Abstract
Cowpea (Vigna unguiculata) cultivation in northern and northeastern Brazil provides an excellent source of nutrients and carbohydrates for the poor and underprivileged. Production surplus leads to its consumption in other regions of Brazil and also as an export commodity. Its capacity to establish relationships with atmospheric nitrogen-fixing bacteria is crucial to the reduction of production costs and the environmental impact of nitrogen fertilizers. This study assessed the symbiotic efficiency of new strains of symbiotic nitrogen-fixing bacteria with cowpea and their tolerance to pH and aluminum. Twenty-seven strains of bacteria from different soils were evaluated under axenic conditions. These strains were compared to the following inoculant strains: INPA03-11B, UFLA03-84 and BR3267 and two controls that were not inoculated (with and without mineral nitrogen). Six strains and the three strains approved as inoculants were selected to increase the dry weight production of the aerial part (DWAP) and were tested in pots with soil that had a high-density of nitrogen-fixing native rhizobia. In this experiment, three strains (UFLA03-164, UFLA03-153, and UFLA03-154) yielded higher DWAP values. These strains grow at pH levels of 5.0, 6.0, 6.8 and at high aluminum concentration levels, reaching 10(9) CFU mL-1. In particular UFLA03-84, UFLA03-153, and UFLA03-164 tolerate up to 20 mmol c dm-3 of Al+3. Inoculation with rhizobial strains, that had been carefully selected according to their ability to nodulate and fix N2, combined with their ability to compete in soils that are acidic and contain high levels of Al, is a cheaper and more sustainable alternative that can be made available to farmers than mineral fertilizers.
Vigna unguiculata; Bradyrhizobium; Burkholderia; Native rhizobia
AGRICULTURAL MICROBIOLOGY
Cowpea symbiotic efficiency, pH and aluminum tolerance in nitrogen-fixing bacteria
Bruno Lima SoaresI; Paulo Avelar Ademar FerreiraI; Silvia Maria de Oliveira-LongattiI,II; Leandro Marciano MarraI; Marcia RufiniI; Messias José Bastos de AndradeIII; Fatima Maria de Souza MoreiraI,II,* * Corresponding author < fmoreira@dcs.ufla.br>
IFederal University of Lavras - Dept. of Soil Science, C.P. 3037 - 37200-000 - Lavras, MG - Brazil
IIFederal University of Lavras - Dept. of Biology
IIIFederal University of Lavras - Dept. of Agronomy
ABSTRACT
Cowpea (Vigna unguiculata) cultivation in northern and northeastern Brazil provides an excellent source of nutrients and carbohydrates for the poor and underprivileged. Production surplus leads to its consumption in other regions of Brazil and also as an export commodity. Its capacity to establish relationships with atmospheric nitrogen-fixing bacteria is crucial to the reduction of production costs and the environmental impact of nitrogen fertilizers. This study assessed the symbiotic efficiency of new strains of symbiotic nitrogen-fixing bacteria with cowpea and their tolerance to pH and aluminum. Twenty-seven strains of bacteria from different soils were evaluated under axenic conditions. These strains were compared to the following inoculant strains: INPA03-11B, UFLA03-84 and BR3267 and two controls that were not inoculated (with and without mineral nitrogen). Six strains and the three strains approved as inoculants were selected to increase the dry weight production of the aerial part (DWAP) and were tested in pots with soil that had a high-density of nitrogen-fixing native rhizobia. In this experiment, three strains (UFLA03-164, UFLA03-153, and UFLA03-154) yielded higher DWAP values. These strains grow at pH levels of 5.0, 6.0, 6.8 and at high aluminum concentration levels, reaching 109 CFU mL1. In particular UFLA03-84, UFLA03-153, and UFLA03-164 tolerate up to 20 mmolc dm3 of Al+3. Inoculation with rhizobial strains, that had been carefully selected according to their ability to nodulate and fix N2, combined with their ability to compete in soils that are acidic and contain high levels of Al, is a cheaper and more sustainable alternative that can be made available to farmers than mineral fertilizers.
Keywords:Vigna unguiculata, Bradyrhizobium, Burkholderia, Native rhizobia
Introduction
Cowpea (Vigna unguiculata) is an important crop in northern and northeastern Brazil, because it is an excellent source of nutrients and carbohydrates for the poor and underprivileged that produces and consumes it. Seventy percent of all beans produced in northern and northeastern Brazil is cowpea (Santos et al., 2000), but this crop is continually expanding into other areas in Brazil. The production of cowpeas is based on very low inputs and use of marginal soils that are phosphorus- and nitrogen (N)-deficient. Therefore, current yields in Brazil are very low: between 400 and 500 kg ha-1. To increase productivity, cowpea cultivation may be associated with nitrogen-fixing bacteria that provide its partial or total nitrogen fertilization. This would reduce production costs and prevent environmental pollution caused by the manufacture and misuse of nitrogen-based fertilizers. Several researchers have demonstrated the ability of these bacteria to provide N to crops with yields similar to 70 kg ha-1 of N-urea (Soares et al., 2006; Lacerda et al., 2004) or 50 kg N-urea ha-1 (Almeida et al., 2010).
