ABSTRACT
Caused by the vascular fungus Ceratocystis fimbriata, mango wilt is considered to be one of the most serious threats in mango-producing regions worldwide. However, changes in leaf gas exchange level and the mechanisms underlying host responses to this fungal infection remain poorly described. This study aimed to evaluate potential changes in the leaf gas exchange of different mango cultivars (Ubá, Espada, Haden and Tommy Atkins) in response to two Brazilian isolates of C. fimbriata (CEBS15 and MSAK16) to non-invasively assess cultivar variability in relation to the basal level of resistance to mango wilt. Both isolates, regardless of the cultivar, caused reductions in stomatal conductance and, thus, a reduction in CO2 assimilation via diffusive limitations. Taking into account the full length of the internal lesion and the radial colonization of the stem tissues, both isolates showed equivalent aggressiveness when inoculated into the Haden and Tommy Atkins cultivars. Conversely, when compared to the CEBS15 isolate of C. fimbriata, the MSAK16 isolate was more aggressive in cv. Espada and less aggressive in cv. Ubá.
Mangifera indica; mango wilt; photosynthesis; vascular disease
Introduction
Caused by the vascular fungus Ceratocystis fimbriata (Halsted, 1890Halsted, B.D. 1890. Some Fungous Diseases of the Sweet Potato. New Jersey Agricultural College Experiment Station, New Brunswick, NJ, USA. (Bulletin, 76).), mango wilt is considered to be one of the most serious threats in mango-producing regions worldwide (Masood and Saeed, 2012Masood, A.; Saeed, S. 2012. Bark beetle, Hypocryphalus mangiferae Stebbing (Coleoptera: Curculionidae: Scolytinae) is a vector of mango sudden death disease in Pakistan. Pakistan Journal of Botany 44: 813-820.), often leading to tree death and the decline of entire orchards (Ribeiro, 1997Ribeiro, I.J.A. 1997. Mango (Mangifera indica L.) diseases = Doenças da manga (Mangifera indica L.). p. 511-524. In: Kimati, H.; Amorim, L.; Bergamin Filho, A.; Camargo, L.E.A.; Rezende, J.A.M., eds. Phytopathology manual: diseases of cultivated plants. Agronômica Ceres, São Paulo, SP, Brazil (in Portuguese).; Masood and Saeed, 2012Masood, A.; Saeed, S. 2012. Bark beetle, Hypocryphalus mangiferae Stebbing (Coleoptera: Curculionidae: Scolytinae) is a vector of mango sudden death disease in Pakistan. Pakistan Journal of Botany 44: 813-820.). During infections by vascular pathogens, including C. fimbriata, vascular disorders usually lead to a decrease in hydraulic conductivity and the ensuing leaf water deprivation substantially affects plant growth and metabolism as a whole, reducing leaf gas exchange and inducing large alterations in source-sink relationships (Flexas et al., 2004Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmes, J.; Gulias, J.; Lefi, E.; Martinez-Canellas, S.F.; Moreno, M.T.; Ribas-Carbo, M.; Riera, D.; Sampol, B.; Medrano, H. 2004. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Annals of Applied Biology 144: 273-491., 2007Flexas, J.; Diaz-Espejo, A.; Galmes, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. 2007. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment 30: 1284-1298.; Roitsch, 1999).
Crop yield has a high dependence on biomass production (Johnson, 1987Johnson, K.B. 1987. Defoliation, disease and growth: a reply. Phytopathology 77: 1495-1497.), since the amount of carbohydrate supplied to fruits during their formation depends directly on the amount of assimilates produced during the photosynthetic process (Fishman and Génard, 1998Fishman, S.; Génard, M. 1998. A biophysical model of fruit growth: simulation of seasonal and diurnal dynamics of mass. Plant, Cell and Environment 21: 739-752.; Rosati et al., 1999Rosati, A.; Esparza, G.; DeJong, D.M.; Pearcy, R.W. 1999. Influence of canopy light environment and nitrogen availability on leaf photosynthetic characteristics and photosynthetic nitrogen-use efficiency of field-grown nectarine trees. Tree Physiology 20: 271-276.; Le Roux et al., 2001Le Roux, X.; Walcroft, A.S.; Daudet, F.A.; Sinoquet, H.; Chaves, M.M.; Rodrigues, A.; Osorio, L. 2001. Photosynthetic light acclimation in peach leaves: importance of changes in mass: area ratio, nitrogen content, and leaf nitrogen partitioning. Tree Physiology 21: 377-386.). Thus, because photosynthesis, and ultimately the source-sink regulation of sugar partitioning, is related to (and depends on) a healthy leaf area and the integrity of the plant, it may be assumed that, as a consequence of the alterations in leaf gas exchange caused by disease, considerable reductions in crop yield result from pathogen invasion and establishment in plant tissues.
Photosynthesis may be considered a component of the integrated plant system, and thus, it is one of the physiological processes that is most sensitive to several abiotic and biotic stresses (Berry and Bjorkman, 1980Berry, J.; Bjorkman, O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491-543.; Baastians, 1991Baastians, L. 1991. Ratio between virtual and visual lesion size as a measure to describe reduction in leaf photosynthesis of rice due to leaf blast. Phytophatology 81: 611-615.; Elings et al., 1999Elings, A.; Rossing, W.A.H.; Werf, W. van der. 1999. Virtual lesion extension: a measure to quantify the effects of bacterial blight on rice leaf CO2 exchange. Phytopathology 89: 789-795.; Aucique-Perez et al., 2014Aucique-Perez, C.E.; Rodrigues, F.A.; Moreira, W.R.; DaMatta, F.M. 2014. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytophatology 104: 143-149.). Therefore, the assessment of changes in leaf gas exchange may assist in elucidating the high variability between mango cultivars in terms of their basal level of resistance to mango wilt. A better understanding of the relationship between C. fimbriata isolates and mango cultivars will be very important for plant breeding programs whose goal is screening accessions for resistance to the disease. Hence, considering the substantial yield losses in producing areas and the consequent need for more information about its effect over mango physiology, the main goal of this study was to assess the contrasting behavior of a number of mango cultivars against infection by certain C. fimbriata isolates in terms of plant leaf gas exchange performance.
