were differences (p ≤ 0.01) for heterosis medium and varietal heterosis of group 1 (G1), and specific heterosis (p ≤ 0.05), while for grain yield differences were obtained for all the effects (p ≤ 0.05). For plant mean height, just group 1 lines presented heterotic effects, while, for grain yield, this conclusion is valid for lines of groups 1 and 2. The lines with higher varietal heterosis effect are more genetically distant from each other, or their alleles presented greater dominance deviations, compared to those of lower heterotic effect. Parents with greater genetic diversity are required in crosses aiming transgressive segregation. The significance of the effect of specific heterosis for grain yield and plant mean height shows that the parents presented non allelic genes with epistatic interaction. The interactions dominant × dominant, dominant × additive and additive × dominant are not inheritable, just being useful in hybrids.
Comparing hybrid means with those of the parents (Table 6), it can be observed that hybrids were higher yielding than the parents. For diameter of the hypocotyl and plant architecture grade, the means of the hybrids were similar to those of the parents and for plant mean height, the hybrids were shorter than the parents. These results indicate the existence of heterosis for grain yield and plant mean height, resulting from positive dominance deviations for the genes for grain yield and negative for plant mean height.
The heterosis values in the crosses varied in magnitude and signal (Table 7). In group 1, cross A525 × L2 is superior for grain yield. This cross presented heterosis of 1397 kg ha1, surpassing the mean of both parents in 56 %. The combinations A170 × VC6 (48.5 %) and A525 × BRSMG Majestoso (47.8 %) also presented high heterosis for grain yield.
The crosses with greater heterosis value for plant mean height were BRS Supremo × BRSMG Majestoso and BRS Horizonte × VC6, with a heterosis value of 7.17 cm (16.2 %) and 4.17 cm (9.6 %), respectively, (Table 7). However, the hybrids with greater heterosis did not always present the highest means due to the fact that the superiority of a hybrid depends on both the amount of heterozygous loci and on the means of the parents.
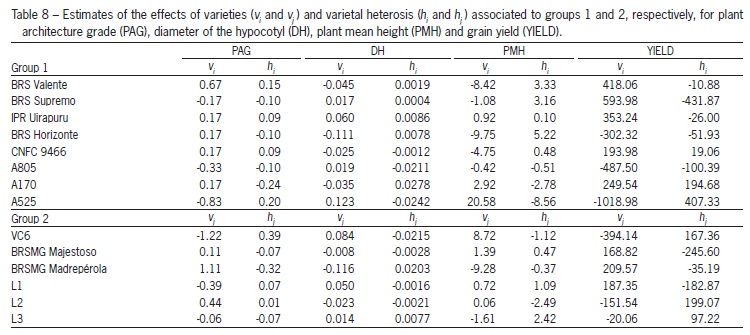
The estimates of the variety effects (vi and vj) of the parents of each group are presented in Table 8. The per se effect of a parent is an indicative of frequency of its favorable alleles. In the case of grain yield, varieties BRS Supremo (group1) and BRSMG Madrepérola (group 2) presented the highest per se effects, indicating a greater concentration of favorable alleles in those parents. For plant architecture grade, diameter of the hypocotyl, and plant mean height, group 1 line A525, and group 2 line VC6, presented the largest frequency of favorable alleles. For plant architecture grade negative effects is an indication of a great concentration of favorable alleles.
In group 1, considering the effects of varietal heterosis for grain yield (Table 8), A525, L2 and VC6 presented the highest values. While, for plant architecture grade and diameter of the hypocotyl, A170 and, for plant mean height, BRS Horizonte were superior. In group 2, BRSMG Madrepérola and L3 presented a greater estimate of varietal heterosis, being BRSMG Madrepérola the best for plant architecture grade and diameter of the hypocotyls, while L3 for plant mean height. Gonçalves-Vidigal et al. (2008) observed heterosis for common bean grain yield and also noted that the hybridization of cultivars belonging to distinct commercial groups favors higher heterosis. Similar results were obtained by Foolad and Bassiri (1983), Gutiérrez and Singh (1985), Nienhuis and Singh (1986), who also verified heterosis for grain yield.
It can be concluded that grain yield and the traits related to plant architecture present great complexity, making selection of erect and high yielding plants difficult. However, the selection of erect plants with a higher diameter of the hypocotyls can be carried out at early generations, due to the action of the additive effect genes. For grain yield and plant mean height, selection must be done preferably, in more advanced generations, as there is greater contribution of dominance effects for these traits. In the F4 generation dominance deviations are reduced by 87.5 %. Thus, it recommended that bulks should be opened at this generation, aiming to select high yielding lines that also have erect plant architecture.
Acknowledgements
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), for the financial support of the research activities in the Common Bean Breeding Program of the Federal University of Viçosa, Brazil.
Received March 08, 2012
Accepted February 05, 2013
Edited by: Antonio Augusto Franco Garcia
- Acquaah, G.; Adams, M.W.; Kelly, J.D. 1991. Identification of effective indicators of erect plant architecture in dry bean. Crop Science 31: 261-264.
- Adams, M.W. 1982. Plant architecture and yield breeding. Iowa State Journal of Research 56: 225-254.
- Allard, R.W. 1960. Principles of Plant Breeding. John Wiley, New York, NY, USA.