Although cowpea is considered a promiscuous species for its ability to nodulate with several bacterial species and genera (Moreira, 2008), the maximum yield is obtained with strains belonging to Bradyrhizobium genus (Almeida et al., 2010; Chagas Junior et al., 2010; Costa et al., 2011; Lacerda et al., 2004; Moreira, 2006; Soares et al., 2006; Zilli et al., 2006). Soil characteristics, such as pH and Al3+, may compromise symbiotic efficiency and plant development. Ph values below 5.0 are reported to be deleterious for nodulation and nitrogen fixation (Appunu and Dhar, 2006; Mukherjee and Asanuma, 1998). On the other hand, four strains of Bradyrhizobium, that are approved as inoculants to the following: Glycine max (BR29, SEMIA587), Vigna unguicuata (INPA3-11B) and Enterolobium contortisiliquum (BR4406), all grow satisfactorily in pH values of 5.0; 6.0 and 6.8 (Miguel and Moreira, 2001; Barberi et al., 2004). Therefore, selection of bacteria with greater tolerance to different soil acidities and Al3+ concentrations is essential to maximizing symbiotic efficiency.
The diversity of soils and climates ensures a variety of native bacteria adapted to these diverse conditions. Moreover, in this edaphoclimatic diversity, the N2-fixing capacity of bacteria might significantly differ between strains, and thus require more appropriate selection under certain conditions. This study assessed the symbiotic efficiency of 27 bacterial strains isolated from different locations, as well as their tolerance to pH and Al3+ as compared to the three strains approved as inoculants.
Materials and Methods
The evaluation of strains efficiency comprised the first two steps in the methodology usually applied for this purpose: sterilized Leonard jars and non-sterilized pots containing soil.
Strain efficiency under axenic conditions
The first experiment was performed in Leonard jars in a greenhouse (Vincent, 1970), between 25 June 2007 and 16 Aug 2007. The experimental design was completely randomized, consisting of three replicates of 32 treatments. These treatments were inoculation with each one of the 27 strains (Table 1) and the three strains approved as inoculants for cowpea cultivation by the Brazilian Ministry of Agriculture Livestock and Supply (MAPA) (INPA 03-11B, UFLA 03-84 and BR 3267), and two non-inoculated controls, one without (C) and one with mineral N (C+N). N was supplied three times at 10-day intervals beginning 15 days after emergence, for a total treatment of 210 mg N (NH4NO3) per jar. A 1:1 mixture of sand (250 mL) and vermiculite (250 mL) was placed in the upper part of the Leonard jar; 4-fold diluted Jensen's nutrient solution was placed at the bottom (0.2 g L-1 K2HPO4; 0.2 g L-1 MgSO4·7H2O, 0.2 g L-1 NaCl, 1 g L-1 CaHPO4, 0.1 g L-1 FeCl3·6H2O; 2.86 mg L-1 H3BO3; 2.03 mg L-1 MnSO4·4H2O; 0.22 mg L-1 ZnSO4·7H2O; 0.08 mg L-1 CuSO4·5H2O; and 0.09 mg L-1 Na2MoO4·H2O). The nutrient solutions and jars were autoclaved for 1 h at 1.5 kg cm-2, 127 ºC.
Cowpea seeds were superficially disinfected with 98 % alcohol for 30 s, followed by 2 % sodium hypochlorite for 2 min. The seeds were washed six times with sterile distilled water to remove the residues of previous treatments. Then the seeds were immersed in sterile distilled water for two hours. Germination occurred on petri dishes where the seeds were placed with paper film and sterile moistened cotton and maintained in a growth chamber at 28 ºC for 24 h.
The cowpea variety used for the experiment was BR-17 Gurgueia. After sterilization and disinfection, four pre-germinated seeds were placed in each jar. These were then inoculated with 1 mL bacteria during the log phase of growth (109 cells mL-1) cultured in medium 79 (Fred and Waksman, 1928). In the non-inoculated control and in the absence of mineral N, only 1 mL sterile medium 79 was added. Ten days after germination, the seedlings were thinned, leaving two plants per jar. After seeding, a 2.0 cm layer of a sterile mixture of sand, benzene, and paraffin (5:1:0.015) was placed in the jar to avoid contamination.
Solution levels were periodically replenished with sterile Jensen nutrient solution. After 52 days, at maximum flowering, plants were harvested to assess dry weight of the aerial part (DWAP), number of nodules (NN), dry weight of nodules (DWN), and N accumulation in the aerial part (NAAP). Data were analyzed using the analysis of variance method as per Sisvar 5.3. Values for NN and DWN were previously transformed into (X + 0.5)0.5.
