Abstract
Cowpea (Vigna unguiculata) is an important grain-producing legume that can forego nitrogen fertilization by establishing an efficient symbiosis with nitrogen-fixing bacteria. Although inoculating strains have already been selected for this species, little is known about the genotypic and symbiotic diversity of native rhizobia. Recently, Bradyrhizobium has been shown to be the genus most frequently trapped by cowpea in agricultural soils of the Amazon region. We investigated the genetic and symbiotic diversity of 148 bacterial strains with different phenotypic and cultural properties isolated from the nodules of the trap species cowpea, which was inoculated with samples from soils under agroforestry systems from the western Amazon. Sixty non-nodulating strains indicated a high frequency of endophytic strains in the nodules. The 88 authenticated strains had varying symbiotic efficiency. The SPAD (Soil Plant Analysis Development) index (indirect measurement of chlorophyll content) was more efficient at evaluating the contribution of symbiotic N2-fixation than shoot dry matter under axenic conditions. Cowpea-nodulating bacteria exhibited a high level of genetic diversity, with 68 genotypes identified by BOX-PCR. Sequencing of the 16S rRNA gene showed a predominance of the genus Bradyrhizobium, which accounted for 70 % of all strains sequenced. Other genera identified were Rhizobium, Ochrobactrum, Paenibacillus, Bosea, Bacillus, Enterobacter, and Stenotrophomonas. These results support the promiscuity of cowpea and demonstrate the high genetic and symbiotic diversity of rhizobia in soils under agroforestry systems, with some strains exhibiting potential for use as inoculants. The predominance of Bradyrhizobium in land uses with different plant communities and soil characteristics reflects the adaptation of this genus to the Amazon region.
legume-nodulating bacteria; biodiversity; symbiotic promiscuity; chlorophyll content
AGRICULTURAL MICROBIOLOGY
Symbiotic nitrogen-fixing bacterial populations trapped from soils under agroforestry systems in the Western Amazon
Paula Marcela Duque Jaramillo§ § Present address: Universidade de Brasília Depto de Biologia Celular, Campus Universitário Darcy Ribeiro 70910-900 Brasília, DF Brasil. ; Amanda Azarias Guimarães; Ligiane Aparecida Florentino§§ §§ Present address: Universidade José do Rosário Vellano, Faculdade de Agronomia, Rod. MG 179 km 0 Campus Universitário - 37130-000, Alfenas, MG Brasil. ; Karina Barroso Silva; Rafaela Simão Abrahão Nóbrega¶ ¶ Present address: Universidade Federal do Recôncavo da Bahia Centro de Ciências Agrárias, Ambientais e Biológicas Rua Rui Barbosa 710, Centro 44380-000 Cruz das Almas, BA Brasil. ; Fatima Maria de Souza Moreira* * Corresponding author < fmoreira@dcs.ufla.br> Edited by: Cláudio Marcelo Gonçalves de Oliveira
Universidade Federal de Lavras Depto de Ciência do Solo, C.P. 3037 37200-000 Lavras, MG Brasil
ABSTRACT
Cowpea (Vigna unguiculata) is an important grain-producing legume that can forego nitrogen fertilization by establishing an efficient symbiosis with nitrogen-fixing bacteria. Although inoculating strains have already been selected for this species, little is known about the genotypic and symbiotic diversity of native rhizobia. Recently, Bradyrhizobium has been shown to be the genus most frequently trapped by cowpea in agricultural soils of the Amazon region. We investigated the genetic and symbiotic diversity of 148 bacterial strains with different phenotypic and cultural properties isolated from the nodules of the trap species cowpea, which was inoculated with samples from soils under agroforestry systems from the western Amazon. Sixty non-nodulating strains indicated a high frequency of endophytic strains in the nodules. The 88 authenticated strains had varying symbiotic efficiency. The SPAD (Soil Plant Analysis Development) index (indirect measurement of chlorophyll content) was more efficient at evaluating the contribution of symbiotic N2-fixation than shoot dry matter under axenic conditions. Cowpea-nodulating bacteria exhibited a high level of genetic diversity, with 68 genotypes identified by BOX-PCR. Sequencing of the 16S rRNA gene showed a predominance of the genus Bradyrhizobium, which accounted for 70 % of all strains sequenced. Other genera identified were Rhizobium, Ochrobactrum, Paenibacillus, Bosea, Bacillus, Enterobacter, and Stenotrophomonas. These results support the promiscuity of cowpea and demonstrate the high genetic and symbiotic diversity of rhizobia in soils under agroforestry systems, with some strains exhibiting potential for use as inoculants. The predominance of Bradyrhizobium in land uses with different plant communities and soil characteristics reflects the adaptation of this genus to the Amazon region.
Keywords: legume-nodulating bacteria, biodiversity, symbiotic promiscuity, chlorophyll content
Introduction
Agroecosystems generally support lower plant species diversity than ecosystems without human intervention. The reduction in above-ground diversity can have a marked influence on the diversity of edaphic organisms, affecting the biochemical processes essential for the sustainability of the forest. Agroforestry systems that seek to minimize these effects have been implemented by many communities in the Amazon, including those of the upper Solimões River (http://www.biosbrasil.ufla.br/). Several researchers conducted studies in the Amazon biome and have demonstrated the great diversity of N2-fixing legume-nodulating bacteria (LNB) strains isolated from nodules in the field or by using different species as trap plants under controlled conditions to capture LNB from the soil (Guimarães et al., 2012; Chagas Junior et al., 2010; Lima et al., 2009; Moreira et al., 1998; Moreira et al., 1993).
