ABSTRACT
Despite the widespread distribution of the Cassava common mosaic virus (CsCMV) in Brazil, little is known about the losses it causes in yield. The effect of CsCMV on different varieties was evaluated by reference to several agronomic traits. Four field trials were established in 2012/2013 and 2013/2014 using six varieties of cassava. Following mechanical inoculation with CsCMV, the presence of the virus was confirmed using the ELISA assay. The evaluated traits were plant height (PH), dry matter content (DMC), harvest index (HI), aerial part yield (APY), root yield (RoY), and starch yield (StY) in both inoculated and non-inoculated plants. Overall, the presence of the virus contributed little to the reduction in PH, HI, and DMC across the varieties, with PH being significantly reduced by 9.2 and 7.0 % in the BGM0212 and BRS Kiriris varieties, respectively. In contrast, APY, RoY, and StY were reduced by 30.2, 29.3, and 30.0 %, in the virus-infected plants respectively. While the BRS Kiriris and BRS Jari varieties suffered the highest reductions overall and were considered highly susceptible to CsCMV, none of the traits suffered reductions in the inoculated BRS Formosa plants. Although RoY and StY were reduced in inoculated plants of BRS Tapioqueira, crop yield for this variety was the highest. Thus, BRS Formosa and BRS Tapioqueira exhibited tolerance against CsCMV, which warrants further investigation.
Keywords:
Manihot esculenta Crantz; viruses; root yield; disease
Introduction
Cassava (Manihot esculenta Crantz) is the fourth-most important source of calories worldwide, behind only wheat, corn, and rice (Legg et al., 2014aLegg, J.P.; Somado, E.A.; Barker, I.; Beach, L.; Ceballos, H.; Cuellar, W.; Elkhoury, W.; Gerling, D.; Helsen, J.; Hershey, C.; Jarvis, A.; Kulakow, P.; Kumar, P.L.; Lorenzen, J.; Lynam, J.; McMahon, M.; Gowda, M.; Miano, D.; Mtunda, K.; Natwuruhunga, P.; Okogbenin, E.; Pezo, P.; Terry, E.; Thiele, G.; Thresh, M.; Wadsworth, J.; Walsh, S.; Winter, S.; Tohme, J.; Fauquet, C.M. 2014a. A global alliance declaring war on cassava viruses in Africa. Food Security 6: 231-248.). Currently, about 20 million hectares are planted with cassava, with a total production of 260 million tons distributed over approximately 80 countries. The main cassava-producing countries are Nigeria, Thailand, Indonesia, and Brazil (FAO, 2014Food and Agriculture Organization [FAO]. 2014. FAOSTAT database gateway. Available at: http://faostat.fao.org/site/567/default.aspx [Accessed Feb 15, 2015]
http://faostat.fao.org/site/567/default....
).
In many regions of the world including Brazil, cassava is grown in unsuitable areas, such as in highly erodible soils; yet, even under these conditions, this species is able to achieve relatively high yields compared to other plant species (Tonukari et al., 2015Tonukari, N.J.; Ezedom, T.; Enuma, C.C.; Sakpa, S.O.; Avwioroko, O.J.; Eraga, L.; Odiyoma, E. 2015. White gold: cassava as an industrial base. American Journal of Plant Sciences 6: 972-979.). However, the yield potential of cassava can reach 90 t ha−1 of fresh roots when using improved and adapted cultivars, and proper cultural and management practices to control pests and diseases (El-Sharkawy, 2012El-Sharkawy, M.A. 2012. Stress-tolerant cassava: the role of integrative ecophysiology-breeding research in crop improvement. Open Journal of Soil Science 2: 162-186.).
Numerous factors can compromise the production of cassava, but one of the main causes of the low root yield in Brazil is the use of cuttings with low genetic potential and poor phytosanitary quality. Quantitative and qualitative losses in propagation material, as well as losses of fresh roots, can occur because of its vegetative propagation that results in accumulation of pests and pathogens - especially viruses - during successive cutting cycles (Sastry and Zitter, 2014Sastry, K.S.; Zitter, T.A. 2014. Management of virus and viroid diseases of crops in the tropics. p. 149-480. In: Sastry, K.S.; Zitter, T.A., eds. Plant virus and viroid diseases in the tropics. Springer, Berlin, Germany.).