Strain efficiency in pots with soil
This experiment was performed in a greenhouse, from 11 Feb 2008 to 11 May 2008 in plastic pots (5.0 dm3). The soil was a Haplic Cambisol, collected in the municipality of Itutinga-MG (21º23'29" S; 44º39'13" W), with Brachiaria (Urochloa decumbens) cover and no leguminous plants. The soil was obtained from the arable layer (0 to 20 cm), air-dried, conditioned, homogenized and passed through a 4-mm sieve before use as a substrate. The chemical characteristics of the soil prior to liming and fertilization were: pH in H2O (1:2.5) 5.8; P (Mehlich 1) 2.3 mg dm-3; K+ (Mehlich 1) 58 mg dm-3; Ca2+ 15 mmolc dm-3; Mg2+ 8 mmolc dm-3; Al3+ 0 mmolc dm-3; H+Al 29 mmolc dm-3 (extracted by 1 M KCl); Sum of Bases 25 mmolc dm-3; effective Cation Exchange Capacity (CEC) 25 mmolc dm-3; potential CEC pH 7 53 mmolc dm-3; aluminum saturation 0 %; base saturation 45 %; organic matter (2 M sulfuric acid + 5 M sodium dichromate) 30 g kg-1.
The base saturation method was used to calculate the amount of lime in order to increase the base saturation to 60 %. Dolomitic lime was applied 30 days before sowing, while maintaining soil humidity in the field capacity to allow the reaction to occur. In all plots, fertilization was carried out with 300, 300, 40, 0.8, 1.5, 3.6, 5.0, and 0.15 mg dm-3 K, P, S, B, Cu, Mn, Zn, and Mo, respectively (Malavolta et al., 1989). The sources were Super Simple phosphate [3Ca(H2PO4)2], Potassium Sulfate (K2SO4), Boric Acid (H3BO3), Copper Sulfate (CuSO4), Manganese Sulfate (MnSO4), Zinc Sulfate (ZnSO4), and Sodium Molybdate (Na2MoO4).
The experimental design was completely randomized and consisted of three replicates and 11 treatments with the strains that demonstrated the best capacity to induce dry weight of the aerial part (DWAP) in experiment 1, in addition to three strains approved as inoculants for cowpea cultivation (UFLA 03-84, INPA 03-11B, and BR 3267) and two non-inoculated controls, without (C) and with mineral N [C+N; 500 mg/ pot of NH4NO3. Mineral N was divided into two applications (at sowing and 20 days after emergence)]. The cowpea used in the experiment was BR-17 Gurguéia.
After sterilization and disinfection as previously described, pre-germinated seeds were distributed four per pot and inoculated with 1 mL bacteria (109 cells mL-1) grown in medium 79 (Fred and Waksman, 1928) and added during log-phase growth. In the non-inoculated control and without addition of mineral N, only 1 mL sterile medium 79 was added. Ten days after germination, the plants were thinned, leaving two per pot. Plants were harvested at maximum flowering to assess dry weight of the aerial part (DWAP), number of nodules (NN), dry weight of nodule (DWN), and N accumulation in the aerial part (NAAP). Data were analyzed by the analysis of variance method as per Sisvar 5.3.
Rhizobia native populations density of soil
The experiment was conducted in Leonard jars (Vincent, 1970), from 15 Feb 2008 to 15 Mar 2008 to assess the most probable number of native soil rhizobia in experiment 2. The experimental design was completely randomized, and consisted of three replicates of eight treatments. The treatments were 1-mL inoculations of 10-1 to 10-6 serial dilutions of soil suspension and two non-inoculated controls, one without (C) and one with mineral N (C+N). At the control C+ N, N was supplied three times at 10-day intervals, 15 days after emergence, for a total treatment of 210 mg N (NH4NO3) per jar. The preparation of Leonard jars and seeds was performed as described in experiment 1.
To estimate the most probable number (MPN) of rhizobia cells in the three collected samples the MPNES (most probable number estimate) program was used after recording the presence (positive) and absence (negative) of rhizobia in each dilution (Woomer et al. 1988). Data including dry weight in the aerial part (DWAP), dry weight of nodules (DWN), and number of nodules (NN) were compared by the analysis of variance method in Sisvar 5.3.
Strain tolerance to pH and Al
The three strains with better symbiotic efficiency in soil pots, together with UFLA 03-84, INPA 03-11B, and BR 3267, were evaluated for their tolerance to acidity and aluminum (Al3+) in liquid culture medium. Isolated colonies from each strain were inoculated in medium 79 (Fred and Waksman, 1928) without the bromothymol blue dye and grown until they reached an optical density and incubated to optical density (OD560) of 0.5 at 560 nm.
A 1 mL inoculum was transferred into 100 mL culture medium 79 supplemented with HEPES (1.3 mg L-1 N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid) and MES [1.1 mg L-1 2-(N-morpholino) ethane sulfonic acid] (Cole and Elkan, 1973), with pH adjusted with HCl 2 mol L-1 to 5.0, 6.0, and 6.9. The influence of Al3+ was evaluated by adding 0, 5, 10, and 20 mmolc L-1 Al3+ as AlCl3·6H2O to the medium before autoclaving, followed by adjustment to pH 4.5. Strains were grown subjected to shaking at 120 rpm at 28 ºC. Colony forming units (CFU) were counted by using serial dilutions as described by Miles et al. (1938). Aliquots of 20 µL were collected at intervals of 24, 48, 72, 96, 120, 144, 168, and 192 h incubation. The number of CFUs was assessed by directly counting the number of colonies on the plates at each dilution.
