ABSTRACT
Citrus canker, caused by Xanthomonas citri subsp. citri (Xcc), has an important economic impact on the citrus industry. Extensive information is available about the disease but, nevertheless, the study of plant-pathogen interactions could provide new information in the understanding of citrus canker disease. A new isolate has been identified, Xcc AT, which has a high genetic similarity (> 90 %) to the virulent Xcc T strain based on genetic clustering analyses of the rep-PCR fingerprinting patterns, but it does not produce cankerous lesions in Citrus limon. In this study, we compared C. limon responses to Xcc AT and to the virulent Xcc T strain at both histological and transcriptional levels. Histologically, leaves inoculated with Xcc AT exhibited neither a typical disordering of the spongy mesophyll, nor a swelling of epidermis. A particular content (undetermined) was also found in mesophyll cells near the stomata, together with increased starch accumulation. The transcriptomic profiles were compared by cDNA-AFLP technique. A total of 121 fragments derived from transcript (TDF) were either specifically induced or repressed by the isolates, and 62 were sequenced. Analysis of global expression identified different classes of genes known to be involved in plant-pathogen interactions. This study constitutes the first approach of the specific interaction between the avirulent Xcc AT isolate and C. limon.
Keywords:
cDNA-AFLP; gene expression; canker; plant defense; microscopy
Introduction
Asiatic citrus canker, caused by the bacterial plant pathogen Xanthomonas citri subsp. citri (Xcc), is a widespread disease that affects most commercial citrus species and varieties. Xcc infects hosts through natural openings, such as stomata and lesions in plant tissue, producing pustule-like cankers on leaves, stems and fruits (Brunings and Gabriel, 2003Brunings, A.M.; Gabriel, D.W. 2003. Xanthomonas citri: breaking the surface. Molecular Plant Pathology 4: 141-157.) and defoliation, blemished fruit, premature abscission and fruit drop in severe infections, all of which results in crop loss. Because Xcc is a quarantine pathogen in many countries, the impact of the disease on commerce is the most serious consequence, as there are restrictions on interstate and international fruit trade from canker-affected areas are imposed (Gottwald et al., 2002Gottwald, T.R.; Graham, J.H.; Schubert, T.S. 2002. Citrus canker: the pathogen and its impact. Plant Health Progress. DOI: 10.1094/PHP-2002-0812-01-RV.
https://doi.org/10.1094/PHP-2002-0812-01...
; 2009Gottwald, T.; Graham, J.; Bock, C.; Bonnc, G.; Civerolo, E.; Irey, M.; Leite, R.; McColluma, G.; Parker, P.; Ramallo, J.; Riley, T.; Schubert, T.; Stein, B.; Taylor, E. 2009. The epidemiological significance of post-packinghouse survival of Xanthomonas citri subsp. citri for dissemination of Asiatic citrus canker via infected fruit. Crop Protection 28: 508-524.).
The disease is present in the Americas, where it first appeared in Brazil, and soon spread to other important citrus producing countries, such as Argentina, one of the world's largest lemon producers. Extensive information is already available about the disease, its epidemiology, the susceptibility of host genotypes and, in particular, the Xcc pathogen (Gottwald, 1993Gottwald, T.R. 1993. Differential host range reaction of Citrus and citrus relatives to citrus canker and citrus bacterial spot determined by leaf mesophyll susceptibility. Plant Disease 77: 1004.; Graham et al., 2004Graham, J.H.; Gottwald, T.R.; Cubero J.; Achor, D.S. 2004. Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Molecular Plant Pathology 5: 1-15.; Van Sluys et al., 2002Van Sluys, M.; Monteiro-Vitorello, C.B.; Camargo, L.E.; Menck, C.F.M.; Silva, A.C.R.; Ferro, J.A.; Oliveira, M.C.; Setubal, J.C.; Kitajima, J.P.; Simpson, A.J. 2002. Comparative genomic analysis of plant-associated bacteria. Annual Review of Phytopathology 40: 169-189.; Vojnov et al., 2010Vojnov, A.A.; Amaral, A.M.; Dow, J.M.; Maxwell, J.; Castagnaro, A.P.; Marano, M.R. 2010. Bacteria causing important diseases of citrus utilise distinct modes of pathogenesis to attack a common host. Applied Microbiology and Biotechnology 87: 467-477.). However. the study of plant-pathogen interactions could provide new information in the understanding of citrus canker disease.