There are at least 15 species of virus that can infect cassava (Legg et al., 2014bLegg, J.P.; Lava Kumar, P.; Makeshkumar, T.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. 2014b. Cassava virus diseases: biology, epidemiology and management. Advances in Virus Research 91: 85-142.). However, the main viruses in Latin America are the Cassava vein mosaic virus (CsVMV), Cassava frogskin disease (CFSD), and Cassava common mosaic virus (CsCMV). The main symptoms of CsCMV are light green chlorotic areas in the leaves, interspersed with dark green areas, sparsely distributed in the leaf blade (Silva et al., 2011Silva, J.M.; Carnelossi, P.R.; Bijora, T.; Facco, C.U.; Picoli, M.H.S.; Souto, E.R.; Oliveira, A.J.B.; Almeida, A.M.R. 2011. Immunocapture-RT-PCR detection of Cassava common mosaic virus in cassava obtained from meristem-tip culture in Paraná state. Tropical Plant Pathology 36: 271-275.). CsCMV is widespread in Latin America, and although still little reported, its typical symptoms can interfere with the photosynthesis of plants and consequently with its development, and thus cause serious losses in root yield and the quality of the propagation material.
Similar to other Potexviruses, CsCMV has no known vector. Therefore, its spread has been associated with mechanical transmission via cutting tools used in the cultivation and preparation of planting material (Legg et al., 2014bLegg, J.P.; Lava Kumar, P.; Makeshkumar, T.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. 2014b. Cassava virus diseases: biology, epidemiology and management. Advances in Virus Research 91: 85-142.). Various control methods have been suggested, but the most widely recommended is the use of virus-free propagation material and resistant varieties (Sastry and Zitter, 2014Sastry, K.S.; Zitter, T.A. 2014. Management of virus and viroid diseases of crops in the tropics. p. 149-480. In: Sastry, K.S.; Zitter, T.A., eds. Plant virus and viroid diseases in the tropics. Springer, Berlin, Germany.). Therefore, this work aimed to evaluate the reaction of a number of commercial cassava varieties to CsCMV, and to estimate the virus-induced losses caused as reflected in certain agronomic traits.
Materials and Methods
Plant material and experimental design
Five cassava varieties developed by Embrapa Cassava and Fruits (Brazilian Agricultural Research Corporation, Cruz das Almas, Bahia, Brazil) were evaluated: BRS Jari, BRS Formosa, BRS Tapioqueira, BRS Kiriris, and BRS Poti Branca, and one landrace widely used as bitter cassava in the “Reconcavo” region of Bahia (BGM0212).
Two field experiments were conducted in 2012/2013 and two in 2013/2014 at two locations: Location 1: 12°40’19” S, 39°06’22” W, 220 m above sea level; and Location 2: 12°39’32” S, 39°05’19” W, 245 m above sea level. These two locations were in the municipality of Cruz das Almas, Bahia. The climate is tropical, hot, and humid - Aw to Am, according to the Köppen classification.
The experimental layout was a randomized block in a 6 × 2 factorial design (six varieties and plants inoculated and non-inoculated with CsCMV), with three blocks, each block consisting of a two rows in a plot with eight plants each. Planting was done at the beginning of the rainy season (June) in the region using 20 cm long cuttings, distributed in furrows about 10 cm deep, with 0.90 m spacing between the rows and 0.80 m between the plants. The crop management practices followed regional recommendations.
Inoculation and indexing
To ensure that the propagation material used in the experiments was free of viruses, an initial index using part of the mother plants (leaf or stem) was formulated by indirect ELISA analyses for CsCMV (Mowat and Dawson, 1987Mowat, W.P.; Dawson, S. 1987. Detection of plant viruses by ELISA using crude sap extracts and unfractionated antisera. Journal of Virology Methods 15: 233-247.) and polymerase chain reaction (PCR) for CsVMV (Calvert et al., 1995Calvert, L.A.; Ospina, M.D.; Shepherd, R.J. 1995. Characterization of cassava vein mosaic virus: a distinct plant pararetrovirus. Journal of General Virology 76: 1271-1276.), respectively.
For the inoculation, the CsCMV strain was kept active in cassava cuttings grown in a greenhouse. For the mechanical inoculation, leaf samples were crushed and diluted 1:5 (w/v) in 0.02 M sodium phosphate buffer, pH 7.0, containing 0.02 M sodium sulfite, using celite as an abrasive. The inoculation was carried out mechanically using gauze approximately 30 days after planting, when the plants had produced three fully expanded leaves. One month prior to harvesting, another indexing for CsCMV was formulated to confirm the presence of the virus in the inoculated plots and its absence in the (non-inoculated) control. Reactions that corresponded to twice the absorbance values recorded for the average of healthy plants (used as controls) were considered positive for CsCMV, based on indirect ELISA analysis (Cuervo Ibáñez et al., 2010Cuervo Ibáñez, M.; Moreno, M.G.; Flor, N.C.; Ramírez, J.L.; Medina, C.A.; Debouck, D.; Martínez, J. 2010. Handbook of Procedures of the Germplasm Health Laboratory. International Center for Tropical Agriculture, Cali, Colombia.).