Materials and Methods
Plant material
Approximately one-year old mango plants, cultivars Espada, Haden, Ubá, and Tommy Atkins, were obtained from a commercial orchard in Dona Euzébia municipality, in the state of Minas Gerais, Brazil (-21o18’59” S, -42o48’38” W, and 222 m). All cultivars were grafted onto plants from the cultivar Imbú, which is widely used as rootstock in the Zona da Mata region, Minas Gerais, Brazil. Saplings were transplanted into plastic pots containing 8 kg of substrate consisting of a mixture of soil, sand and manure in a ratio of 2:1:1. The plants were maintained in a greenhouse (temperature of 30 ± 2 °C and relative humidity of 70 ± 5 %) for two months before the beginning of the experiments. The plants were irrigated and fertilized as needed.
Inoculation procedure
The procedure was largely performed as previously described (Bispo et al., 2015Bispo, W.M.S.; Araújo, L.; Bermúdez-Cardona, M.B.; Cacique, I.S.; DaMatta, F.M.; Rodrigues, F.A. 2015. Ceratocystis fimbriata-induced changes in the antioxidative system of mango cultivars. Plant Pathology 64: 627-637.). The CEBS15 and MSAK16 isolates of C. fimbriata were used to inoculate the plants. These isolates were obtained from symptomatic mango plants collected, respectively, from the following municipalities in Brazil: Brejo Santo, in the state of Ceará (07°29’34” S, 38°59’06” W, and 381 m), and Aquidauana, in the state of Mato Grosso do Sul (20°28’15” S, 55°47’13” W, 147 m). The isolates were preserved according to Castellani's method (Dhingra and Sinclair, 1995Dhingra, O.D.; Sinclair, J.B., eds. 1995. Basic Plant Pathology Methods. Lewis Publisher, Boca Raton, FL, USA.). Plugs of malt extract-agar medium containing fungal mycelia were transferred to Petri dishes containing potato dextrose agar (PDA). After three days, the PDA plugs containing fungal mycelia were transferred to new Petri dishes containing the same culture medium and maintained in an incubator chamber (temperature of 25 °C with a 12-h photoperiod) for 14 days. The plants were inoculated according to Al-Sadi et al. (2010)Al-Sadi, A.M.; Al-Ouweisi, F.A.; Al-Shariani, N.K.; Al-Adawi, A.O.; Kaplan, E.J.; Deadman, M.L. 2010. Histological changes in mango seedlings following infection with Ceratocystis manginecans, the cause of mango decline. Journal of Phytopathology 158: 738-743. with a few modifications. Stem disks (10-mm diameter and approximately 2-mm wide) were removed from the stems using a punch at approximately 5 cm above the graft scar. A PDA plug (10-mm diameter) obtained from a 14-day-old fungal colony was carefully placed in the punch hole. Each hole containing a PDA plug with fungal mycelia was carefully covered with a piece of moistened cotton and then wrapped with parafilm to maintain adequate moisture for fungal infection. The holes on the stems of the plants receiving only PDA medium plugs served as the control treatment.
Disease assessments
Disease development was evaluated at 28 days after inoculation (dai). The upward, downward and radial colonization of the stem tissues by fungal hyphae were determined by measuring the distance (length in cm) from the inoculation site to the edge of the internal necrotic tissue using a digital caliper. The upward relative lesion length (URLL) and the downward relative lesion length (DRLL) were determined as the ratio between the length from the graft scar to the top of the stem (LGST) and the lesion length (LL) in the same interval (upward and downward) from the inoculation point according to the following formula: URLL or DRLL = LL × 100/LGST. The plants were standardized to 20-cm long (the distance from the graft scar to the top of the stem). The radial fungal colonization (RFC) was determined as the length of necrotic tissue in relation to the total stem diameter × 100. The number of wilted leaves from the total number of leaves per plant of the replication of each treatment was determined at 28 dai according to Al-Sadi et al., (2010)Al-Sadi, A.M.; Al-Ouweisi, F.A.; Al-Shariani, N.K.; Al-Adawi, A.O.; Kaplan, E.J.; Deadman, M.L. 2010. Histological changes in mango seedlings following infection with Ceratocystis manginecans, the cause of mango decline. Journal of Phytopathology 158: 738-743..
Leaf gas exchange measurements
The leaf gas exchange parameters were determined using a portable open-flow gas exchange system. The net carbon assimilation rate (A), stomatal conductance to water vapor (gs), internal-to-ambient CO2 concentration ratio (Ci/Ca) and transpiration rate (E) were measured in the fully expanded leaves of six plants from each treatment at 7, 14, 21 and 28 dai. The measurements were obtained at ambient temperature and CO2 conditions under artificial light (1000 µmol photons m−2 s−1) from approximately 08h00 to 12h00.
Experimental design and statistics
A 4 × 3 factorial experiment, consisting of four cultivars and plants that were not inoculated, inoculated with isolate MSAK16 or inoculated with isolate CEBS15 of C. fimbriata, was arranged in a completely randomized design. A total of five plants per treatment were used to evaluate the leaf gas exchange parameters at each sampling time (7, 14, 21 and 28 dai) and the disease indices at the end of the experimental period (28 dai). Each replication consisted of an individual potted plant. The experiment was conducted twice. Data from all of the variables were subjected to an analysis of variance (ANOVA), and the means of the treatments were compared by Tukey test (p ≤ 0.05). To analyze disease indices, the means obtained for plants inoculated with the C. fimbriata isolates were compared using the Student’s t test (p ≤ 0.05) with SAS (Statistical Analysis System, version 9.02).