Cowpea [Vigna unguiculata (L.) Walp] is an important food crop in Africa and Asia, and the most widely grown grain legume in northern and northeast Brazil, where it is grown primarily by small producers and represents one of the main subsistence crops. This species is considered promiscuous and can establish symbiosis with several species and genera of LNB belonging to the alphaproteobacteria and betaproteobacteria classes (Moreira, 2006). Nevertheless, cowpea can respond to inoculation with select strains of the genus Bradyrhizobium with an increase in productivity (Guimarães et al., 2012). The use of cowpea in studies assessing LNB diversity in soils is relevant because it also allows for the identification of native strains that are adapted to local conditions and potentially useful as inoculants. These studies also assess the need for inoculation when efficient native strains are not available.
Recently, Bradyrhizobium was shown to be the predominant microsymbiont of cowpea in agricultural soils from the Amazon (Guimarães et al., 2012). Agroforestry systems in the same region have varying soil characteristics (Moreira et al., 2009) as well as varying diversity of plant species, which could affect rhizobia diversity. The objective of this study was to investigate the genetic and symbiotic diversity of nitrogen-fixing bacteria from agroforestry systems in the upper Solimões River, western Amazon. Additionally, the indirect measurement of chlorophyll content (SPAD index) was evaluated as a measure of N2-fixation for the first time in cowpea.
Materials and Methods
Strain origin
Strains were isolated from soil samples collected from the municipality of Benjamin Constant, Amazonas State (4°21'-4°26' S and 69°36'-70°1' W), near the border of Brazil, Colombia, and Peru. The area comprises the town of Benjamin Constant and the communities of Guanabara II and Nova Aliança.
Six windows were distributed in the area: windows 1 and 2 in Guanabara II, windows 3, 4, and 5 in Nova Aliança, and window 6 in Benjamin Constant, where many chemical, physical, and biological studies of soils have been carried out by the BiosBrasil project (http://www.biosbrasil.ufla.br). The windows comprise the six land use systems representative of the region: primary forest, secondary forest (late successional state), secondary forest (early regeneration state), agroforestry systems, agriculture, and pasture. The location of sampling points and, sampling procedures were described by Moreira et al. (2009) and Guimarães et al. (2012).
A total of 148 strains were obtained from the following sampling points under agroforestry systems: 24, 22, 25, 20, 17, 24A, and 17A at window 2 (Guanabara II); and 66, 67A, and 67 at window 5 (Nova Aliança) (http://www.biosbrasil.ufla.br). The chemical characteristics of the soil samples were in the following ranges: pH in water: 4.6-5.5; K+: 34-124 mg dm3; P: 1.4-10 mg dm3; S: 3.3-173 mg dm3; Al3+: 5-36 mmolc dm3; Ca2+: 4.6-12 mg dm3; and Mg2+: 1.0-3.6 mg dm3. Average sum of bases ranged from 65 to 158 mmolc dm3. Micronutrient levels were as follows: Fe2+: 75.6-231.7 mg dm3; Zn2+: 2.0-14.6 mg dm3; Mn2+: 11.2-99.7 mg dm3; B: 0.0-0.7 mg dm3; and Cu2+: 0.8-3.7 mg dm3. Average organic matter content was 18 g kg1; and base saturation ranged from 30 to 62 %. Moreira et al. (2009) provides more details of these soils as compared to other land use systems in the same area. No commercial bacterial inoculants were applied to any of these soils.
At the agroforestry sampling points the following genera known to establish symbiosis with rhizobia were found: Acacia, Calopogonium, Centrosema, Dalbergia, Derris, Desmodium, Entada, Inga, Machaerium, Mimosa, Pueraria, Piptadenia, Pityrocarpa, Swartzia, and Tachigali; the tree species Inga edulis was the most abundant, followed by the herb Calopogonium mucunoides.
Cultural characteristics of each strain were evaluated in 79 media (Fred and Waksman, 1928) as described previously (Guimarães et al., 2012; Moreira et al., 1993).
Strain authentication and symbiotic efficiency
Authentication as symbiotic nitrogen-fixing bacteria was verified for all 148 strains via procedures described in detail by Guimarães et al. (2012), in two successive experiments carried out in axenic conditions with nutrient solution (Hoagland and Arnon, 1950) in the greenhouse. The experiments were conducted over a period of 35 days, and 75 and 73 strains were analyzed in the first and second experiments, respectively. Strains approved as inoculants to cowpea by the Brazilian Ministry of Agriculture were included as positive controls (Moreira, 2006; Martins et al., 2003).
Symbiotic efficiency of nitrogen-fixing bacteria was also evaluated with the SPAD (Soil Plant Analysis Development) index of leaves of the middle and upper thirds of the plant, determined using a Minolta SPAD-502 chlorophyll meter that was previously calibrated according to the manufacturer's instructions by averaging 30 readings per plant. The indirect measurement of chlorophyll content using Minolta SPAD-502 chlorophyll meters has been validated for crops such as maize, beans, and soybean, demonstrating a positive, linear correlation between SPAD index values and the nitrogen content of plants and other related variables (Pan and Smith, 2000; Argenta et al., 2002; Poustini et al., 2007; Remans et al., 2008; Vollmann et al., 2011).