Assessments and data analysis
The experiments were harvested manually10-11 months after planting. At this time, the evaluated traits were: 1) plant height, using five plants randomly collected per plot from ground level up to the top of the plant with the aid of a graduated ruler; 2) aerial part yield, determined by weighing the shoots and stems produced per plot (t ha−1); 3) root yield, obtained by weighing all of the storage roots grown per plot (t ha−1); 4) harvest index, the ratio between the weight of the roots and the total plant biomass; 5) dry matter content, measured using the gravimetric method; and 6) starch yield, measured by multiplying the starch content (%) by root yield (t ha−1). Dry matter and starch content of the roots were measured according to Kawano et al. (1987)Kawano, K.; Fukuda, W.M.G.; Cenpukdee, U. 1987. Genetic and environmental effects on dry matter content of cassava root. Crop Science 27: 69-74..
The main and interaction effects were analyzed by fitting a general linear mixed model with the varieties and inoculation status (inoculated or not) as fixed effects and i) environment, ii) the block, and iii) the block of the field experimental design nested within each environment as random effects. Means of the variables, for which an effect was significant in the ANOVA, were compared based on Fisher's least significance difference (LSD) method. These analyses were conducted using the SAS PROC-MIXED procedure. The differences, in percentage, between non-inoculated and CsCMV-inoculated plants were used to compute the relative reduction for each trait.
Results and Discussion
Effects of CsCMV on agronomic traits
For all variables analysed treatment × variety interaction was not significant, suggesting that the response to the virus infection was the same for all varieties. However, the single effects of variety and environment were significant for all traits (Table 1). In contrast, there was no effect of CsCMV inoculation on the harvest index and dry matter content.
Summary of the analysis of variance for the main and interaction effects of varieties and treatment (non-inoculated or CsCMV-inoculated) on plant height (PH), aerial part yield (APY), root yield (RoY), harvest index (HI), dry matter content (DMC), and starch yield (StY) traits of cassava plants grown in four environments.
CsCMV-infected plants showed decreasing yield for the aerial part, root yield, and starch yield (Figure 1 and Table 2). This reduction in biomass production may be related to the presence of the classic symptoms of CsCMV, for which leaves show mosaic and chlorotic symptoms that may progress to atrophy of plants in more severe conditions (Calvert and Thresh, 2002Calvert, L.A.; Thresh, J.M. 2002. The viruses and virus diseases of cassava. p. 237-260. In: Hillocks, R.J.; Thresh, J.M.; Bellotti, C., eds. Cassava: biology, production and utilization. CAB International, Wallingford, UK.). Since the virus colonizes the plant systemically, chlorotic leaves can compromise photosynthesis and consequently the production of photosynthate for biomass accumulation.
Boxplot of plant height (PH), aerial part yield(APY), root yield (RoY), harvest index (HI), dry matter content (DMC), and starch yield (StY) traits in non-inoculated or CsCMV-inoculated plants. Means connected by the same letter do not differ significantly at p ≥ 0.05 by LSD test.
Mean relative reductions caused by Cassava common mosaic virus (CsCMV) inoculated in cassava plants compared to non-inoculated plants for several agronomic traits: plant height (PH), aerial part yield (APY), root yield (RoY), harvest index (HI), dry matter content (DMC), and starch yield (StY).
Unlike other cassava viruses, such as Cassava brown streak disease (CBSD), CsCMV does not reduce the quality of the roots and make them unmarketable. However, there were significant losses in the yield of the aerial part, roots, and starch. In contrast, there were no differences between the inoculated and non-inoculated plants for the harvest index and dry matter content in the roots (Table 2). These last two traits were therefore less affected by the presence of CsCMV. In contrast to our results, a viral infection caused by Barley yellow dwarf virus decreased plant height, number of tillers, dry weight of shoots, and total weight of grain when two wheat varieties were infested with viruliferous aphids (Cezare et al., 2011Cezare, D.G.; Schons, J.; Lau, D. 2011. Analysis of resistance/ tolerance of the wheat cultivar BRS Timbaúva to Barley yellow dwarf virus (BYDV-PAV). Tropical Plant Pathology 36: 249-255 (in Portuguese, with abstract in English).). Therefore, the deleterious effects caused by viral diseases are highly dependent on the host and traits measured.