Results
Analysis of variance
At least one of the factors cultivar, plant inoculation, and their interaction were significant for A, gs, Ci/Ca, E, URLL, DRLL, RLL, and RFC, especially from 21 dai onward (Table 1).
− Analysis of variance of the effects of cultivars (C) and plant inoculation (I) and their interaction in the net CO2 assimilation rate (A), stomatal conductance (gs), internal-to-ambient CO2 concentration ratio (Ci/Ca) and transpiration rate (E), as well as the upward relative lesion length (URLL), downward relative lesion length (DRLL), total relative lesion length (RLL) and radial fungal colonization (RFC) of the disease indices at 7, 14, 21 and 28 days after inoculation.
Disease indices
There were no significant differences between the cultivars evaluated against the CEBS15 isolate of C. fimbriata (Figure 1A-D). Despite the fact that plants from cv. Haden presented greater URLL in comparison to cv. Tommy Atkins in response to the MSAK16 isolate, there were no differences (p > 0.05) between cvs. Haden and Tommy Atkins in response to the CEBS15 and MSAK16 isolates of C. fimbriata for the disease indices. Except for DRLL, plants from cv. Espada inoculated with the MSAK16 isolate showed the highest values in contrast to plants from cv. Ubá (approximately 90 % for URLL, 83 % for RLL and 67 % for RFC) (Figure 1A, C, D). The CEBS15 isolate of C. fimbriata was less aggressive than the MSAK16 isolate on cv. Espada according to the disease indices (approximately 35 % for URLL, 25 % for RLL and 14 % for RFC) (Figure 1A-D) evaluated. The MSAK16 isolate was less aggressive than the CEBS15 isolate in cv. Ubá, with differences between indices of approximately 75 % for URLL, 45 % for DRLL, 65 % for RLL, and 45 % for RFC (Figure 1A-D).
− The upward relative lesion length (URLL) (A), downward relative lesion length (DRLL) (B), total relative lesion length (RLL) (C) and radial fungal colonization (RFC) (D) in the stem tissues of mango plants from cultivars Espada, Uba, Haden and Tommy Atkins inoculated with the CEBS15 and MSAK16 isolates of Ceratocystis fimbriata. Means followed by the same lowercase letters are not significantly different according to Tukey´s test (p ≤ 0.05) for the CEBS15 isolate. Means followed by the same uppercase letters are not significantly different according to Tukey´s test (p ≤ 0.05) for the MSAK16 isolate. The means of the plants inoculated with MSAK16 and CEBS15 for each cultivar followed by * at each evaluation time are significantly different according to Student’s t test (p ≤ 0.05). The error bars represent the standard error of the mean. n = 5.
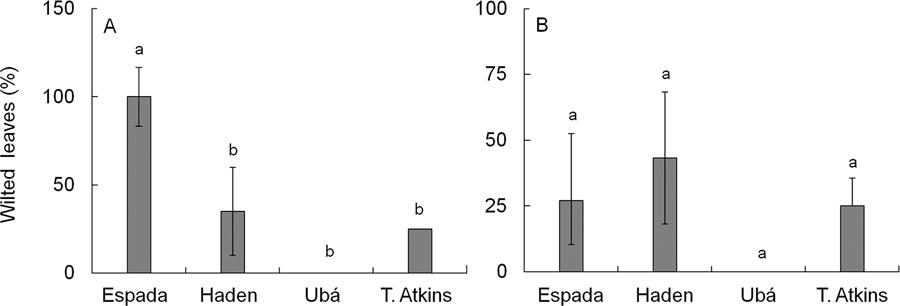
Wilted leaves
Plants from cv. Espada inoculated with the MSAK16 isolate of C. fimbriata presented 100 % wilted leaves at 28 dai (Figure 2A). There were no differences (p > 0.05) between cultivars for the plants inoculated with the CEBS15 isolate (Figure 2B).
− Percentage of wilted leaves in plants from cultivars Espada, Ubá, Haden and Tommy Atkins inoculated with the MSAK16 (A) or CEBS15 (B) isolates of Ceratocystis fimbriata. Means followed by the same letter are not significantly different according to Tukey´s test (p ≤ 0.05). The error bars represent the standard error of the mean. n = 5.
Leaf gas exchange parameters
Net carbon assimilation rate (A) - There was no changes (p > 0.05) in A for plants inoculated with the CEBS15 isolate of C. fimbriata at any of the sampling times regardless of the cultivar (Figure 3A-D). In general, plants inoculated with the MSAK16 isolate of C. fimbriata showed the lowest values for A (40 % lower in comparison to cv. Ubá at 14 dai). For plants from cv. Ubá inoculated with the CEBS15 isolate of C. fimbriata, A was 33, 14 and 59 % lower compared to the non-inoculated plants at 14, 21 and 28 dai, respectively (Figure 3B). For plants from cv. Haden inoculated with the CEBS15 isolate, the reductions in A were nearly 50 % at 21 dai and approximately 100 % at 28 dai in comparison to the non-inoculated plants (Figure 3C). For plants from cv. Tommy Atkins inoculated with the CEBS15 isolate, A was reduced by 35, 55 and 65 % at 14, 21 and 28 dai, respectively, when compared to the non-inoculated plants (Figure 3D). Plants from cv. Espada inoculated with the isolate MSAK16 died at 21 dai, therefore A was not evaluated at 21 and 28 dai (Figure 3A).
− Net carbon assimilation rate (A) of plants from cultivars Espada (A), Ubá (B), Haden (C) and Tommy Atkins (D) that were non-inoculated (control, black bars) or inoculated with the MSAK16 (MS, grey bars) or CEBS15 (CE, white bars) isolates, at 7, 14, 21 and 28 dai. Means followed by the same lower case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a horizontal comparison of bars from different inoculation treatments for the same cultivar at each evaluation time. Means followed by the same upper case letters do not differ significantly according to Tukey’s test (p ≤ 0.05) following a vertical comparison of bars from different cultivars for the same inoculation treatment at each evaluation time. The error bars represent the standard error of the mean. n = 5.