All data were tested for normality. The results were analyzed by analysis of variance (ANOVA) with the number of nodules (NN) transformed to the square root of (x + 1) as recommended by SAS Learning Edition 2.0. Mean values were grouped by the Scott-Knott test (p ≤ 0.05) using SISVAR (Ferreira, 2011).
Characterization of genetic diversity by BOX-PCR
The genetic diversity of 73 strains, out of a total of 88 authenticated as cowpea-nodulating strains, was evaluated by BOX-PCR as described by Guimarães et al. (2012), following previous described procedures (Pule-Meulenberg et al., 2010; Rademaker et al., 1997; Versalovic et al., 1994). The following type strains were included: Cupriavidus taiwanensis (LMG19424T), Burkholderia sabiae (BR3405), Sinorhizobiumsp. (BR6806), Azorhizobium doebereinerae (BR5401T), Bradyrhizobium sp. (UFLA 03-84), Rhizobium tropici (CIAT899T), and Azorhizobium caulinodans (ORS571T).
The genetic diversity of the strains was analyzed by the presence or absence of polymorphic bands in the gel. The data were grouped with the Unweighted Pair Group Mean Arithmetic Method (UPGMA) algorithm and Jaccard coefficient using BioNumerics 6.5 software (Applied Maths, Sint-Martens-Latem, Belgium).
Characterization of genetic diversity by sequencing of the 16S rRNA gene
A total of 22 strains, including at least one from each cultural group and representative of genotypes determined by BOX-PCR, were selected for sequencing of the 16S rRNA gene. Bacterial growth conditions, extraction of genomic DNA (Ausubel et al., 2005), DNA quantification, and PCR conditions (Lane, 1991) are described in Guimarães et al. (2012).
The quality of sequences was verified using Phred and submitted to BLAST for comparison with other sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/GenBank). Only sequences greater than 400 bp in length were used in the phylogenetic analysis. Sequence alignment was performed with ClustalW, and the phylogenetic tree was constructed using the neighbor-joining method in the Kimura 2 model (Krasova-Wade et al., 2003; Saitou and Nei, 1987) using the parameters in MEGA version 5 (Tamura et al., 2011). A bootstrap confidence analysis was performed with 1000 replications.
Nucleotide sequence accession numbers
The sequences determined in this work have been deposited in GenBank under accession numbers KC113601 to KC113623.
Results
The 148 strains clustered into nine groups according to growth rate (GR) and pH (Figure 1). Among the fast-growing strains, 46 (31 %) acidified the medium (FA), six (4 %) did not alter the pH of the medium (FN), and 22 (15 %) alkalinized the medium (FAL). Of the intermediate-growing strains, eight (5 %) acidified the medium (IA), 25 (17 %) did not alter the pH of the medium (IN), and 25 (17 %) alkalinized the medium (IAL). Among the slow-growing strains, two (1 %) acidified the medium (SA), two (1 %) did not alter the pH of the medium (SN), and 12 (8 %) alkalinized the medium (SAL).
Of the 148 strains evaluated in the two authentication experiments, 88 (59 %) exhibited a capacity to nodulate. Strains that failed to nodulate were predominantly fast-growing, and among these, the most frequent were those that acidified the medium (FA). Controls with and without nitrogen supplementation (52.5 mg N L1) showed no nodulation, indicating no external contamination in the experiment, which is a requirement for this type of study.
No strain had shoot dry matter (SDM) similar to the control with high nitrogen content [corresponding to the highest relative efficiency (RE); statistical "group a"]. Strains exhibited varying REs and were grouped by the Scott-Knott test into two groups: intermediate (statistical group "b"), which comprised 12 strains, and inefficient (statistical group "c"), which comprised 80 strains and the control without nitrogen supplementation. Among the reference strains, which have been used as cowpea inoculants, the BR 3267 strain (RE = 56 %) performed best. Among the land use system strains, UFLA 03-282 (RE = 79 %), which was identified as Rhizobium etli with 99 % similarity (Table 1), demonstrated the highest efficiency.
According to the SPAD index, the reference strains UFLA 03-84, INPA 03-11B, and BR 3267, a group of 51 strains, and the control supplemented with nitrogen demonstrated the highest values (statistical group "a"), indicating that these strains were effective at fixing nitrogen, which was not reflected in the SDM. The other 16 strains were considered inefficient (statistical group "c"), as they were similar to the control without N supplementation. The remaining 21 strains had intermediate efficiency (statistical group "b"). Significant and positive correlation coefficients were obtained for SPAD index with both SDM (r = 0.47, p < 0.01) and relative efficiency (r = 0.52, p < 0.01).