Results
Strain efficiency under axenic conditions
In the experiment conducted in Leonard jars, four groups based on the means were identified for the production of dry weight in the aerial part (DWAP) of cowpea plants (Table 2). In the first group, only the C+N control yielded higher values. In the second group, two strains, UFLA 03-164 and UFLA 03-153, showed DWAP values lower than 28 % and 40 %, respectively, in comparison to the nitrogen control. The third group was formed by strains recommended as inoculants for cowpea cultivation (UFLA 03-84, INPA03-11B, and BR 3267) and four strains (UFLA 03-154, UFLA 03-21, UFLA 03-165, and UFLA 04-885), all of which yielded DWAP higher than the control without mineral N, although these values were lower than the yields provided by strains in the second group.
Eighteen strains yielded similar numbers of nodules (p < 0.05) as the strains approved as inoculants for cowpea (UFLA 03-84, INPA 03-11B, and BR 3267). The non-inoculated controls (with and without mineral nitrogen) produced no nodules, indicating the absence of contamination (Table 2).The six strains with higher DWAP values included strains UFLA 03-164 and UFLA 04-0885 that had DWN lower than the four other strains (Table 2). UFLA 03-164 and UFLA 03-153 have nitrogen accumulation in the aerial part similar to the nitrogen fertilizer control. In the three other treatments, UFLA 03-154, UFLA 04-885, and UFLA 03-165 were included in the group of UFLA 03-84 (Table 2).
Strain efficiency in plots with soil and native population density of soil rhizobia
In the experiment carried out in pots with soil in the greenhouse to evaluate the efficient of each strain, UFLA 03-164, UFLA 3-154, and UFLA 03-153 excelled in promoting higher DWAP, with values similar to those of nitrogen-fertilized controls and those inoculated with a MAPA-approved strain, INPA 03-11B (Table 3). Only UFLA 03-153 showed higher NN values; and was included in the BR 3267 group. UFLA 03-154 was in the lower group, although it did not differ from INPA03-11B (Table 3).
No difference in DWN was noted among treatments (p < 0.05), except for the nitrogen-fertilizer control, which developed a few small nodules only.
Nitrogen accumulation in the aerial part was higher in the nitrogen-fertilizer control, thus differing from the other treatments. The control without mineral nitrogen and inoculation showed DMAP, DMN, and NAAP values similar to those provided by BR 3267 and UFLA 03-84 (Table 3). In the evaluation of the native population (Table 4) nodulation occurred up to dilution 10-6, and the DWAP in the dilution 10-1 up to 10-3 was no different (p < 0.05) from the N-fertilizer control indicating a highly efficient native population and a high density of soil rhizobia.
Tolerance to pH and Aluminum "in vitro"
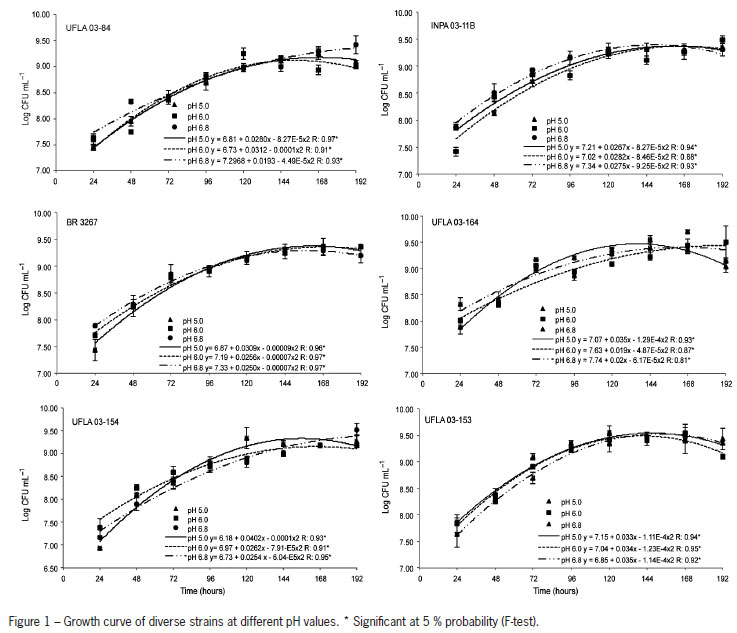
The strains grew efficiently at different pH values (Figure 1). At pH 5.0, all strains exceeded 109 CFU mL-1, indicating a good capacity to tolerate acidic conditions. However, these values were not reached at the same time. UFLA 03-164 had values higher than 109 CFU mL-1, at 72 h, whereas INPA 03-11, UFLA 03-153, and UFLA 03-154 reached these values only at 96 h. UFLA 03-84 presented similar values only at 144 h. At pH 6.0, growth above 109 CFU mL-1 was faster with UFLA 03-153, UFLA 03-164, and BR 3267 at 96 h; for UFLA 03-84 and INPA 03-11B, these values were reached at 120 h. At pH 6.9, the maximum number of cells was more rapidly obtained (96 h) with UFLA 03-153, UFLA 03-164, and BR 3267, followed by INPA 03-11B at 120 h and UFLA 03-84 and UFLA 03-164 at 144 h. UFLA 03-164 generated a greater increase in pH, from pH 5.0 to pH 5.95, in the culture medium. However, all the strains increased the pH (Table 5). This increase was more significant when the strains were grown at pH 5.0 and 6.0.