Relative losses caused by CsCMV
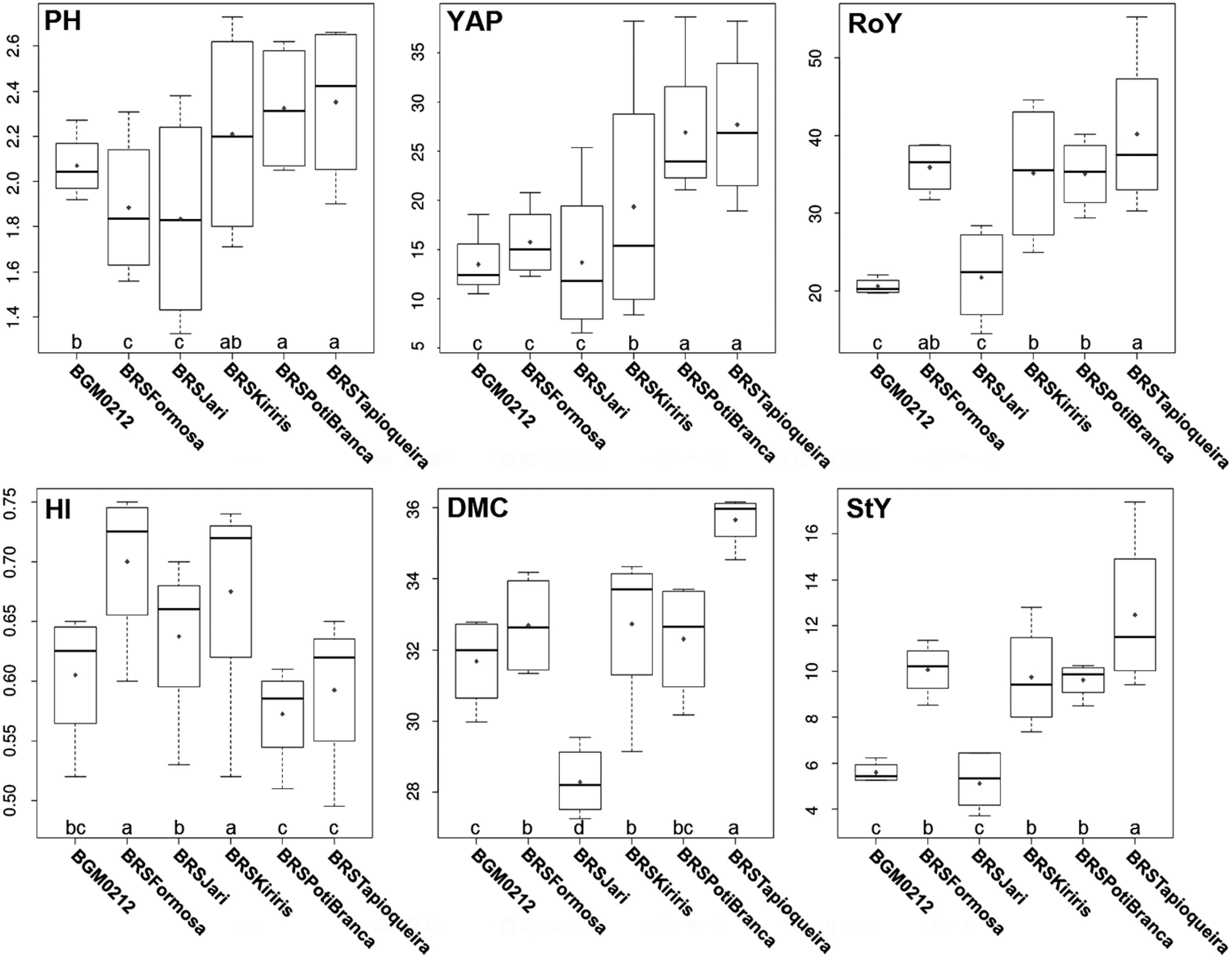
Estimates of the relative losses caused by CsCMV for the six agronomic traits are presented in Table 2. Although there was a slight reduction in plant height of all six cassava varieties which were inoculated, statistically significant differences between inoculated and non-inoculated plants were observed only in the BGM0212 (- 9.2 %) and BRS Kiriris (- 7.0 %) varieties. In inoculated and non-inoculated plants, the taller varieties were BRS Tapioqueira, BRS Poti Branca, and BRS Kiriris (Figure 2). In wheat, reductions in plant height ranged from 4.2 to 27.5 % depending on the variety as a result of SBWMV (Soil-borne wheat mosaic virus) (Dalbosco et al., 2002Dalbosco, M.; Schons, J.; Prestes, A.M.; Cecchetti, D. 2002. Effects of soil-borne wheat mosaic virus on yield in wheat and triticale cultivars. Fitopatologia Brasileira 27: 53-57 (in Portuguese, with abstract in English).).