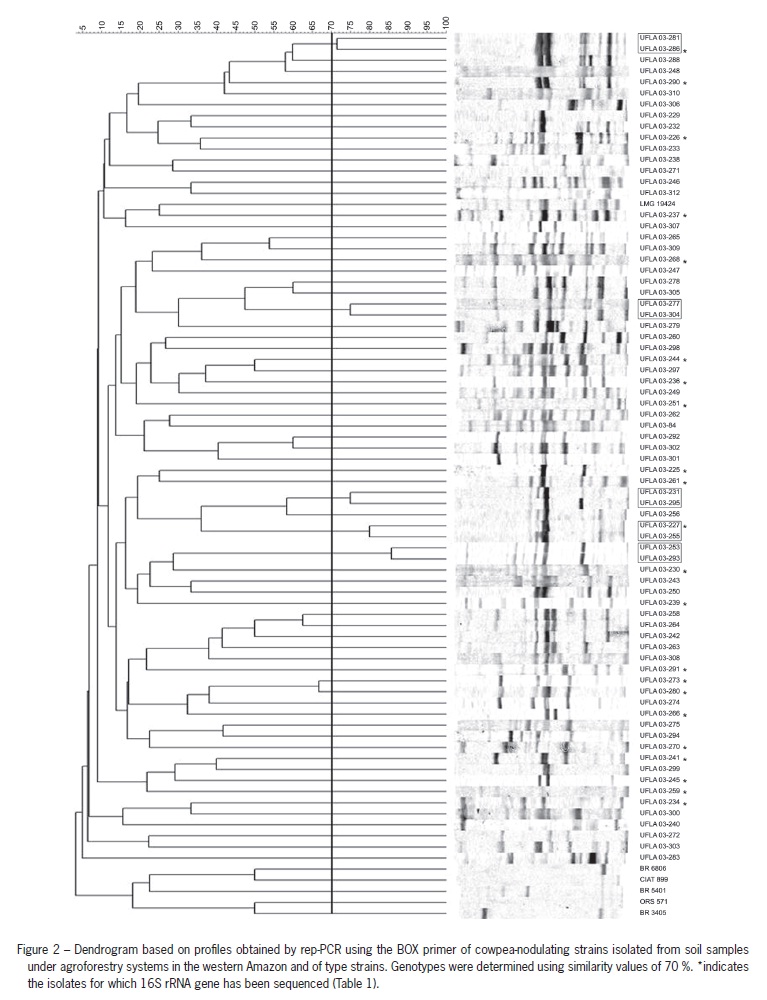
A total of 75 genotypes, most of which were restricted to only one strain, were obtained after clustering the BOX-PCR profiles with over 70 % similarity (Figure 2). The 73 strains from the agroforestry system, which were distributed among 68 genotypes, did not exhibit a banding pattern similar to any of the seven reference/type strains evaluated, indicating a high level of diversity among them.
After genotypes were determined by BOX-PCR, 23 strains were selected for phylogenetic analysis and partial sequencing of the region 3' - 5' that encodes the 16S rRNA gene. These sequences ranged between 416 bp and 850 bp and exhibited 96-100 % identity with existing sequences in GenBank (Table 1). The phylogenetic relationships between the sequences are shown in Figure 3.
Sequencing of the 16S rRNA gene indicated that all sampling sites exhibited great diversity (Table 1). The largest number of strains (70 %) belonged to the genus Bradyrhizobium, and most of these were Bradyrhizobium sp. at varying percentages of similarity. Other genera identified were: Rhizobium, Ochrobactrum, Paenibacillus, Bosea, Bacillus, Enterobacter, and Stenotrophomonas.
Discussion
The fact that non-nodulating strains were predominantly fast-growing indicates that these strains were internal or external contaminants of the nodules that grew faster than the rhizobia during the isolation process because nodules were firm, exhibited no signs of decomposition, and were harvested from fully-grown plants that were not in senescence.
The SPAD index, an indirect measurement of chlorophyll content, confirmed the N2 efficiency of various strains that presented the highest SDM, and the SPAD index and SDM were significantly correlated. However, while a total of 51 strains (out of 88) were considered efficient by the SPAD index (because they were similar to the control with mineral nitrogen), none reached the SDM of the mineral nitrogen. This occurred probably due to the short period plants were allow to grow (35 days), during which nitrogen uptake was not yet converted to dry matter. Thus, although the measurement of SDM per se is fast and less expensive, SPAD is a more effective method for evaluating symbiotic N2 fixation under axenic conditions over shorter periods. On the other hand, the equipment to measure the SPAD index is not expensive and can provide a faster analysis than N analysis by Kjeldahl method. These results corroborate results obtained in pots with Phaseolus vulgaris (Poustini et al., 2007). Under field conditions significant correlations between SPAD index and yields were obtained for maize (Argenta et al., 2002), beans (Remans et al., 2008), and soybean (Pan and Smith, 2000; Volmann et al., 2011).
A high diversity of the native rhizobia populations of agroforestry was revealed by rep-PCR, a high-resolution technique used to assess bacterial diversity (Versalovic et al., 1994). Rep-PCR is considered to be a universal tool for studies of genomic variation in prokaryotes and reflects the variability of the entire genome (Igual et al., 2001). In our study, BOX-PCR, which is a specific type of rep-PCR, proved to be an efficient technique for assessing the diversity of nitrogen-fixing bacteria that nodulate cowpea from agroforestry systems. The high diversity of strains was confirmed by 16S rRNA sequence, although 16S rRNA gene sequencing does not offer good species-level resolution among members of the Bradyrhizobium, thus requiring further testing to identify species belonging to this genus (Guimarães et al., 2012; Willems et al., 2001).