In the aluminum tolerance test (Figure 2) at pH 4.5 in the absence of Al3+, three strains (UFLA 03-84, INPA 03-11B, and BR 3267) grew satisfactorily, reaching more than 109 CFU mL-1 at 96 h; UFLA 03-153 reached this value at 120 h. UFLA 03-154 and UFLA 03-164 reached 108.90 CFU mL-1 and 108.86 CFU mL-1 at 144 h and showed a higher tolerance to pH 4.5 in the absence of Al+3. Addition of 5 mmolc dm-3 of Al3+ prevented all strains from reaching 109 CFU mL-1, although some strains reached 108 CFU mL-1 at 120 h. This indicates high adaptability to acidic pH and to low concentrations of Al3+. The only exception was UFLA 03-154, which showed limited growth at 5 mmolc dm-3. For concentrations of 10 mmolc dm-3 of Al+3, all strains, except UFLA 3-154, appeared to be tolerant, with values reaching around 108.0 UFC mL-1. For concentrations of 20 mmolc Al+3 only strains UFLA3-84, UFLA 3-153 and UFLA 3-164 were able to grow, although less than at lower Al+3 concentrations. There was no relationship between the origin of the strains, i.e., the soil characteristics from where they were isolated (Table 1) and their tolerance to acidity and aluminum.
Discussion
The DWAP of the six strains studied were lower than the C+N (p < 0.05), but were similar to the three bacterial strains approved as inoculants for cultivation. Lima et al. (2005) used Leonard jars for experiments performed in the spring/summer and found that UFLA 04-885 and UFLA 03-84 belong to the same symbiotic efficiency group in comparison to controls treated with mineral nitrogen. This suggested that even under axenic conditions, climate effects might impact strain efficiency, in contrast to experiments carried out in the autumn/winter. DWAP positively correlated with DWN (r: 0.42; p < 0.01), as observed by Fernandes et al. (2003), and with N accumulation (r: 0.93; p < 0.01). Positive correlation was also observed between DWN and N accumulation (r: 0.56; p < 0.01), thus confirming the data obtained by Döbereiner et al. (1966), Fernandes and Fernandes (2000), and Lima et al. (2005).
The soil used in the pots had a high density of native rhizobia, including strains that can fix nitrogen when associated with cowpea. In this experiment, UFLA 3-153, UFLA 3-154, UFLA 0-164, and INPA 03-11B were similar to C+N. The high efficiency of INPA 03-11B has also been observed by other authors in field experiments. Costa et al. (2011), Lacerda et al. (2004) and Soares et al. (2006) showed INPA 03-11B has been included in the same control group that received nitrogen fertilization. Costa et al. (2011), also showed that UFLA 03-153 has been included in this group.
C+ N treatment was higher than inoculated strains in Leonard jars condition (Table 2). However, in pots with soil C+ N treatment was similar to inoculant strains (Table 3). This happens because plant response to mineral N is faster than to inoculation. However, when more time is given for plants to develop, and this includes the conditions for this development, responses become similar. Nitrogen treatment similar to strain inoculation also occurs in the field when yields are evaluated (Soares et al., 2006).
The native population of rhizobia efficiently fixed nitrogen under controlled condition, with DWAP values similar to C+N. Density of these native populations can be considered high: from 2 × 105 to 4 × 106 CFU per gram of soil. These are usual values for soils, since the native population of rhizobia can reach up to 107 CFU per gram (Hirsch, 1996). Martins et al. (2003) observed 104 CFU per gram of soil after the second year of rhizobial inoculation in cowpea cultivations in the northeast of Brazil and Soares et al. (2006) showed native populations to be about 23 and 38 CFU per gram of soil in southeast Brazil. Considering the high numbers of native populations, we have to prove that the inoculated strains in soil were the ones that induced higher production of DWAP. Thus, the patterns of root infection by INPA 03-11B, UFLA 03-153, C+N, and C (Figure 3) treatments were compared.
Roots in the C+N had no nodules, due to inhibition by mineral nitrogen (Figure 3A). Figure 3B shows the pattern of nodulation of control by native soil bacterial that were distributed throughout all roots, thus differing from the INPA 03-11B and UFLA 03-153 inoculation treatments, which yielded a pattern of infection at the base of the shoot, typical of a cell concentration derived from inoculation (Figures 3C and 3D).
UFLA 3-153 and UFLA 0-164 belongs to Bradyrhizobium genus, as well as INPA 3-11B, UFLA3-84 and Br3267, the strains currently approved by MAPA as cowpea inoculants (Table 1). UFLA 03-154 belongs to the genus Burkholderia. Nodulation of cowpea by Burkholderia strain UFLA 03-216 was reported previously (Guimarães et al., 2012). However, this strain was inefficient in terms of symbiotic nitrogen fixation with cowpea. Thus, this is the first report of the soil symbiotic efficiency of this genus in cowpea. Nodulation by unusual genera such as Enterobacter, Pseudomonas, Acinetobacter corroborates findings previously reported (Guimarães et al., 2012; Marra et al., 2012). This nodulation was confirmed by Koch´s postulates, i.e. pure cultures were re inoculated under axenic conditions and reisolated from nodules. Besides, contamination could be detected by low quality of sequences, which was not the case. Bacterial endophytes of nodules may become symbiotic by horizontal gene transfer from symbiotic bacteria (Li et al., 2008; Shiraishi et al., 2010).