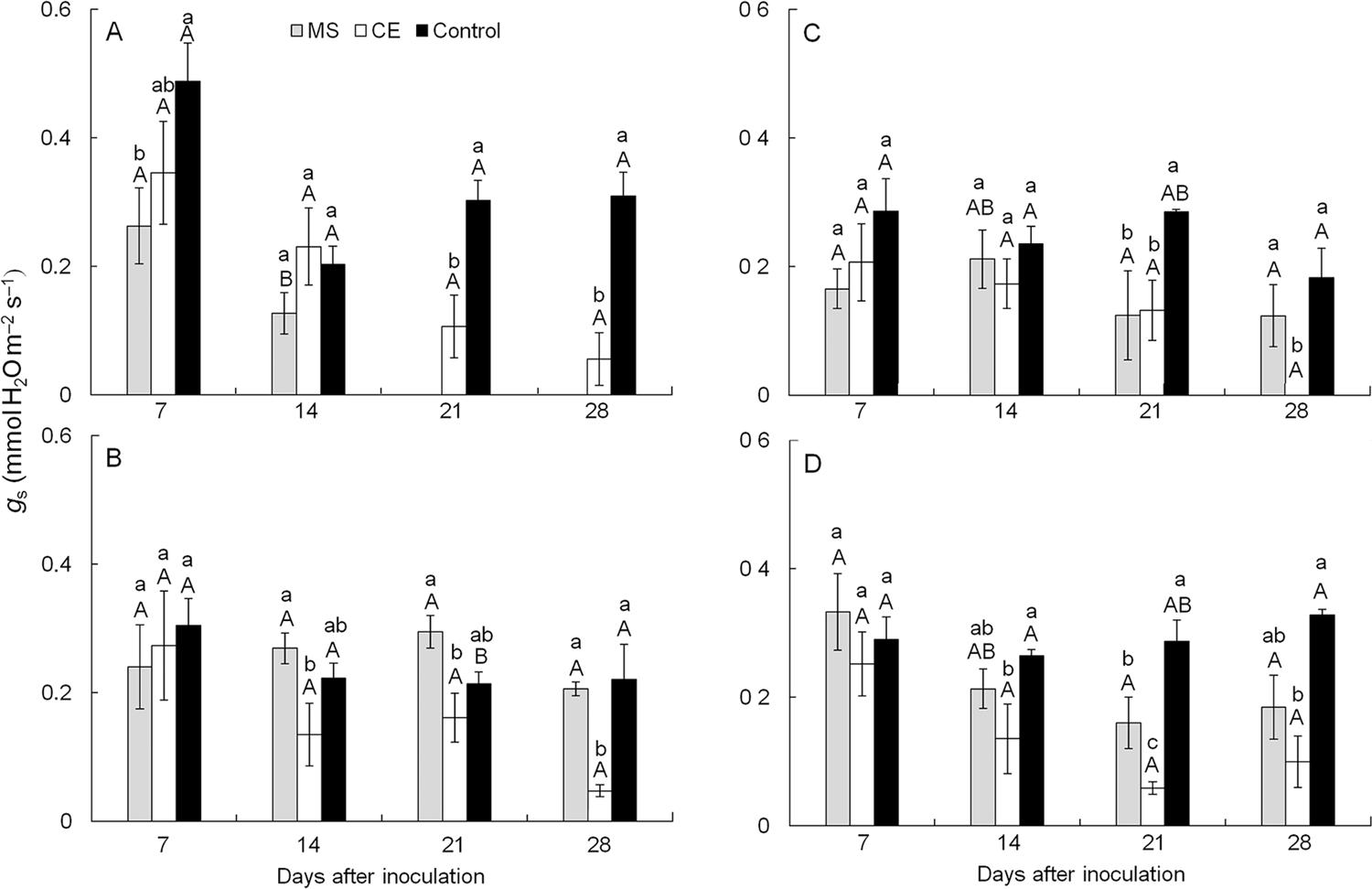
Stomatal conductance to water vapor (gs) - There was no changes (p > 0.05) in gs for plants inoculated with the CEBS15 isolate of C. fimbriata at any of the sampling times regardless of the cultivar (Figure 4A-D). In general, plants from cv. Espada inoculated with the MSAK16 isolate showed the lowest values for gs (53 % lower in comparison to cv. Ubá at 14 dai) (Figure 4A). For plants from cv. Ubá inoculated with the CEBS15 isolate of C. fimbriata, gs was 39, 24 and 78 % lower than the non-inoculated plants at 14, 21 and 28 dai, respectively (Figure 4B). For CEBS15-inoculated plants from cv. Haden, the reductions in gs were nearly 55 % at 21 dai and approximately 100 % at 28 dai in comparison to the non-inoculated plants (Figure 4C). For cv. Tommy Atkins, gs was reduced by 48, 79 and 69 % (at 14, 21 and 28 dai) in plants inoculated with the CEBS15 isolate when compared to the non-inoculated plants (Figure 4D). Plants from cv. Espada inoculated with the MSAK16 isolate died at 21 dai, therefore gs was not evaluated at 21 and 28 dai (Figure 4A).
− Stomatal conductance to water vapor (gs) of plants from cultivars Espada (A), Ubá (B), Haden (C) and Tommy Atkins (D) that were non-inoculated (control, black bars) or inoculated with the MSAK16 (MS, grey bars) or CEBS15 (CE, white bars) isolates, at 7, 14, 21 and 28 dai. Means followed by the same lower case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a horizontal comparison of bars from different inoculation treatments for the same cultivar at each evaluation time. Means followed by the same upper case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a vertical comparison of bars from different cultivars for the same inoculation treatment at each evaluation time. The error bars represent the standard error of the mean. n = 5.