In addition to Bradyrhizobium spp., other species and genera were identified in this study: Bacillus pumilus, Paenibacillus daejeonensis, Paenibacillus humicus, Enterobacter cloacae, Bosea thiooxidans, Stenotrophomonas maltophillia, Rhizobium etli, and Ochrobactrum anthropi. The latter two species belong to N2-fixing, legume-nodulating bacterial genera, although O. anthropi has not previously been reported as such. Lima et al. (2009) reported that the genus Stenotrophomonas is potentially legume-nodulating in the same study area as in the present study, but there are no reports on the nodulation capacity of the other species, and further work is needed to confirm that they are indeed LNBs and not non-symbiotic endophytes that are commonly isolated from legume nodules (Sprent, 2009; Gyaneshwar et al., 2011). Their taxonomic position and nodulation capacity should be assessed in future studies. Nevertheless, there are reports that these species promote plant growth through other processes. Silva et al. (2007) studied the effects of different Paenibacillus macerans, Paenibacillus durus, Paenibacillus polymyxa, and B. pumilus strains on the symbiosis between Bradyrhizobium and cowpea, and showed that these strains stimulated nodulation and improved nitrogen fixation efficiency.
Few studies in Brazil have assessed the genotypic diversity of native populations that establish symbiotic relationships with cowpea. Zilli et al. (2006) sequenced the 16S rRNA gene from 14 strains: 11 isolated from cowpea nodules of plants grown in Cerrado soils in Piauí state (BR); one isolated from the semi-arid region of Pernambuco state (BR); one isolated from the Integrated Agroecology Production System (Sistema Integrado de Produção Agroecológica - SIPA) in Seropédica, state of Rio de Janeiro, Brazil (22º48'S; 43º41'S, 33 m a.s.l); and one strain (BR2001) previously approved by the Brazilian Ministry of Agriculture (MAPA) as a cowpea inoculant but later replaced by the reference strains cited in this study. Of these 14 strains, nine grouped with Bradyrhizobium elkanii (ATTC49852//USDA 76T) with a similarity of 98 %, three with Bradyrhizobium japonicum type strain (ATCC10324T/LMG 6138) and B. liaoningense (ATCC700350/LMG18230) strains, and two grouped indirectly with B. elkanii and with the group formed by B. japonicum and B. liaoningense. The diversity of 20 rhizobia isolates from upland and floodplain soils based on morphological, physiological, and symbiotic characteristics was reported in another study conducted in the Amazon using cowpea as a trap plant (Chagas Junior et al., 2010). However, genetic diversity and identification of rhizobia were not provided in the study.
A few strains, isolated from nodules collected from cowpea growing in the field, were identified as Bradyrhizobium by 16S-23S rDNA IGS sequencing, after screening of a larger number of nodules (88 and 270) by PCR-RFLP analysis of this gene in Senegal, West Africa (Krasova-Wade et al., 2004) (four strains) and South Africa, Ghana, and Botswana (Pule-Meulenberg et al., 2010) (13 strains). It may be that the small number of strains sequenced and bias in obtaining isolates, such as restriction to slow-growing alkalinizing isolates, hindered the isolation of other genera.
Lima et al. (2009) identified rhizobia from different land use systems in the western Amazon, including the same agroforestry system sampling points of this study, using siratro (Macroptilium atropurpureum) as a trap plant. The authors identified the following species in the agroforestry system: R. etli, R. tropici, R. galegae, Rhizobium sp., Sinorhizobium medicae, Burkholderia sp., Azorhizobium sp., Bradyrhizobium sp., B. elkanii, B. japonicum, and Mesorhizobium sp. Some, but not all, of these species were also identified in the present study, in addition to other genera (Paenibacillus, Stenotrophomonas, Enterobacter, Bosea, and Ochrobactrum). These results illustrate the need to use more than one species of trap plant when assessing the diversity of nitrogen-fixing, legume-nodulating bacteria in native populations; moreover, siratro captures a higher diversity of legume-nodulating bacteria than cowpea.
Guimarães et al. (2012) found high genetic and symbiotic diversity of strains isolated from agricultural soils in the same sampling area by using cowpea as trap plant. Although they also found Bradyrhizobium to be a predominant genus among isolates, other genera were found (Rhizobium, Burkolderia, and Achromobacter). However, a lower diversity was found when compared to agroforestry systems, probably due to the higher diversity of legume genera in agroforestry systems (15) than in agricultural systems (6). Thus, the diversity of phenotypes, genotypes, and species of legume-nodulating bacteria found in this study is probably associated with the diversity of legumes in this land use system, because they belong to genera that have already been reported to host a great diversity of legume-nodulating bacteria (Moreira, 2006). Even with the more diverse plant composition and soil attributes in agroforestry than in agriculture, the genus Bradyrhizobium was the most frequent trapped by cowpea, although this species is also able to nodulate with many other genera like Burkholderia, Azorhizobium, Mesorhizobium, Sinorhizobium, and Rhizobium (Moreira, 2006), Achromobacter (Guimarães et al., 2012), and Cupriavidus (Silva et al., 2012).
In conclusion, cowpea was found to nodulate with a high diversity of nitrogen-fixing bacteria genotypes isolated from soils under agroforestry systems. Most genotypes (70 %) were identified as belonging to different species in the genus Bradyrhizobium, while the remaining genotypes belonged to the genera Rhizobium, Ochrobactrum, Stenotrophomonas, Paenibacillus, Enterobacter, and Bosea. The nodulating strains exhibited varying N2-fixing efficiencies, but most of them established an efficient symbiosis with cowpea. The SPAD index was more efficient than SDM at evaluating the contribution of symbiotic N2-fixation under axenic conditions over shorter periods.