Chiesa et al. (2013)Chiesa, M.; Siciliano, M.F.; Ornella, L.; Roeschlin, R.A.; Favaro, M.A.; Pino Delgado, N.; Sendín, L.N.; Orce, I.G.; Ploper, L.D.; Vojnov, A.A.; Gadea Vacas, J.; Filippone, M.P.; Castagnaro, A.P.; Marano, M.R. 2013. Characterization of a variant of Xanthomonas citri subsp. citri that triggers a host-specific defense response. Phytopathology 103: 555-564. identified a new Xcc isolate with a high genetic similarity (> 90 %) to the virulent Xcc T strain based on genetic clustering analyses of the fingerprinting patterns generated by BOX, repetitive extragenic palindromic (REP), and enterobacterial repetitive intergenic consensus (ERIC) polymerase chain reaction (PCR) (repetitive elements-based [rep]-PCRs). Named Xcc AT, this isolate does not produce cankerous lesions in a host-specific interaction with C. limon, and is deficient in extracellular polysaccharide (EPS) xanthan production and induces an oxidative burst and a defense response in C. limon, independent of supplementation with exogenous xanthan (Chiesa et al., 2013Chiesa, M.; Siciliano, M.F.; Ornella, L.; Roeschlin, R.A.; Favaro, M.A.; Pino Delgado, N.; Sendín, L.N.; Orce, I.G.; Ploper, L.D.; Vojnov, A.A.; Gadea Vacas, J.; Filippone, M.P.; Castagnaro, A.P.; Marano, M.R. 2013. Characterization of a variant of Xanthomonas citri subsp. citri that triggers a host-specific defense response. Phytopathology 103: 555-564.).
In this study, we compared C. limon responses to Xcc AT and the virulent Xcc T strain at both histological and transcriptional levels. The close genetic relationship between these two isolates could serve to understand the mechanism underlying resistance and susceptibility.
Materials and Methods
Plant material and inoculum preparation
Six-month plants of Citrus limon (L.) Burm cv. Frost Eureka Nuclear were used to reproduce canker disease under controlled growth conditions. Plant leaves were inoculated with a bacterial suspension of Xcc AT or virulent Xcc T strain with a concentration of 108 CFU mL−1, using swab methods to mimic a natural Xcc infection process (Sendín et al., 2011Sendín, L.N.; Filippone, M.P.; Orce, I.G.; Rigano, L.; Enrique, R.; Peña, L.; Vojnov, A.A.; Marano, M.R.; Castagnaro, A.P. 2011. Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathology 61: 648-657.). Inoculation with magnesium chloride (MgCl2) was used as control. After inoculation, plants were covered with plastic bags and kept overnight, and then transferred to a growth chamber at 28 °C, with 70 % relative humidity and a 16 h photoperiod.
Light microscopy and cDNA-AFLP analysis
For histological analyses, samples of inoculated leaves were taken at 0, 3 and 7 days post-inoculation (dpi). Cross sections of these samples were stained with safranin, methyl blue and iodine (D'Ambrogio de Argüeso, 1986D' Ambrogio de Argüeso, A. 1986. Manual of Vegetal Histology Technics. Ed. Hemisferio Sur, Buenos Aires, Argentina.), mounted on slides in a solution of glycerin / water (50/50) and observed in a light microscope.