Internal-to-ambient CO2concentration ratio (Ci/Ca) - Overall, there were no differences between mango cultivars in terms of Ci/Ca values regardless of the isolate of C. fimbriata (Figure 5A-D). For plants from cv. Ubá inoculated with the CEBS15 isolate, Ci/Ca was 8 and 17 % lower compared to the non-inoculated plants at 21 and 28 dai, respectively (Figure 5B). For plants from cv. Haden inoculated with the CEBS15 isolate, the reductions in Ci/Ca were nearly 25 % at 21 dai and approximately 36 % at 28 dai in comparison to the non-inoculated plants (Figure 5C). For plants from cv. Tommy Atkins inoculated with the CEBS15 isolate of C. fimbriata, Ci/Ca was reduced by 14, 24 and 24 % at 14, 21 and 28 dai, respectively, when compared to the non-inoculated plants (Figure 5D). Plants from cv. Espada inoculated with the MSAK16 isolate died at 21 dai, therefore Ci/Ca was not evaluated at 21 and 28 dai (Figure 5A).
− Internal-to-ambient CO2 concentration ratio (Ci/Ca) of plants from cultivars Espada (A), Ubá (B), Haden (C) and Tommy Atkins (D) that were non-inoculated (control, black bars) or inoculated with the MSAK16 (MS, grey bars) or CEBS15 (CE, white bars) isolates, at 7, 14, 21 and 28 dai. Means followed by the same lower case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a horizontal comparison of bars from different inoculation treatments for the same cultivar at each evaluation time. Means followed by the same upper case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a vertical comparison of bars from different cultivars for the same inoculation treatment at each evaluation time. The error bars represent the standard error of the mean. n = 5.
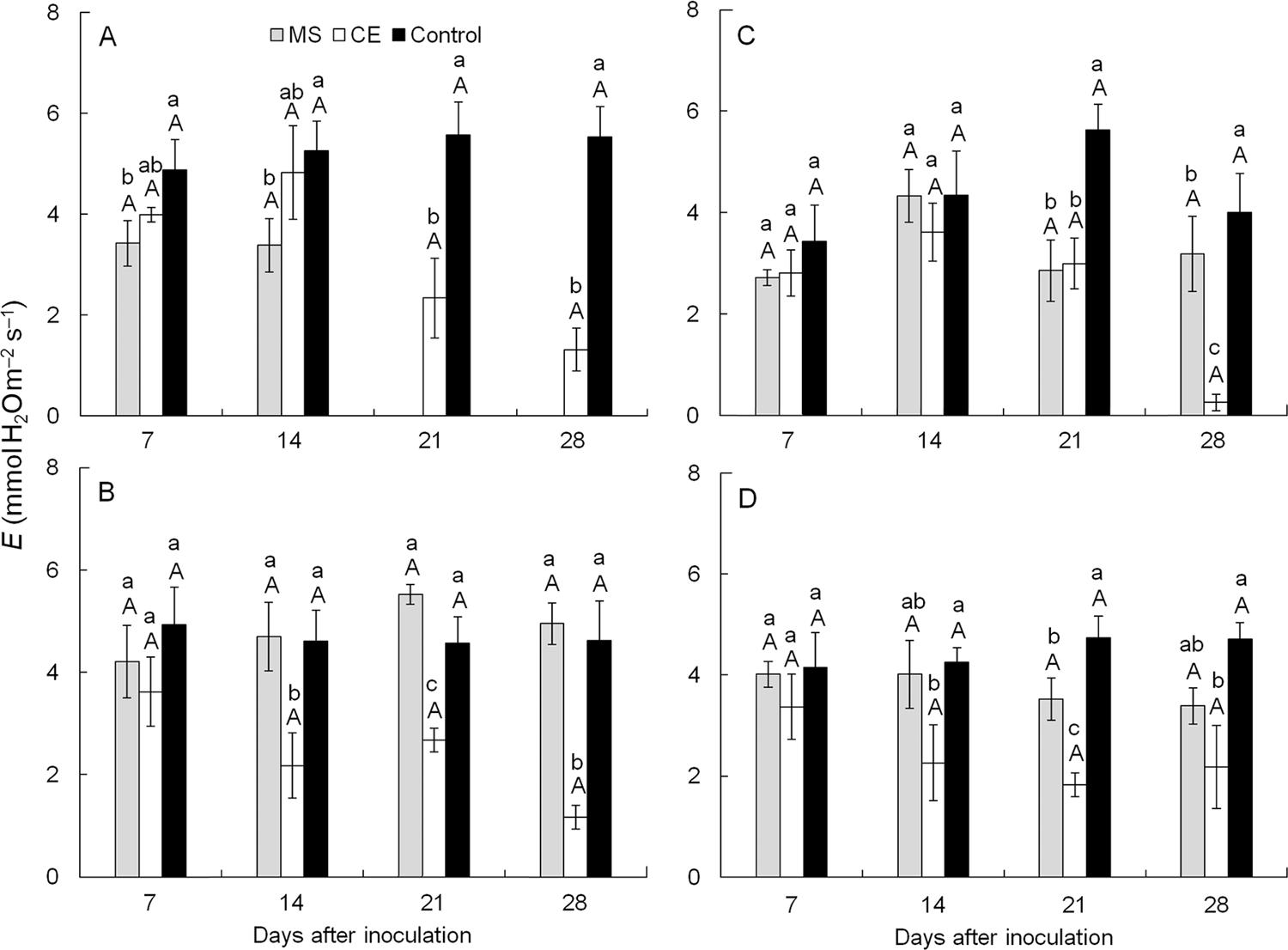
Transpiration rate (E) - There were no changes (p > 0.05) in E for plants inoculated with the CEBS15 isolate of C. fimbriata at any of the sampling times regardless of the cultivar (Figure 6A-D). Furthermore, non-inoculated plants from all of the cultivars displayed no differences in E during the course of evaluation. For plants from cv. Ubá inoculated with the CEBS15 isolate, E was 52, 41 and 74 % lower compared to the non-inoculated plants at 14, 21 and 28 dai, respectively (Figure 6B). For plants from cv. Haden inoculated with the CEBS15 isolate, the reductions in E were nearly 50 % at 21 dai and approximately 100 % at 28 dai in comparison to the non-inoculated plants (Figure 6C). For cv. Tommy Atkins plants inoculated with the CEBS15 isolate, E was reduced by 46, 61 and 53 % at 14, 21 and 28 dai, respectively, when compared to the non-inoculated plants (Figure 6D). Plants from cv. Espada inoculated with the isolate MSAK16 died at 21 dai, therefore E was not evaluated at 21 and 28 dai (Figure 6A).