Boxplot of plant height (PH), aerial part yield (YAP), root yield (RoY), harvest index (HI), dry matter content (DMC), and starch yield (StY) traits for each cassava variety. Means connected by the same letter do not differ significantly at p ≥ 0.05 by LSD test.
As to yield of the aerial plant part, only the BRS Formosa variety showed no difference between non-inoculated and CsCMV-inoculated plants (Table 2). For the remaining varieties, reduction in yield of the aerial part caused by CsCMV ranged from 13.5 % (BRS Tapioqueira) to 30.2 % (BRS Kiriris). According to Pazarlar et al. (2013)Pazarlar, S.; Gümüs, M.; Öztekin, G.B. 2013. The effects of tobacco mosaic virus infection on growth and physiological parameters in some pepper varieties (Capsicum annuum L.). Notulae Botanicae Horti Agrobotanici 41: 427-433., many viruses can cause significant reductions in the shoot, whereas the photosynthetic activities that provide energy for growth and plant defense against pests and diseases are dramatically reduced. Therefore, agronomic traits such as leaf number and total leaf area decrease and compromise the overall growth of the plant, and hence its biomass production.
As was the case with aerial part yield, there was a reduction in root yield in inoculated plants of all cassava varieties, except for BRS Formosa variety (Table 2). The greatest reduction in root yield caused by CsCMV (≈ 30 %) was observed in the BRS Kiriris and BRS Jari varieties. These losses in root yield in our study are higher than 10 to 20 % as reported by Fukuda (1993)Fukuda, C. 1993. Cassava disease = Doenças da mandioca. p. 53-56. In: EMBRAPA. Instruções práticas para o cultivo de mandioca. Centro Nacional de Mandioca e Fruticultura, Cruz das Almas, BA, Brazil (in Portuguese).. According to Calvert and Thresh (2002)Calvert, L.A.; Thresh, J.M. 2002. The viruses and virus diseases of cassava. p. 237-260. In: Hillocks, R.J.; Thresh, J.M.; Bellotti, C., eds. Cassava: biology, production and utilization. CAB International, Wallingford, UK., there is limited information about the properties, distribution, effects, and importance of most cassava viruses. In addition, a long period of co-evolution between pathogen and host has probably selected forms of tolerance to several neotropical cassava viruses, and lower levels of deleterious effects on the infected plants. In current highly competitive cassava production systems, relative losses in root yield caused by CsCMV in magnitudes of 30 % are unacceptably high. Among other cassava viruses, only Cassava mosaic disease (CMD) and CBSD cause great reduction in root yield, eventually reaching 80 % (Moses et al., 2007Moses, E.; Asafu-Agyei, J.N.; Adubofour, K.; Adusei, A. 2007. Guide to Identification and Control of Cassava Diseases. CSIR Crops Research Institute, Kumasi, Ghana.) and 100 % (Kaweesi et al., 2014Kaweesi, T.; Kawuki, R.; Kyaligonza, V.; Baguma, Y.; Tusiime, G.; Ferguson, M.E. 2014. Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virology Journal 11: 216.), respectively.
Virus-inoculated plants did not differ from the non-inoculated level in terms of harvest index and dry matter content (Figure 1 and Table 2). In general, dry matter content in cassava is influenced by factors such as variety, age at harvest, and environmental conditions (Kizito et al., 2007Kizito, E.B.; Stljung, A.C.R.W.; Egwang, T.; Gullberg, U.; Fregene, M.; Westerbergh, A.A. 2007. Quantitative trait loci controlling cyanogenic glucoside and dry matter content in cassava (Manihot esculenta Crantz) roots. Hereditas 144: 129-136.). However, according to Oliveira et al. (2015)Oliveira, E.J.; Aidar, S.T.; Morgante, C.V.; Chaves, A.R.M.; Cruz, J.L.; Coelho Filho, M.A. 2015. Genetic parameters for drought-tolerance in cassava. Pesquisa Agropecuária Brasileira 50: 233-241., the range of dry matter content is relatively narrow and the genetic component has a greater effect on the expression of these traits. Therefore, due to biotic stresses, small changes are expected in dry matter content, unless there is intense plant defoliation, which will require energy from the roots to restore the leaves, and a consequently rapid reduction of dry matter content. Indeed, CsCMV does not cause leaf drop, which explains, at least in part, the fact that dry matter content suffers only a relatively small reduction in virus-infected plants.