- Brothers, M.E.; Kelly, J.D. 1993. Interrelationship of plant architecture and yield components in the pinto bean ideotype. Crop Science 33: 1234-1238.
- Collicchio, E.; Ramalho, M.A.P.; Abreu, A.F.B. 1997 Association between plant architecture of the bean plant and seed size. Pesquisa Agropecuária Brasileira 32: 297-304 (in Portuguese, with abstract in English).
- Coyne, D.P. 1980. Modification of plant architecture and crop yield by breeding. Hortscience 15: 244-247.
- Cruz, C.D. 2006. Genes Program: Biometrics. UFV, Viçosa, MG, Brazil (in Portuguese).
- Davison, A.C.; Hinkley, D.V. 1997. Bootstrap Methods and Their Application. Cambridge University Press, New York, NY, USA.
- Dawo, M.I.; Sanders, F.E.; Pilbeam, D.J. 2007. Yield, yield components and plant architecture in the F3 generation of common bean (Phaseolus vulgaris L.) derived from a cross between the determinate cultivar 'Prelude' and an indeterminate landrace. Euphytica 156: 77-87.
- Falconer, D.S. 1981. Introduction to Quantitative Genetics. 2ed. Longman, London, UK.
- Foolad, M.R.; Bassiri, A. 1983. Estimates of combining ability, reciprocal effects and heterosis for yield and yield components in a common bean diallel cross. The Journal of Agricultural Science 100: 103-108.
- Gardner, C.O.; Eberhart, S.A. 1966. Analysis and interpretation of the variety cross diallel and related populations. Biometrics 22: 439-452.
- Geraldi, I.O.; Miranda Filho, J.B. 1988. Adapted models for the analysis of combining ability of varieties in partial diallel crosses. Brazilian Journal of Genetics 11: 419-430.
- Gonçalves‑Vidigal, M.C.; Silvério, L.; Elias, H.T.; Vidigal Filho, P.S.; Kvitschal, M.V.; Retuci, V.S.; Silva, C.R. 2008. Combining ability and heterosis in common bean cultivars. Pesquisa Agropecuária Brasileira 43: 1143-1150.
- Griffing, B. 1956. Concept of general and specific combining ability in relation to diallel crossing systems. Australian Journal of Biological Sciences 9: 463-493.
- Gutiérrez, J.A.; Singh, S.P. 1985. Heterosis and inbreeding depression in dry bush beans, Phaseolus vulgaris L. Canadian Journal of Plant Science 65: 243-249.
- Hallauer, A.R.; Miranda Filho, J.B. 1988. Quantitative Genetics in Maize Breeding. 2ed. Iowa State University Press, Ames, IA, USA.
- Izquierdo, J.A.; Hosfield, G.L. 1983. The relationship of seed filling to yield among dry beans with differing architectural forms. Journal of the American Society for Horticultural Science 108: 106-111.
- Kelly, J.D. 2001. Remaking bean plant architecture for efficient production. Advances in Agronomy 7: 109-143.
- Kelly, J.D.; Adams, M.W. 1987. Phenotypic recurrent selection in ideotype breeding of pinto beans. Euphytica 36: 69-80.
- Kempthorne, O.; Curnow, R.N. 1961. The partial diallel cross. Biometrics 17: 229-250.
- Kurek, A.J.; Carvalho, F.I.F.; Assmann, I.C.; Cruz, P.J. 2001. Combining ability as an efficiency criterion in the parental bean selection. Pesquisa Agropecuária Brasileira 36: 645-651 (in Portuguese, with abstract in English).
- Machado, C.F.; Santos, J.B.; Nunes, G.H.S.; Ramalho, M.A.P. 2002. Choice of common bean parents based on combining ability estimates. Genetics and Molecular Biology 25: 179‑183.
- Miranda Filho, J.B.; Geraldi, I.O. 1984. An adapted model for the analysis of partial diallel cross. Brazilian Journal of Genetics 7: 677-688.
- Nienhuis, J.; Singh, S.P. 1986. Combining hability analyses and relationships among yield, yield components and architectural traits in dry bean. Crop Science 26: 21-27.
- Nienhuis, J.; Singh, S.P. 1988. Genetics of seed yield and its components in common bean (Phaseolus vulgaris L.) of Middle-American origin. I. General combining ability. Plant Breeding 101: 143-154.
- Ragagnin, V.A.; Souza, T.L.P.O.; Sanglard, D.A.; Arruda, K.M.A.; Costa, M.R.; Alzate-Marin, A.L.; Carneiro, J.E.S.; Moreira, M.A.; Barros, E.G. 2009. Development and agronomic performance of common bean lines simultaneously resistant to anthracnose, angular leaf spot and rust. Plant Breeding 128: 156-163.
- Sprague, G.F.; Tatum, L.A. 1942. General vs. specific combining ability in single crosses of corn. Journal of the American Society of Agronomy 34: 923-932.
- Torres, E.A.; Geraldi, I.O. 2007. Partial diallel analysis of agronomic characters in rice (Oryza sativa L.). Genetics and Molecular Biology 30: 605-613.
Publication Dates
-
Publication in this collection
14 May 2013 -
Date of issue
June 2013
History
-
Received
08 Mar 2012 -
Accepted
05 Feb 2013