− Transpiration rate (E) of plants from cultivars Espada (A), Ubá (B), Haden (C) and Tommy Atkins (D) that were non-inoculated (control, black bars) or inoculated with MSAK16 (MS, grey bars) or CEBS15 (CE, white bars) isolates, at 7, 14, 21 and 28 dai. Means followed by the same lower case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a horizontal comparison of bars from different inoculation treatments for the same cultivar at each evaluation time. Means followed by the same upper case letters are not significantly different according to Tukey’s test (p ≤ 0.05) following a vertical comparison of bars from different cultivars for the same inoculation treatment at each evaluation time. The error bars represent the standard error of the mean. n = 5.
Discussion
Before mango plants infected by C. fimbriata exhibit typical symptoms of wilting and drying leaves, extensive necrosis in the inner tissues of the stem and root are the main signs of disease progression (Viégas, 1960Viégas, A.P. 1960. Mango wilt. Bragantia 19: 163-182 (in Portuguese, with abstract in English).). Thus, an evaluation of internal symptoms appears to be appropriate for determining the progress of disease prior to the onset of visible symptoms. Studies such as those conducted by Al-Sadi et al., (2010)Al-Sadi, A.M.; Al-Ouweisi, F.A.; Al-Shariani, N.K.; Al-Adawi, A.O.; Kaplan, E.J.; Deadman, M.L. 2010. Histological changes in mango seedlings following infection with Ceratocystis manginecans, the cause of mango decline. Journal of Phytopathology 158: 738-743. and Araújo et al., (2014)Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata. Plant Pathology 63: 1282-1295. clearly illustrate the pathogen infection process at the microscopic level and endorse the measurement of acropetal, basipetal, and radial pathogen growth as an effective strategy for evaluating the spread of disease.
In this study, there were no differences overall according to the disease indices (URLL, DRLL, RLL and RFC) between the cultivars tested in response to the CEBS15 isolate of C. fimbriata. However, following infection with the MSAK16 isolate, plants from cv. Espada showed marked susceptibility, with premature death of plants from 21 dai onward, especially in comparison to cv. Ubá, which showed the lowest proportion of colonized stem tissues. Considering the full length of the internal lesion and the radial colonization of the stem tissues, the aggressiveness of the two C. fimbriata isolates was quite similar for the plants from cvs. Haden and Tommy Atkins. Conversely, when compared to the CEBS15 isolate of C. fimbriata, the MSAK16 isolate was more and less aggressive on cvs. Espada and Ubá, respectively. Based on the observation of interactions between different mango cultivars and different isolates of C. fimbriata, it has been suggested that, rather than differences between the aggressiveness of isolates, the variability observed may be related to different cultivar × isolate interactions, whereby cultivars become more or less susceptible to a given isolate (Rossetto et al., 1996Rossetto, C.J.; Ribeiro, I.J.A.; Sabino, P.B.G.J.C.; Carvalho, R.P.L.; Kubo, R.; Oliveira, A.S. 1996. Mango pests = Insetos da manga. p. 145-166. In: São José, A.R.; Souza, I.V.B.; Martins Filho, J.; Morais, O.M., eds. Manga: production technology and market. Universidade Estadual do Sudoeste da Bahia, Vitória da Conquista, BA, Brazil (in Portuguese).). In fact, this hypothesis is consistent with the results obtained by Araújo et al., (2014)Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata. Plant Pathology 63: 1282-1295., who classified Ubá as a highly resistant and cv. Espada as a highly susceptible cultivar regardless of the invading isolate, based on RLL and RFC disease indices.