Among the varieties, only BRS Formosa suffered no reduction in starch yield when inoculated with the virus (Table 2), while the other varieties suffered significant reductions that ranged from 12.9 % to 30.0 %. As in the case of root yield, BRS Kiriris and BRS Jari varieties suffered the greatest reduction in starch yield due to CsCMV (over 28 %). Regardless of the virus infection, BRS Tapioqueira had the highest starch yield: 13.5 and 11.5 t ha−1 in non-inoculated and CsCMV-infected plants, respectively. Numerically, the second-highest starch yield was measured in BRS Formosa, with 10.7 and 9.5 t ha−1 in non-inoculated and CsCMV-inoculated plants, respectively (data not shown).
According to Legg et al. (2014b)Legg, J.P.; Lava Kumar, P.; Makeshkumar, T.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. 2014b. Cassava virus diseases: biology, epidemiology and management. Advances in Virus Research 91: 85-142., the importance of viral infections are usually ignored or underestimated, unless the yield losses compromise food security. Our observations confirm previous reports (Silva et al., 2011Silva, J.M.; Carnelossi, P.R.; Bijora, T.; Facco, C.U.; Picoli, M.H.S.; Souto, E.R.; Oliveira, A.J.B.; Almeida, A.M.R. 2011. Immunocapture-RT-PCR detection of Cassava common mosaic virus in cassava obtained from meristem-tip culture in Paraná state. Tropical Plant Pathology 36: 271-275.), in which CsCMV is spread across all cassava varieties grown in the northwestern state of Paraná (Brazil). There, the disease is considered of secondary importance and one that does not severely compromise yields. However, a reduction in starch yield of 28 % is quite high where there is a need to ensure income for farmers who exclusively cultivate this crop for the starch industry. Therefore, our results suggest the need to adopt control measures, such as varieties that are less susceptible.
Tolerance vs. susceptibility of cassava varieties
CsCMV-inoculated plants of the BRS Formosa did not differ in any of the agronomic traits compared to non-inoculated plants. BRS Tapioqueira was quite productive for root and starch yield regardless of the inoculation (36.9 t ha−1 of roots and 11.5 t ha−1 of starch). These results suggest a relationship between the presence of levels of CsCMV tolerance and reduction in disease symptoms, whose mechanisms in these two varieties need to be more comprehensively investigated in future research. In wheat, Cezare et al. (2011)Cezare, D.G.; Schons, J.; Lau, D. 2011. Analysis of resistance/ tolerance of the wheat cultivar BRS Timbaúva to Barley yellow dwarf virus (BYDV-PAV). Tropical Plant Pathology 36: 249-255 (in Portuguese, with abstract in English). demonstrated that the BRS Timbaúva variety was as susceptible to viral infection as Embrapa 16, but in all trials the damage caused by Barley yellow dwarf virus (BYDV) and the reduction in grain yield in BRS Timbaúva were low, suggesting some degree of tolerance to the virus-vector complex. Our results suggest that BRS Tapioqueira and BRS Formosa varieties are good options for cassava farmers.