Shiraishi et al. (2010) detected occurrence of nodulation in Robinia pseudoacacia by Pseudomonas sp. and the presence of nodA, nifH and nifHD in this genus. Shiraishi et al. (2010) also demonstrated that nodA genes from Pseudomonas, Agrobacterium, Burkholderia and Mesorhizobium isolates, from the same soil, had high genetic relationship. Further studies looking for the presence of nodulation and nitrogen fixation genes must confirm their taxonomic position of our strains.
All the strains studied were tolerant to acidity, although they grew at different rates (Figure 1). Graham et al. (1994) showed that Bradyrhizobium strains grow on solid media at pH 4.25 although with a late growth. The variation in time to maximum bacterial growth might be related to membrane permeability (Correa and Barneix, 1997). Chen et al. (1993 a,b) suggest that tolerance to acidity is related to the ability to maintain the cytoplasmic pH unchanged.
The strains isolated from bauxite mining areas, where the pH of the soil is between 5.13 and 7.5, seem to have acquired adaptation mechanisms for growth at pH 5.0; the strains recommended as inoculants were isolated from Amazonian acid soils (INPA 03-11B and UFLA 03-84). Ferreira et al. 2012 showed Rhizobium strains from the same origin were highly tolerant to acidity and up to 1 mmol Al3+ L-1. At varying Al+3 concentrations bacterial growth yielded different changes in medium pH (Table 5). However, all alkalinized in the media, a characteristic typical of Bradyrhizobium genus. Tolerance to pH and acidity may differ among strains, as Cunningham and Munns (1984) reported that production of exopolysaccharides might coincide with increased acid tolerance. R. miluonense isolated from the acidic soil of the Amazon showed high tolerance to acidity and aluminum, an effect that might be related to plasma membrane permeability (Ferreira et al., 2012).
The adaptation of tropical Bradyrhizobium strains to acidity was also verified previously. Miguel and Moreira (2001) observed that four Bradyrhizobium strains grew better at pH 6.0. Barberi et al. (2004) showed that B. elkanii BR 29 grew better at pH 6.8 or 5.5 supplemented with calcium. Our results show that UFLA 03-153, UFLA 03-154 and UFLA 03-164 are also adapted to acidity and highly efficient in symbiotic N2 fixation with cowpea. Besides, UFLA 03-153 and UFLA 03-164 are also tolerant to high Al+3 concentrations. Thus, they should be assessed regarding agronomic efficiency in the field, in both acidic and neutral soil. The good performance of inoculant strain UFLA3-84 in various soils must be due to its tolerance to various antibiotics produced by other microorganisms (Florentino et al., 2012) as well as its adaptation to acidity and high alumimum contents in soil.
Conclusions
Bradyrhizobium strains UFLA3-164, UFLA3-153, and Burkholderia strain UFLA3-154 isolated from bauxite mining areas, have high nitrogen-fixing capacity in symbiosis with cowpea and are good competitors with native soil rhizobia, and are thus recommended for field experiments.
All strains grow well at pH 5.0, 6.0, and 6.8. In particular, UFLA 03-84, UFLA 03-153, and UFLA 03-164 grow in media supplemented with up to 20 mmolc Al+3 dm-3.
Inoculation with rhizobial strains that have been carefully selected according to their ability to nodulate and fix N2, combined with their ability to compete in soils that are acidic and contain high levels of Al, is a cheaper and more sustainable alternative that can be made available to farmers than mineral N fertilizers.
Acknowledgments
We thank CAPES (Coordination for the Improvement of Higher Level Personnel), Fapemig (Foundation for Research Support of the State of Minas Gerais) and CNPq (Brazilian National Council for Scientific and Technological Development) for student fellowships, CNPq for a research fellowship and grant, and project GEF/UNEP-GF2715-02 (CSM-BGBD) for financial support. This paper presents part of the findings of the international project "Conservation and Management of Below-Ground Biodiversity" being implemented in seven tropical countries-Brazil, Cote d'Ivoire, India, Indonesia, Kenya, Mexico, and Uganda. This project was coordinated by the Tropical Soil Biology and Fertility Institute of CIAT (TSBF-CIAT) with co-financing from the Global Environmental Facility (GEF), and implementation support from the United Nations Environment Program (UNEP). Brazilian Co-executing Institution was Federal Universidade of Lavras in Brazil project CSM-BGBD was named BiosBrasil. Views expressed in this publication are those of their authors and do not necessary reflect those of the authors' organization, the United Nations Environment Programme and the Global Environmental Facility.