It is known that C. fimbriata hyphae grow faster through the cortical parenchyma and xylem vessels of plants from susceptible cultivars and, thus, induce the concomitant and intense formation of polysaccharide gels and tyloses as host defense responses (Araújo et al., 2014Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata. Plant Pathology 63: 1282-1295.). As previously reported by Araújo et al., (2014)Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata. Plant Pathology 63: 1282-1295., C. fimbriata extensively colonize the pith parenchyma in the radial direction in the stem tissues of plants from susceptible mango cultivars, such as Espada and Haden; in contrast, in resistant cultivars, the fungal hyphae barely reach these cells. The formation of these defense structures, together with the tissue colonization itself and increasing structural damages, ultimately lead to an increase in sap flow resistance and may result in an altered plant hydration status (Nogués et al., 2002Nogués, S.; Cotxarrera, L.; Alegre, L.; Trillas, M.I. 2002. Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytologist 154: 461-470.; Dong et al., 2012Dong, X.; Ling, N.; Wang, M.; Shen, Q.; Guo, S. 2012. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium-infected banana plants. Plant Physiology and Biochemistry 60: 171-179.; Park et al., 2013Park, J.H.; Juzwik, J.; Cavender-Bares, J. 2013. Multiple Ceratocystis smalleyi infections associated with reduced stem water transport in bitternut hickory. Phytopathology 103: 565-574.). In due course, the alterations in water availability may lead to reductions in photosynthesis, transpiration rates, leaf longevity and integrity (Nogués et al., 2002Nogués, S.; Cotxarrera, L.; Alegre, L.; Trillas, M.I. 2002. Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytologist 154: 461-470.) as observed for a series of other pathosystems that also involve the mango-C. fimbriata interaction (Bispo et al., 2015Bispo, W.M.S.; Araújo, L.; Bermúdez-Cardona, M.B.; Cacique, I.S.; DaMatta, F.M.; Rodrigues, F.A. 2015. Ceratocystis fimbriata-induced changes in the antioxidative system of mango cultivars. Plant Pathology 64: 627-637.). In fact, in the present study, decreases in A, gs, Ci/Ca and E, in association with wilting leaves, were a general response of the mango cultivars to infection by C. fimbriata. Although plants from cvs. Ubá and Tommy Atkins inoculated with the MSAK16 isolate of C. fimbriata exhibited leaf gas exchange parameters similar to their non-inoculated counterparts (control), the Haden and Espada cultivars showed drastic declines in the evaluated parameters and overt plant death from 21 dai onward, showing considerably more susceptibility to development of the disease when inoculated with this fungal isolate. In turn, for plants from the cultivars Ubá, Haden and Tommy Atkins, changes were more likely to occur from 14 dai onward in response to infection with the CEBS 15 fungal isolate. Thus, regardless of the isolate used for plant inoculation, the changes observed in leaf gas exchange parameters were likely associated with the net flux of CO2 into the stomata because decreases in stomatal conductance were mostly accompanied by decreases in the Ci/Ca ratio. According to Mansfield et al., (1990)Mansfield, T.A.; Hetherington, A.M.; Atkinson, C.J. 1990. Some current aspects of stomatal physiology. Annual Review of Plant Physiology and Plant Molecular Biology 41: 55-75. and Farooq et al., (2009)Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185-212., the first response of plants suffering from water privation is stomatal closure to prevent loss of water through the transpiration process.
The reduction in gs also appears to function as a main determinant for the drop in carbon assimilation under conditions of mild to moderate stress (Yokota et al., 2002Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. 2002. Citrulline and DRIP-1 protein (ArgE Homologue) in drought tolerance of wild watermelon. Annals of Botany 89: 825-832.; Farooq et al., 2009Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185-212.). The transpiration process and uptake of atmospheric CO2 are competing effects that utilize the same pathway to leave and enter the leaf mesophyll via the stomatal pore (Hopkins and Hüner, 2004Hopkins, W.G.; Hüner, N.P.A. 2004. Introduction to Plant Physiology. Wiley, New York, NY, USA.). As a result of limited carbon assimilation, decreases in the consumption of electrons released from water and enhancement of the photorespiratory processes are expected to occur (Noctor et al., 2002Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. 2002. Drought and oxidative load in wheat leaves: a predominant role for photorespiration? Annals of Botany 89: 841-850.; Chaves et al., 2003Chaves, M.M.; Maroco, J.P.; Pereira, J.S. 2003. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology 30: 239-264.). These alterations generally lead to the onset of excess excitation energy, which facilitates the formation and accumulation of reactive oxygen species, thereby resulting in potential oxidative stress (Noctor et al., 2002Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. 2002. Drought and oxidative load in wheat leaves: a predominant role for photorespiration? Annals of Botany 89: 841-850.; Chaves et al., 2003Chaves, M.M.; Maroco, J.P.; Pereira, J.S. 2003. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology 30: 239-264.; Bispo et al., 2015Bispo, W.M.S.; Araújo, L.; Bermúdez-Cardona, M.B.; Cacique, I.S.; DaMatta, F.M.; Rodrigues, F.A. 2015. Ceratocystis fimbriata-induced changes in the antioxidative system of mango cultivars. Plant Pathology 64: 627-637.). In this way, plants from the most susceptible cultivars may have dealt with the development of derived oxidative stress coupled with potential water privation favoring, therefore, the development of disease symptoms in contrast to plants from the most resistant cultivars to which the damage in vascular tissues was less pronounced as highlighted by the diseases indices studied. According to Araújo et al., (2014Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata. Plant Pathology 63: 1282-1295., 2015Araújo, L.; Bispo, W.M.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. 2015. Induction of the phenylpropanoid pathway by acibenzolar-S-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Disease 99: 447-459.), the capacity of mango plants to produce different types of phenolics on the stem were closely related to their level of basal resistance against C. fimbriata infection.
Considering the coupling of data for disease evaluation and changes in leaf gas exchange parameters resulting from infection, it may be assumed that there are significant differences between cultivars in terms of resistance responses. Therefore, an evaluation of leaf gas exchange provides a reliable indication of the general metabolic status of the host in a non-invasive manner. In general, regardless of the inoculated isolate, alterations in gas exchange parameters were progressively observed during the development of disease in all mango cultivars, but whether they were more or less pronounced was dependent on the interaction. This approach may aid in the selection resistant cultivars that are more prone to the alleviation of disease symptoms and maintenance of plant production.
Acknowledgements
Prof. F.A. Rodrigues thanks the Brazilian National Council for Scientific and Technological Development (CNPq) for his fellowship. Dra. W.M.S. Bispo and Dr. L. Araújo were supported by the CNPq. The authors thank Prof. A.C. Alfenas and Dr. L.S.S. Oliveira for kindly providing the isolates of C. fimbriata used in this study. This study was supported by a grant from Vale S.A. to Prof. F.A. Rodrigues.
References
- Al-Sadi, A.M.; Al-Ouweisi, F.A.; Al-Shariani, N.K.; Al-Adawi, A.O.; Kaplan, E.J.; Deadman, M.L. 2010. Histological changes in mango seedlings following infection with Ceratocystis manginecans, the cause of mango decline. Journal of Phytopathology 158: 738-743.