Acknowledgements
We thank CAPES and CNPq for student fellowships, CNPq for a research fellowship and grant, and the GEF/UNEP-GF2715-02 (CSM-BGBD) project for financial support. This work presents part of the findings of the international project "Conservation and Management of Below-Ground Biodiversity" implemented in seven tropical countries: Brazil, Cote d'Ivoire, India, Indonesia, Kenya, Mexico, and Uganda. This project is coordinated by the Tropical Soil Biology and Fertility Institute of CIAT (TSBF-CIAT), with co-financing from the Global Environmental Facility (GEF), and implementation support from the United Nations Environment Program (UNEP). The Brazilian Co-executing Institution was Universidade Federal de Lavras, and in Brazil the CSM-BGBD project was named BiosBrasil. Views expressed in this publication are those of their authors and do not necessary reflect those of the authors' organization, the United Nations Environment Program, and the Global Environmental Facility.
References
Argenta, G.; Silva, P.R.F.; Mielniczuk, J.; Botollini, C.G. 2002. Plant parameters as indicators of nitrogen status in maize. Pesquisa Agropecuária Brasileira 37: 519-527.
Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. 2005. Current Protocols in Molecular Biology. Wiley, New York, NY, USA.
Chagas Junior, A.F.; Oliveira, L.A.; Oliveira, N.A. 2010. Phenotypic characterization of rhizobia strains isolated from Amazonian soils and symbiotic efficiency in cowpea. Acta Scientiarum Agronomy 32: 161-169.
Ferreira, D.F. 2011. Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35: 1039-1042.
Fred, E.B.; Waksman, S.A. 1928. Laboratory Manual of General Microbiology. McGraw-Hill, New York, NY, USA.
Guimarães, A.A.; Jaramillo, P.M.D.; Nóbrega, R.S.A.; Florentino, L.A.; Silva, K.B.; Moreira, F.M.S. 2012. Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Applied and Environmental Microbiology 78: 6726-6733.
Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W-M.; Elliot, G.M.; Bontemps, C.; Santos, P.E.; Gross, E.; Reis Jr, F.B.; Sprent, J.I.; Young, J.P.W.; James, E.K. 2011. Legume-nodulating betaproteobacteria: diversity, host range and future prospects. Molecular Plant-Microbe Interactions 24: 1276-1288.
Hoagland, D.; Arnon, D.I. 1950. The water culture method for growing plants without soil. University of California, Berkeley, CA, USA. (AES Circular, 347).
Igual, J.M.; Valverde, A.; Cervantes, E.; Velásquez, E. 2001. Phosphate-solubilizing bacteria as inoculants for agriculture: use of updates molecular techniques in their study. Agronomie 21: 561-568.
Krasova-Wade, T.; Ndoye, I.; Braconnier, S.; Sarr, B.; de Lajudie, P.; Neyra, M. 2003. Diversity of indigenous bradyrhizobia associated with three cowpea cultivars (Vigna unguiculata (L.) Walp.) grown under limited and favorable water conditions in Senegal (West Africa). African Journal of Biotechnology 2: 13-22.
Lane, D.J. 1991. 16S/23S rRNA sequencing, p. 115-148. In: Stackebrandt E.; Goodfellow M., eds. Nucleic acid techniques in bacterial systematics. Wiley, New York, NY, USA.
Lima, A.S.; Nóbrega, R.S.A.; Barberi, A.; Silva, K.; Ferreira, D.F.; Moreira, F.M.S. 2009. Nitrogen-fixing bacteria communities occurring in soils under different uses in the western Amazon Region as indicated by nodulation of siratro (Macroptilium atropurpureum). Plant and Soil 319: 127-145.
Martins, L.M.V.; Xavier, G.R.; Rangel, F.W.; Ribeiro, J.R.A.; Neves, M.C.P.; Morgado, L.B.; Rumjanek, N.G. 2003. Contribution of biological nitrogen fixation to cowpea: a strategy for improving grain yield in the semi-arid region of Brazil. Biology and Fertility of Soils 38: 333-339.
Moreira, F.M.S.; Nóbrega, R.S.A.; Jesus, E.C.; Ferreira, D.F.; Perez, D.V. 2009. Differentiation in the fertility of inceptisols as related to land use in the upper Solimões river region, western Amazon. Science of the Total Environment 408: 349-355.
Moreira, F.M.S. 2006. Nitrogen-fixing Leguminosae nodulating bacteria, 280 p. In: Moreira, F.M.S.; Siqueira, J.O.; Brussaard, L., eds. Soil biodiversity in Amazonian and other Brazilian ecosystems. CAB International, Wallingford, UK.
Moreira, F.M.S.; Gillis, M.; Pot, B.; Kersters, K.; Franco, A.A. 1993. Characterization of rhizobia isolated from different divergence groups of tropical leguminosae by comparative polyacrylamide gel electrophoresis of their total proteins. Systematic and Applied Microbiology 16: 135-146.
Moreira, F.M.S.; Haukka, K.; Young, J.P.W. 1998. Biodiversity of rhizobia isolated form a wide range of forest legumes in Brazil. Molecular Ecology 7: 889-895.
Pan, B.; Smith, D.L. 2000. Preincubation of B. japonicum cells with genistein reduces the inhibitory effects of mineral nitrogen on soybean nodulation and nitrogen fixation under field conditions. Plant and Soil 223: 235-242.