Cassava viruses can be controlled using a set of control measures, including the adoption of virus-resistant varieties (Asare et al., 2014Asare, P.A.; Galyuon, I.K.A.; Asare-Bediako, E.; Sarfo, J.K.; Tetteh, J.P. 2014. Phenotypic and molecular scrKeening of cassava (Manihot esculenta Crantz) genotypes for resistance to cassava mosaic disease. Journal of General and Molecular Virology 6: 6-18.) or resistance to vectors (Akinbo et al., 2012Akinbo, O.; Labuschagne, M.; Fregene, M. 2012. Introgression of whitefly (Aleurotrachelus socialis) resistance gene from F1 inter-specific hybrids into commercial cassava. Euphytica 183: 19-26.) – as is the case with CMD – as well as roguing and sanitation (Calvert and Thresh, 2002Calvert, L.A.; Thresh, J.M. 2002. The viruses and virus diseases of cassava. p. 237-260. In: Hillocks, R.J.; Thresh, J.M.; Bellotti, C., eds. Cassava: biology, production and utilization. CAB International, Wallingford, UK.). However, the most important way of controlling disease in cassava is to reduce host susceptibility by using resistant varieties. Indeed, Asare et al. (2014)Asare, P.A.; Galyuon, I.K.A.; Asare-Bediako, E.; Sarfo, J.K.; Tetteh, J.P. 2014. Phenotypic and molecular scrKeening of cassava (Manihot esculenta Crantz) genotypes for resistance to cassava mosaic disease. Journal of General and Molecular Virology 6: 6-18. successfully identified sources of CMD tolerance to different viruses in a set of 38 cassava varieties grown in the field in Ghana. In that study, one variety (Capevars) was highly resistant without presenting the virus, while three other varieties (Adehye, Nkabom, and KW085) were tolerant but presented CMD via molecular analysis. In this case, there should be a tolerance mechanism to prevent the establishment or multiplication of CMD in resistant genotypes. In the specific case of CsCMV, the presence of the virus was confirmed in all of the varieties through indirect ELISA testing, with no restraint mechanism being identified to prevent the infection caused by this pathogen.
An important aspect in the evaluation of plant tolerance is knowledge of the virus genetic and pathogenic variability as it is common that plant tolerance is broken down by co- or multiple infections with different virus strains or even by highly virulent strains (Miano et al., 2008Miano, D.; Labonte, D.; Clark, C. 2008. Identification of molecular markers associated with sweet potato resistance to sweet potato virus disease in Kenya. Euphytica 160: 15-24.). This can make a resistant variety become susceptible in other environments.
Therefore, despite the fact that BRS Tapioqueira and BRS Formosa varieties can be considered tolerant to CsCMV, further studies are needed to check tolerance stability across different environments together with knowledge of the genetic variability of CsCMV.
Acknowledgments
The authors thank the Bahia State Foundation for Research Support (FAPESB), Coordination for the Improvement of Higher Level Personnel (CAPES) and the Brazilian National Council for Scientific and Technological Development (CNPq) for financial assistance and scholarship support.
References
- Akinbo, O.; Labuschagne, M.; Fregene, M. 2012. Introgression of whitefly (Aleurotrachelus socialis) resistance gene from F1 inter-specific hybrids into commercial cassava. Euphytica 183: 19-26.
- Asare, P.A.; Galyuon, I.K.A.; Asare-Bediako, E.; Sarfo, J.K.; Tetteh, J.P. 2014. Phenotypic and molecular scrKeening of cassava (Manihot esculenta Crantz) genotypes for resistance to cassava mosaic disease. Journal of General and Molecular Virology 6: 6-18.
- Calvert, L.A.; Ospina, M.D.; Shepherd, R.J. 1995. Characterization of cassava vein mosaic virus: a distinct plant pararetrovirus. Journal of General Virology 76: 1271-1276.
- Calvert, L.A.; Thresh, J.M. 2002. The viruses and virus diseases of cassava. p. 237-260. In: Hillocks, R.J.; Thresh, J.M.; Bellotti, C., eds. Cassava: biology, production and utilization. CAB International, Wallingford, UK.
- Cezare, D.G.; Schons, J.; Lau, D. 2011. Analysis of resistance/ tolerance of the wheat cultivar BRS Timbaúva to Barley yellow dwarf virus (BYDV-PAV). Tropical Plant Pathology 36: 249-255 (in Portuguese, with abstract in English).
- Cuervo Ibáñez, M.; Moreno, M.G.; Flor, N.C.; Ramírez, J.L.; Medina, C.A.; Debouck, D.; Martínez, J. 2010. Handbook of Procedures of the Germplasm Health Laboratory. International Center for Tropical Agriculture, Cali, Colombia.
- Dalbosco, M.; Schons, J.; Prestes, A.M.; Cecchetti, D. 2002. Effects of soil-borne wheat mosaic virus on yield in wheat and triticale cultivars. Fitopatologia Brasileira 27: 53-57 (in Portuguese, with abstract in English).
- El-Sharkawy, M.A. 2012. Stress-tolerant cassava: the role of integrative ecophysiology-breeding research in crop improvement. Open Journal of Soil Science 2: 162-186.