Received August 29, 2013
Accepted December 19, 2013
Edited by: Leônidas Carrijo Azevedo Melo
- Almeida, A.L.G.; Alcantara, R.M.C.M.; Nóbrega, R.S.A.; Nóbrega, J.C.A.; Leite, L.F.C.; Silva, J.A.L. 2010. Yield and nodulation of Vigna unguiculata (L.) Walp. inoculated with rhizobia strains in Bom Jesus, PI. Revista Brasileira de Ciências Agrárias 5:364-369 (in Portuguese, with abstract in English
- Appunu, C.; Dhar, B. 2006. Symbiotic effectiveness of acid-tolerant Bradyrhizobium strains with soybean in low pH soil. African Journal of Biotechnology 5:842-845.
- Barberi, A.; Moreira, F.M.S.; Florentino, L.A.; Rodrigues, M.I.D. 2004. Growth of Bradyrhizobium elkanii Strain BR 29 in culture media with different pH values. Ciência e Agrotecnologia 28:397-405 (in Portuguese, with abstract in English).
- Chagas Junior, A.F.; Rahmeier, W.; Fidelis, R.R.; Chagas, L.F.B. 2010. Agronomic efficiency of rhizobium strains inoculated in cowpea in the Cerrado, Gurupi-TO. Revista Ciência Agronômica 41:709-714 (in Portuguese, with abstract in English).
- Chen, H.; Gartner, E.; Rolfe, B.G. 1993b. Involvement of genes on a megaplasmid in the acid-tolerant phenotype of Rhizobium leguminosarum biovar trifolii. Applied and Environmental Microbiology 59:1058-1064.
- Chen, H.; Richardson, A.E.; Rolfe, B.G. 1993a. Studies of the physiological and genetic basis of acid tolerance in Rhizobium leguminosarum biovar trifolii. Applied and Environmental Microbiology 59:1798-1804.
- Cole, M.A.; Elkan, G.H. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrobial Agents and Chemotherapy 4:248-253.
- Correa, O.S.; Barneix, A.J. 1997. Cellular mechanisms of pH tolerance in Rhizobium loti. World Journal of Microbiology and Biotechnology 13:153-157.
- Costa, E.M.; Nóbrega, R.S.A.; Martins, L.V.; Amaral, F.H.C.; Moreira, F.M.S. 2011. Yield and nodulation of Vigna unguiculata (L.) Walp. inoculated with rhizobia strains in Bom Jesus, PI. Revista Ciência Agronômica 42: 1-7 (in Portuguese, with abstract in English).
- Cunningham, S.D.; Munns, D.N. 1984. The correlation between extracellular polysaccharide production and acid tolerance in Rhizobium Soil Science Society of America Journal 48:1273-1276.
- Döbereiner, J.; Arruda, N.B.; Penteado, A.F. 1966. Evaluation of nitrogen fixation in legumes, by regression of total plant nitrogen on nodule weight. Pesquisa Agropecuária Brasileira 1:233-237 (in Portuguese, with abstract in English).
- Fernandes, M.F.; Fernandes, R.P.M. 2000. Initial screening and partial characterization of rhizobia from a brazilian coastal tableland when associated to pigeonpea. Revista Brasileira de Ciência do Solo 24:321-327 (in Portuguese, with abstract in English).
- Fernandes, M.F.; Fernandes, R.P.M.; Hungria, M. 2003. Genetic characterization of indigenous rhizobia strains from the coastal tableland efficient for the pigeonpea and cowpea crops. Pesquisa Agropecuária Brasileira 38:911-920 (in Portuguese, with abstract in English).
- Ferreira, P.A.A.; Bomfeti, C.A.; Soares, B.L.; Moreira, F.M.S. 2012. Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World Journal of Microbiology and Biotechnology 28:1947-1959.
- Florentino, L.A.; Jaramillo, P.M.D.; Silva, K.B.; Silva, J.S.; Oliveira, S.M.; Moreira, F.M.S. 2012. Physiological and symbiotic diversity of Cupriavidus necator strains isolated from nodules of Leguminosae species. Scientia Agricola 69:247-258.
- Fred, E.B.; Waksman, S.A. 1928. Laboratory manual of General Microbiology: with Special Reference to the Microorganisms of the Soil. McGraw-Hill, New York, NY, USA.
- Graham, P.H.; Draeger, K.J.; Ferrey, M.L.; Conroy, M.J.; Hammer, B.E.; Martinez, E.; Aarons, S.R.; Quinto, C. 1994. Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Canadian Journal of Microbiology 40:198-207.
- Guimarães, A.A.; Jaramillo, P.M.D.; Nóbrega, R.S.A.; Florentino, L.A.; Silva, K.B.; Moreira, F.M.S. 2012. Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the Western Amazon by using Cowpea as the trap plant. Applied and Environmental Microbiology 78:6726-6733.
- Hirsch, R.P. 1996. Population dynamics of indigenous and genetically modified rhizobia in the field. New Phytologist 133:159-171.
- Lacerda, A.M.; Moreira, F.M.S.; Andrade, M.J.B.; Soares, A.L.L. 2004. Yield and nodulation of Cowpea inoculated with selected rhizobia strains. Revista Ceres 51: 67-82 (in Portuguese, with abstract in English).