Poustini, K.; Mabood, F.; Smith, D.L. 2007. Preincubation of Rhizobium leguminosarum bv. phaseoli with jasmonate and genistein signal molecules increases bean (Phaseolus vulgaris L.) nodulation, nitrogen fixation and biomass production. Journal of Agricultural Science and Technology 9: 107-117.
Pule-Meulenberg, F.; Belane, A.K.; Krasova-Wade, T.; Dakora, F.D. 2010. Symbiotic functioning and bradyrhizobial biodiversity of cowpea (Vigna unguiculata L.Walp.) in Africa. BMC Microbiology 10: 89-100.
Rademaker, J.L.W.; Frank, J.L.; Brujin, F.J. 1997. Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting, p. 1-26. In: Akkemans, A.D.L.; van Elsas, J.D.; de Brujin, F.J. Molecular microbial ecology manual. Kluwer Academic, Dordrecht, Netherlands.
Remans, R.; Ramaekers, L.; Schelkens, S.; Hernandez, G.; Garcia, A.; Reyes, J.L.; Mendez, N.; Toscano, V.; Mulling, M.; Galvez, L.; Vanderleyden, J. 2008. Effect of RhizobiumAzospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil 312: 25-37.
Saitou, N.; Nei, M. 1987. The neighbor joining 440 method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406-25.
Silva, K.; Florentino, L.A.; Silva, K.B.; Brandt, E.; Vandamme, P.; Moreira, F.M.S. 2012. Cupriavidus necator isolates are able to fix nitrogen in symbiosis with different legume species. Systematic and Applied Microbiology 35: 175-182.
Silva, V.N.S.; Silva, L.E.S.F.; Martínez, C.R.; Seldin, L.; Burity, H.A.; Figueiredo, M.V.B. 2007. Strains of Paenibacillus promoters of the specific nodulation in the symbiosis Bradyrhizobium - caupi. Acta Scientiarum Agronomy 29: 331-338.
Sprent, J.I. 2009. Legume Nodulation: A Global Perspective. Wiley-Blackwell, Chichester, UK.
Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-2739.
Versalovic, J.; Schneider, M.; Bruijn, F.J.; Lupski, J.R. 1994. Genomic finger-printing of bacteria using repetitive sequence-based polymerase chain re-action. Methods in Molecular and Cellular Biology 5: 25-40.
Vollmann, J.; Sato, T.; Walter, H.; Schweiger, P.; Wagentristl, H. 2011. Soybean di-nitrogen fixation affecting photosynthesis and seed quality characters. Soil, Plant and Food Interactions 496-502.
Willems, A.; Coopman, R.; Gillis, M. 2001. Phylogenetic and DNA-DNA hybridization analyses of Bradyrhizobium species. International Journal of Systematic and Evolutionary Microbiology 51: 111-117.
Zilli, J.E.; Valicheski, R.R.; Rumjanek, N.G.; Simões-Araújo, J.L.; Freire, F.R.F.; Neves, M.C.P. 2006. Symbiotic efficiency of cowpea Bradyrhizobium strains in Cerrado soils. Pesquisa Agropecuária Brasileira 41: 811-818.
Received March 15, 2013
Accepted July 19, 2013
- Argenta, G.; Silva, P.R.F.; Mielniczuk, J.; Botollini, C.G. 2002. Plant parameters as indicators of nitrogen status in maize. Pesquisa Agropecuária Brasileira 37: 519-527.
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. 2005. Current Protocols in Molecular Biology. Wiley, New York, NY, USA.
- Chagas Junior, A.F.; Oliveira, L.A.; Oliveira, N.A. 2010. Phenotypic characterization of rhizobia strains isolated from Amazonian soils and symbiotic efficiency in cowpea. Acta Scientiarum Agronomy 32: 161-169.
- Ferreira, D.F. 2011. Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35: 1039-1042.
- Fred, E.B.; Waksman, S.A. 1928. Laboratory Manual of General Microbiology. McGraw-Hill, New York, NY, USA.
- Guimarães, A.A.; Jaramillo, P.M.D.; Nóbrega, R.S.A.; Florentino, L.A.; Silva, K.B.; Moreira, F.M.S. 2012. Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Applied and Environmental Microbiology 78: 6726-6733.
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W-M.; Elliot, G.M.; Bontemps, C.; Santos, P.E.; Gross, E.; Reis Jr, F.B.; Sprent, J.I.; Young, J.P.W.; James, E.K. 2011. Legume-nodulating betaproteobacteria: diversity, host range and future prospects. Molecular Plant-Microbe Interactions 24: 1276-1288.
- Hoagland, D.; Arnon, D.I. 1950. The water culture method for growing plants without soil. University of California, Berkeley, CA, USA. (AES Circular, 347).
- Igual, J.M.; Valverde, A.; Cervantes, E.; Velásquez, E. 2001. Phosphate-solubilizing bacteria as inoculants for agriculture: use of updates molecular techniques in their study. Agronomie 21: 561-568.
- Krasova-Wade, T.; Ndoye, I.; Braconnier, S.; Sarr, B.; de Lajudie, P.; Neyra, M. 2003. Diversity of indigenous bradyrhizobia associated with three cowpea cultivars (Vigna unguiculata (L.) Walp.) grown under limited and favorable water conditions in Senegal (West Africa). African Journal of Biotechnology 2: 13-22.