- Food and Agriculture Organization [FAO]. 2014. FAOSTAT database gateway. Available at: http://faostat.fao.org/site/567/default.aspx [Accessed Feb 15, 2015]
» http://faostat.fao.org/site/567/default.aspx - Fukuda, C. 1993. Cassava disease = Doenças da mandioca. p. 53-56. In: EMBRAPA. Instruções práticas para o cultivo de mandioca. Centro Nacional de Mandioca e Fruticultura, Cruz das Almas, BA, Brazil (in Portuguese).
- Kawano, K.; Fukuda, W.M.G.; Cenpukdee, U. 1987. Genetic and environmental effects on dry matter content of cassava root. Crop Science 27: 69-74.
- Kaweesi, T.; Kawuki, R.; Kyaligonza, V.; Baguma, Y.; Tusiime, G.; Ferguson, M.E. 2014. Field evaluation of selected cassava genotypes for cassava brown streak disease based on symptom expression and virus load. Virology Journal 11: 216.
- Kizito, E.B.; Stljung, A.C.R.W.; Egwang, T.; Gullberg, U.; Fregene, M.; Westerbergh, A.A. 2007. Quantitative trait loci controlling cyanogenic glucoside and dry matter content in cassava (Manihot esculenta Crantz) roots. Hereditas 144: 129-136.
- Legg, J.P.; Somado, E.A.; Barker, I.; Beach, L.; Ceballos, H.; Cuellar, W.; Elkhoury, W.; Gerling, D.; Helsen, J.; Hershey, C.; Jarvis, A.; Kulakow, P.; Kumar, P.L.; Lorenzen, J.; Lynam, J.; McMahon, M.; Gowda, M.; Miano, D.; Mtunda, K.; Natwuruhunga, P.; Okogbenin, E.; Pezo, P.; Terry, E.; Thiele, G.; Thresh, M.; Wadsworth, J.; Walsh, S.; Winter, S.; Tohme, J.; Fauquet, C.M. 2014a. A global alliance declaring war on cassava viruses in Africa. Food Security 6: 231-248.
- Legg, J.P.; Lava Kumar, P.; Makeshkumar, T.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. 2014b. Cassava virus diseases: biology, epidemiology and management. Advances in Virus Research 91: 85-142.
- Miano, D.; Labonte, D.; Clark, C. 2008. Identification of molecular markers associated with sweet potato resistance to sweet potato virus disease in Kenya. Euphytica 160: 15-24.
- Moses, E.; Asafu-Agyei, J.N.; Adubofour, K.; Adusei, A. 2007. Guide to Identification and Control of Cassava Diseases. CSIR Crops Research Institute, Kumasi, Ghana.
- Mowat, W.P.; Dawson, S. 1987. Detection of plant viruses by ELISA using crude sap extracts and unfractionated antisera. Journal of Virology Methods 15: 233-247.
- Oliveira, E.J.; Aidar, S.T.; Morgante, C.V.; Chaves, A.R.M.; Cruz, J.L.; Coelho Filho, M.A. 2015. Genetic parameters for drought-tolerance in cassava. Pesquisa Agropecuária Brasileira 50: 233-241.
- Pazarlar, S.; Gümüs, M.; Öztekin, G.B. 2013. The effects of tobacco mosaic virus infection on growth and physiological parameters in some pepper varieties (Capsicum annuum L.). Notulae Botanicae Horti Agrobotanici 41: 427-433.
- Sastry, K.S.; Zitter, T.A. 2014. Management of virus and viroid diseases of crops in the tropics. p. 149-480. In: Sastry, K.S.; Zitter, T.A., eds. Plant virus and viroid diseases in the tropics. Springer, Berlin, Germany.
- Silva, J.M.; Carnelossi, P.R.; Bijora, T.; Facco, C.U.; Picoli, M.H.S.; Souto, E.R.; Oliveira, A.J.B.; Almeida, A.M.R. 2011. Immunocapture-RT-PCR detection of Cassava common mosaic virus in cassava obtained from meristem-tip culture in Paraná state. Tropical Plant Pathology 36: 271-275.
- Tonukari, N.J.; Ezedom, T.; Enuma, C.C.; Sakpa, S.O.; Avwioroko, O.J.; Eraga, L.; Odiyoma, E. 2015. White gold: cassava as an industrial base. American Journal of Plant Sciences 6: 972-979.
Edited by
Publication Dates
-
Publication in this collection
Nov-Dec 2016
History
-
Received
30 Sept 2015 -
Accepted
06 Jan 2016