- Araújo, L.; Bispo, W.M.S.; Cacique, I.S.; Cruz, M.F.A.; Rodrigues, F.A. 2014. Histopathological aspects of mango resistance to the infection process of Ceratocystis fimbriata Plant Pathology 63: 1282-1295.
- Araújo, L.; Bispo, W.M.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. 2015. Induction of the phenylpropanoid pathway by acibenzolar-S-methyl and potassium phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Disease 99: 447-459.
- Aucique-Perez, C.E.; Rodrigues, F.A.; Moreira, W.R.; DaMatta, F.M. 2014. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae Phytophatology 104: 143-149.
- Baastians, L. 1991. Ratio between virtual and visual lesion size as a measure to describe reduction in leaf photosynthesis of rice due to leaf blast. Phytophatology 81: 611-615.
- Berry, J.; Bjorkman, O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology 31: 491-543.
- Bispo, W.M.S.; Araújo, L.; Bermúdez-Cardona, M.B.; Cacique, I.S.; DaMatta, F.M.; Rodrigues, F.A. 2015. Ceratocystis fimbriata-induced changes in the antioxidative system of mango cultivars. Plant Pathology 64: 627-637.
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. 2003. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology 30: 239-264.
- Dhingra, O.D.; Sinclair, J.B., eds. 1995. Basic Plant Pathology Methods. Lewis Publisher, Boca Raton, FL, USA.
- Dong, X.; Ling, N.; Wang, M.; Shen, Q.; Guo, S. 2012. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium-infected banana plants. Plant Physiology and Biochemistry 60: 171-179.
- Elings, A.; Rossing, W.A.H.; Werf, W. van der. 1999. Virtual lesion extension: a measure to quantify the effects of bacterial blight on rice leaf CO2 exchange. Phytopathology 89: 789-795.
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. 2009. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development 29: 185-212.
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmes, J.; Gulias, J.; Lefi, E.; Martinez-Canellas, S.F.; Moreno, M.T.; Ribas-Carbo, M.; Riera, D.; Sampol, B.; Medrano, H. 2004. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Annals of Applied Biology 144: 273-491.
- Flexas, J.; Diaz-Espejo, A.; Galmes, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. 2007. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment 30: 1284-1298.
- Fishman, S.; Génard, M. 1998. A biophysical model of fruit growth: simulation of seasonal and diurnal dynamics of mass. Plant, Cell and Environment 21: 739-752.
- Halsted, B.D. 1890. Some Fungous Diseases of the Sweet Potato. New Jersey Agricultural College Experiment Station, New Brunswick, NJ, USA. (Bulletin, 76).
- Hopkins, W.G.; Hüner, N.P.A. 2004. Introduction to Plant Physiology. Wiley, New York, NY, USA.
- Johnson, K.B. 1987. Defoliation, disease and growth: a reply. Phytopathology 77: 1495-1497.
- Le Roux, X.; Walcroft, A.S.; Daudet, F.A.; Sinoquet, H.; Chaves, M.M.; Rodrigues, A.; Osorio, L. 2001. Photosynthetic light acclimation in peach leaves: importance of changes in mass: area ratio, nitrogen content, and leaf nitrogen partitioning. Tree Physiology 21: 377-386.
- Mansfield, T.A.; Hetherington, A.M.; Atkinson, C.J. 1990. Some current aspects of stomatal physiology. Annual Review of Plant Physiology and Plant Molecular Biology 41: 55-75.
- Masood, A.; Saeed, S. 2012. Bark beetle, Hypocryphalus mangiferae Stebbing (Coleoptera: Curculionidae: Scolytinae) is a vector of mango sudden death disease in Pakistan. Pakistan Journal of Botany 44: 813-820.
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. 2002. Drought and oxidative load in wheat leaves: a predominant role for photorespiration? Annals of Botany 89: 841-850.
- Nogués, S.; Cotxarrera, L.; Alegre, L.; Trillas, M.I. 2002. Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytologist 154: 461-470.
- Park, J.H.; Juzwik, J.; Cavender-Bares, J. 2013. Multiple Ceratocystis smalleyi infections associated with reduced stem water transport in bitternut hickory. Phytopathology 103: 565-574.
- Ribeiro, I.J.A. 1997. Mango (Mangifera indica L.) diseases = Doenças da manga (Mangifera indica L.). p. 511-524. In: Kimati, H.; Amorim, L.; Bergamin Filho, A.; Camargo, L.E.A.; Rezende, J.A.M., eds. Phytopathology manual: diseases of cultivated plants. Agronômica Ceres, São Paulo, SP, Brazil (in Portuguese).
- Rosati, A.; Esparza, G.; DeJong, D.M.; Pearcy, R.W. 1999. Influence of canopy light environment and nitrogen availability on leaf photosynthetic characteristics and photosynthetic nitrogen-use efficiency of field-grown nectarine trees. Tree Physiology 20: 271-276.
- Rossetto, C.J.; Ribeiro, I.J.A.; Sabino, P.B.G.J.C.; Carvalho, R.P.L.; Kubo, R.; Oliveira, A.S. 1996. Mango pests = Insetos da manga. p. 145-166. In: São José, A.R.; Souza, I.V.B.; Martins Filho, J.; Morais, O.M., eds. Manga: production technology and market. Universidade Estadual do Sudoeste da Bahia, Vitória da Conquista, BA, Brazil (in Portuguese).
- Viégas, A.P. 1960. Mango wilt. Bragantia 19: 163-182 (in Portuguese, with abstract in English).
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. 2002. Citrulline and DRIP-1 protein (ArgE Homologue) in drought tolerance of wild watermelon. Annals of Botany 89: 825-832.
Edited by
Publication Dates
-
Publication in this collection
Mar-Apr 2016
History
-
Received
26 Jan 2015 -
Accepted
21 July 2015