- Li, J.H.; Wang, E.T.; Chena, W.F.; Chena, W.X. 2008. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biology and Biochemistry 40:238-246.
- Lima, A.S.; Pereira, J.P.R.A.; Moreira, F.M.S. 2005. Phenotypic diversity and symbiotic efficiency of Bradyrhizobium spp. strains from Amazonian soils. Pesquisa Agropecuária Brasileira 40:1095-1104 (in Portuguese, with abstract in English).
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. 1997. Assessment of nutritional status of plants: principles and applications = Avaliação do estado nutricional de plantas: princípios e aplicacões. 2ed. Potafos, Piracicaba, SP, Brazil. (in Portuguese).
- Marra, L.M.; Soares, C.R.F.S.; Oliveira, S.M.; Ferreira, P.A.A.; Soares, B.L.; Carvalho, R.F.; Lima, J.M.; Moreira, F.M.S. 2012. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant and Soil 357:289-307.
- Martins, L.M.V.; Xavier, G.R.; Rangel, F.W.; Ribeiro, J.R.A.; Neves, M.C.P.; Morgado, L.B.; Rumjanek, N.G. 2003. Contribution of biological nitrogen fixation to cowpea: a strategy for improving grain yield in the semi-arid region of Brazil. Biology and Fertility of Soils 38:333-339.
- Miguel, D.L.; Moreira, F.M.S. 2001. Influence of medium and peat pH on the behaviour of bradyrhizobium strains. Revista Brasileira de Ciência do Solo 25:873-883 (in Portuguese, with abstract in English).
- Miles, A,A.; Misra, S.S.; Irwin, J.O. 1938. The estimation of the bactericidal power of the blood. Journal of Hygiene 38:732-749.
- Moreira, F.M.S. 2006. Nitrogen-fixing Leguminosae-nodulating bacteria. In: Moreira, F.M.S.; Siquira, J.O.; Brussaard, L., eds. Soil biodiversity in Amazonian and other Brazilian ecosystems. 3ed. CAB International, Wallingford, UK. p.237-270.
- Moreira, F.M.S. 2008. Nitrogen fixing bacteria nodulating legumes = Bactérias fixadoras de nitrogênio que nodulam leguminosas. In: Moreira, F.M.S.; Siqueira, J.O.; Brussaard, L., eds. Soil biodiversity in Brazilian ecosystems = Biodiversidade do solo em ecossistemas Brasileiros. UFLA, Lavras, MG, Brazil. p.621-680 (in Portuguese).
- Mukherjee, S.K.; Asanuma, S. 1998. Possible role of cellular phosphate pool and subsequent accumulation of inorganic phosphate on the aluminum tolerance in Bradyrhizobium japonicum Soil Biology & Biochemistry 30:1511-1516.
- Oliveira-Longatti, S.M.; Marra, L.M.; Soares, B.L.; Bomfeti, C.A; Silva, K.; Ferreira, P.A.A.; Moreira, F.M.S. 2014. Bacteria isolated from soils of the western Amazon and from rehabilitated bauxite-mining areas have potential as plant growth promoters. World Journal of Microbiology and Biotechnology 30:1239-1250.
- Santos, C.A.F.; Araujo, F.A.; Menezes, E.A. 2000. Yield performance of cowpea genotypes under irrigated and rainfed conditions in Petrolina and Juazeiro, Brazil. Pesquisa Agropecuária Brasileira 35:2229-2234 (in Portuguese, with abstract in English).
- Shiraishi, A.; Matsushita, N.; Hougetsu, T. 2010. Nodulation in black locust by the Gammaproteobacteria Pseudomonas sp. and the Betaproteobacteria Burkholderia sp. Systematic and Applied Microbiology 33:269-274.
- Soares, A.L.L.; Pereira, J.P.A.R.; Ferreira, P.A.A.; Vale, H.M.M.; Lima, A.S.; Andrade, M.J.B.; Moreira, F.M.S. 2006. Agronomic efficiency of selected rhizobia strains and diversity of native nodulating populations in Perdões (MG-Brazil). I. Cowpea. Revista Brasileira de Ciência do Solo 30:795-802 (in Portuguese, with abstract in English).
- Vincent, J.M. 1970. A manual for the practical study of the root-nodule bacteria: a manual for the practical study of the root-nodule bacteria. IBP, Houston, TX, USA. (IBP Handbook).
- Woomer, P.; Singleton, P.W.; Bohlool, B.B. 1988. Ecological indicators of native rhizobia in tropical soils. Applied and Environmental Microbiology 54:1112-1116.
- Zilli, J.E.; Valicheski, R.R.; Rumjanek, N.G.; Simões-Araujo, J.L.; Freire Filho, F.R.; Neves, M.C.P. 2006. Eficiência simbiótica de estirpes de Bradyrhizobium isoladas de solo do Cerrado em Caupi. Pesquisa Agropecuária Brasileira 41:811-818. (in Portuguese, with abstract in English).
Publication Dates
-
Publication in this collection
15 May 2014 -
Date of issue
June 2014
History
-
Accepted
19 Dec 2013 -
Received
29 Aug 2013