For transcriptional analyses, total RNA from inoculated leaves was prepared by LiCl precipitation (Sambrook et al., 1989Sambrook, J.; Fritsch, E.F.; Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual. 2ed. Cold Spring Harbor Laboratory Press, New York, NY, USA.). The cDNA-AFLP-based transcript profiling procedure was followed as prescribed by Vuylsteke et al. (2007)Vuylsteke, M.; Peleman, J.D.; van Eijk, M.J.T. 2007. AFLP-based transcript profiling (cDNA-AFLP) for genome-wide expression analysis. Nature Protocols 2: 1399-1413., but with different restriction enzymes (MseI and EcoRI). Selective amplifications using the primers 5’-GACTGCGTACCAATTCANN-3’ (EcoRI, where NN represents –GG, CT, GC, CC, AG, CA, AC and CG) and 5’-GATGAGTCCTGAGTAACMM-3’ (MseI, where MM represents TT, AT, AC, AA TC, TA, AG and TG) were performed with a total of 64 selective primer combinations. Amplification products were separated on 5 % polyacrylamide gels, reamplified and sequenced. Sequences obtained were compared with those shown by the BLASTn and BLASTx (NCBI, http://www.ncbi.nlm.nih.gov/BLAST), PHYTOZOME (http://www.phytozome.net), “ORANGE (Citrus sinensis) genome annotation” (http://citrus.hzau.edu.cn) and HARVEST (http://cgf.ucdavis.edu) searching engines. The expression level of eight TDFs randomly selected from Table 1 was quantified by quantitative real time retrotranscription-PCR (qRT-PCR) to validate the pattern of TDFs obtained with cDNA-AFLP. qRT-PCR analyses were performed in a 25 μL volume containing 1X master mix (iQ SYBR Green), 0.4 uM of each primer, and 4 μL of 10-fold cDNA dilutions. The relative expression of RNA transcripts was quantified with the threshold cycle value (Ct) obtained from each sample by using the 2-ΔΔCt method (Livak and Schmittggen, 2001Livak, K.J.; Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408.), with a plant cytochrome oxidase (COX) gene for reference gene expression (Li et al., 2005Li, W.; Hartung, J.S.; Levy, L. 2005. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. Journal of Microbiological Methods 66: 104-115.).
Similarities of sequences of Citrus limon differentially expressed in response to either Xanthomonas citri subsp. citri isolates (Xcc T or Xcc AT) infection.
Results and Discussion
Symptomatology and histology reveal differences in the interaction between C. limon and the closely-related Xcc AT and Xcc T
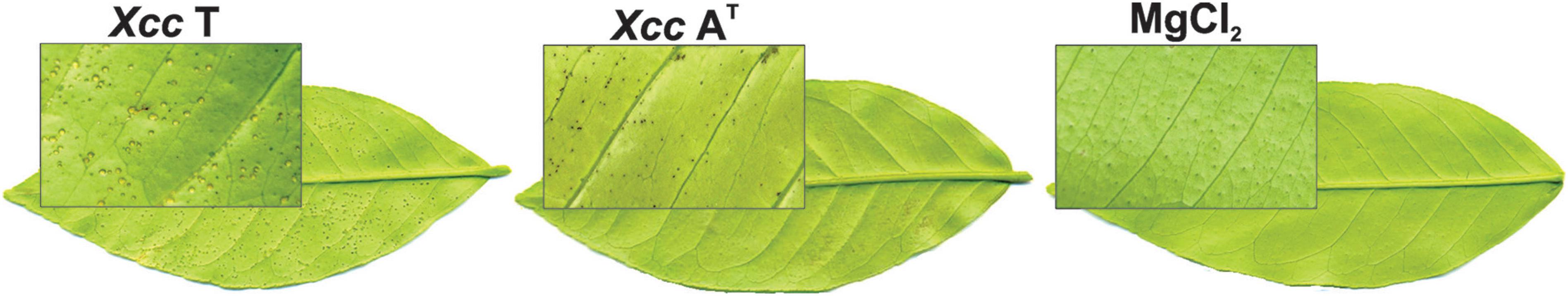
Differential symptomatology was observed in young C. limon leaves treated with avirulent (Xcc AT) or virulent (Xcc T) isolates. Seven days post-inoculation (7 dpi), Xcc T produced the typical circular water soaked lesions, in contrast with leaves inoculated with Xcc AT, where circular dark spots were observed (Figure 1).