- Lane, D.J. 1991. 16S/23S rRNA sequencing, p. 115-148. In: Stackebrandt E.; Goodfellow M., eds. Nucleic acid techniques in bacterial systematics. Wiley, New York, NY, USA.
- Lima, A.S.; Nóbrega, R.S.A.; Barberi, A.; Silva, K.; Ferreira, D.F.; Moreira, F.M.S. 2009. Nitrogen-fixing bacteria communities occurring in soils under different uses in the western Amazon Region as indicated by nodulation of siratro (Macroptilium atropurpureum). Plant and Soil 319: 127-145.
- Martins, L.M.V.; Xavier, G.R.; Rangel, F.W.; Ribeiro, J.R.A.; Neves, M.C.P.; Morgado, L.B.; Rumjanek, N.G. 2003. Contribution of biological nitrogen fixation to cowpea: a strategy for improving grain yield in the semi-arid region of Brazil. Biology and Fertility of Soils 38: 333-339.
- Moreira, F.M.S.; Nóbrega, R.S.A.; Jesus, E.C.; Ferreira, D.F.; Perez, D.V. 2009. Differentiation in the fertility of inceptisols as related to land use in the upper Solimões river region, western Amazon. Science of the Total Environment 408: 349-355.
- Moreira, F.M.S. 2006. Nitrogen-fixing Leguminosae nodulating bacteria, 280 p. In: Moreira, F.M.S.; Siqueira, J.O.; Brussaard, L., eds. Soil biodiversity in Amazonian and other Brazilian ecosystems. CAB International, Wallingford, UK.
- Moreira, F.M.S.; Gillis, M.; Pot, B.; Kersters, K.; Franco, A.A. 1993. Characterization of rhizobia isolated from different divergence groups of tropical leguminosae by comparative polyacrylamide gel electrophoresis of their total proteins. Systematic and Applied Microbiology 16: 135-146.
- Moreira, F.M.S.; Haukka, K.; Young, J.P.W. 1998. Biodiversity of rhizobia isolated form a wide range of forest legumes in Brazil. Molecular Ecology 7: 889-895.
- Pan, B.; Smith, D.L. 2000. Preincubation of B. japonicum cells with genistein reduces the inhibitory effects of mineral nitrogen on soybean nodulation and nitrogen fixation under field conditions. Plant and Soil 223: 235-242.
- Poustini, K.; Mabood, F.; Smith, D.L. 2007. Preincubation of Rhizobium leguminosarum bv. phaseoli with jasmonate and genistein signal molecules increases bean (Phaseolus vulgaris L.) nodulation, nitrogen fixation and biomass production. Journal of Agricultural Science and Technology 9: 107-117.
- Pule-Meulenberg, F.; Belane, A.K.; Krasova-Wade, T.; Dakora, F.D. 2010. Symbiotic functioning and bradyrhizobial biodiversity of cowpea (Vigna unguiculata L.Walp.) in Africa. BMC Microbiology 10: 89-100.
- Rademaker, J.L.W.; Frank, J.L.; Brujin, F.J. 1997. Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting, p. 1-26. In: Akkemans, A.D.L.; van Elsas, J.D.; de Brujin, F.J. Molecular microbial ecology manual. Kluwer Academic, Dordrecht, Netherlands.
- Remans, R.; Ramaekers, L.; Schelkens, S.; Hernandez, G.; Garcia, A.; Reyes, J.L.; Mendez, N.; Toscano, V.; Mulling, M.; Galvez, L.; Vanderleyden, J. 2008. Effect of RhizobiumAzospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil 312: 25-37.
- Saitou, N.; Nei, M. 1987. The neighbor joining 440 method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406-25.
- Silva, K.; Florentino, L.A.; Silva, K.B.; Brandt, E.; Vandamme, P.; Moreira, F.M.S. 2012. Cupriavidus necator isolates are able to fix nitrogen in symbiosis with different legume species. Systematic and Applied Microbiology 35: 175-182.
- Silva, V.N.S.; Silva, L.E.S.F.; Martínez, C.R.; Seldin, L.; Burity, H.A.; Figueiredo, M.V.B. 2007. Strains of Paenibacillus promoters of the specific nodulation in the symbiosis Bradyrhizobium - caupi. Acta Scientiarum Agronomy 29: 331-338.
- Sprent, J.I. 2009. Legume Nodulation: A Global Perspective. Wiley-Blackwell, Chichester, UK.
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-2739.
- Versalovic, J.; Schneider, M.; Bruijn, F.J.; Lupski, J.R. 1994. Genomic finger-printing of bacteria using repetitive sequence-based polymerase chain re-action. Methods in Molecular and Cellular Biology 5: 25-40.
- Vollmann, J.; Sato, T.; Walter, H.; Schweiger, P.; Wagentristl, H. 2011. Soybean di-nitrogen fixation affecting photosynthesis and seed quality characters. Soil, Plant and Food Interactions 496-502.
- Zilli, J.E.; Valicheski, R.R.; Rumjanek, N.G.; Simões-Araújo, J.L.; Freire, F.R.F.; Neves, M.C.P. 2006. Symbiotic efficiency of cowpea Bradyrhizobium strains in Cerrado soils. Pesquisa Agropecuária Brasileira 41: 811-818.
Publication Dates
-
Publication in this collection
03 Dec 2013 -
Date of issue
Dec 2013
History
-
Received
15 Mar 2013 -
Accepted
19 July 2013