Differential symptomatology induced in Citrus limon by Xcc T and Xcc AT. Foliar symptoms were observed at 7 days post-inoculation (dpi) in leaves inoculated with either Xanthomonas citri subsp. citri isolates, Xcc T (left) or Xcc AT (middle), and in mock inoculation with MgCl2 (right).
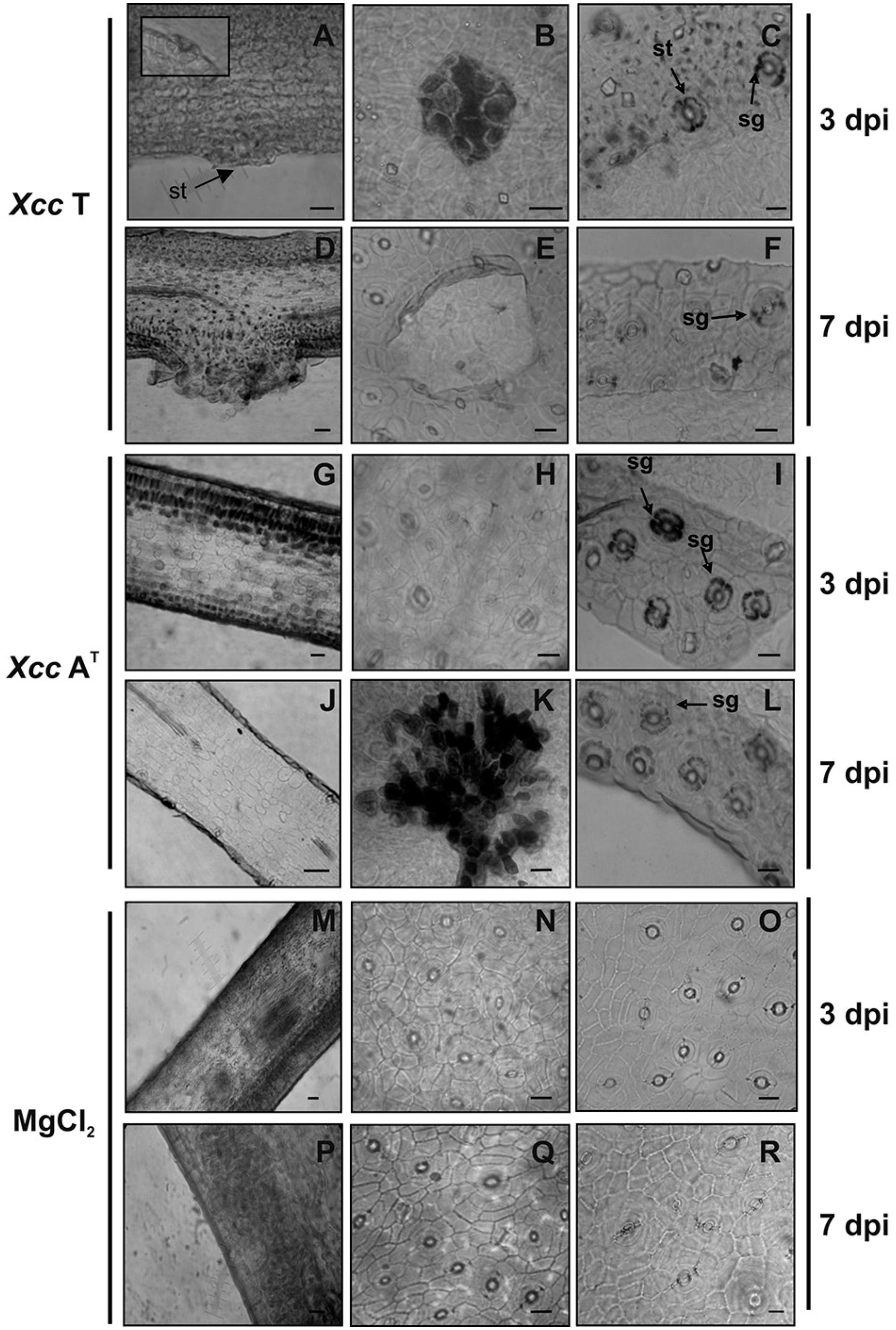
The differential symptomatology observed in C. limon after inoculation with Xcc T or Xcc AT was histologically analyzed by light microscopy. In leaf samples inoculated with Xcc T, it was observed that cells around the stomata were disorderly, intercellular space in the spongy mesophyll region had diminished, and leaf epidermal cells had swollen by 3 dpi (Figure 2A, B). By 7 dpi typical hyperplasia and hypertrophy of mesophyll cells were observed, with a consequent rupture of abaxial epidermis and bacteria oozing at the site of infection (Figure 2D, E). In contrast, in leaf samples inoculated with Xcc AT (Figure 2G, H, J), as well as in control leaves (Figure 2M, N, P, Q), the spongy mesophyll did not show disordering and epidermal cells had not swollen either by 3 dpi nor 7 dpi. However, the mesophyll cells near the stomata showed a particular content by 7 dpi in leaves inoculated with Xcc AT (not determined) (Figure 2K). The starch accumulation was higher in guardian cells of stomata in leaves inoculated with Xcc AT, than in leaf tissue inoculated with Xcc T (Figure 2C, F, I, L). The highest amount of starch was observed at 3 dpi, but it dropped at 7 dpi (Figure 2I, L). In control leaves, cumuli of starch were not observed (Figure 2O, R). This reaction has been cited as a general plant response to different pathogen infections (Bertani et al., 2014Bertani, R.P.; Perera, M.F.; Arias, M.E.; Luque, C.; Funes, C.; González, V.; Cuenya, M.I.; Ploper, D.L.; Castagnaro, A.P. 2014. A study of the sugarcane yellow leaf disease in Argentina. Plant Disease 98: 1036-1042.; Pardo et al., 2012Pardo, E.M.; Grellet, C.F.; Salazar, S.M.; Castagnaro, A.P.; Ricci, J.C.; Arias, M.E. 2012. Histopathology of the resistance to Colletotrichum gloeosporioides of wild strawberries and species related to commercial strawberry. Australian Journal of Crop Science 6: 1147-1153.). Some evidence suggests that the wild Xcc strain presents in its genome a gene coding for a protein similar to natriuretic plant proteins, which are involved in the regulation of cell growth and homeostasis (Gottig et al., 2008Gottig, N.; Garavaglia, B.S.; Daurelio, L.D.; Valentine, A.; Gehring, C.; Orellano, E.G.; Ottado, J. 2008. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proceedings of the National Academy of Sciences of the United States of America 105: 18631-18636.). This protein was shown to alter host physiological responses by means of starch granule degradation in the stomatal guard cells of the plant, causing stomatal openings thereof (Gottig et al., 2008Gottig, N.; Garavaglia, B.S.; Daurelio, L.D.; Valentine, A.; Gehring, C.; Orellano, E.G.; Ottado, J. 2008. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proceedings of the National Academy of Sciences of the United States of America 105: 18631-18636.). It is possible that this protein is defective or absent in Xcc AT, leading to starch accumulation in stoma cells which then hamper the entry of the pathogen.
Histopathologic analysis of Citrus limon leaves inoculated with virulent (Xcc T) or avirulent (Xcc AT) Xanthomonas citri subsp. citri isolates on the 3 and 7 day post-inoculations (dpi). Cross-sections of leaves inoculated with Xcc T (A, D) and Xcc AT (G, J), and control leaves (M, P); a detailed close-up of stomata is included as insert in A. Paradermal views of leaves inoculated with Xcc T (B, E), Xcc AT (H, K), or control leaves (N, Q). Histochemical Lugol test in leaves inoculated with Xcc T (C, F), Xcc AT (I, L) or control leaves (O, R). Arrows indicate stomata (st) or starch accumulation (sg); scale bars: 15 µm.
The avirulent Xcc AT elicits a distinct gene expression pattern in the interaction with C. limon
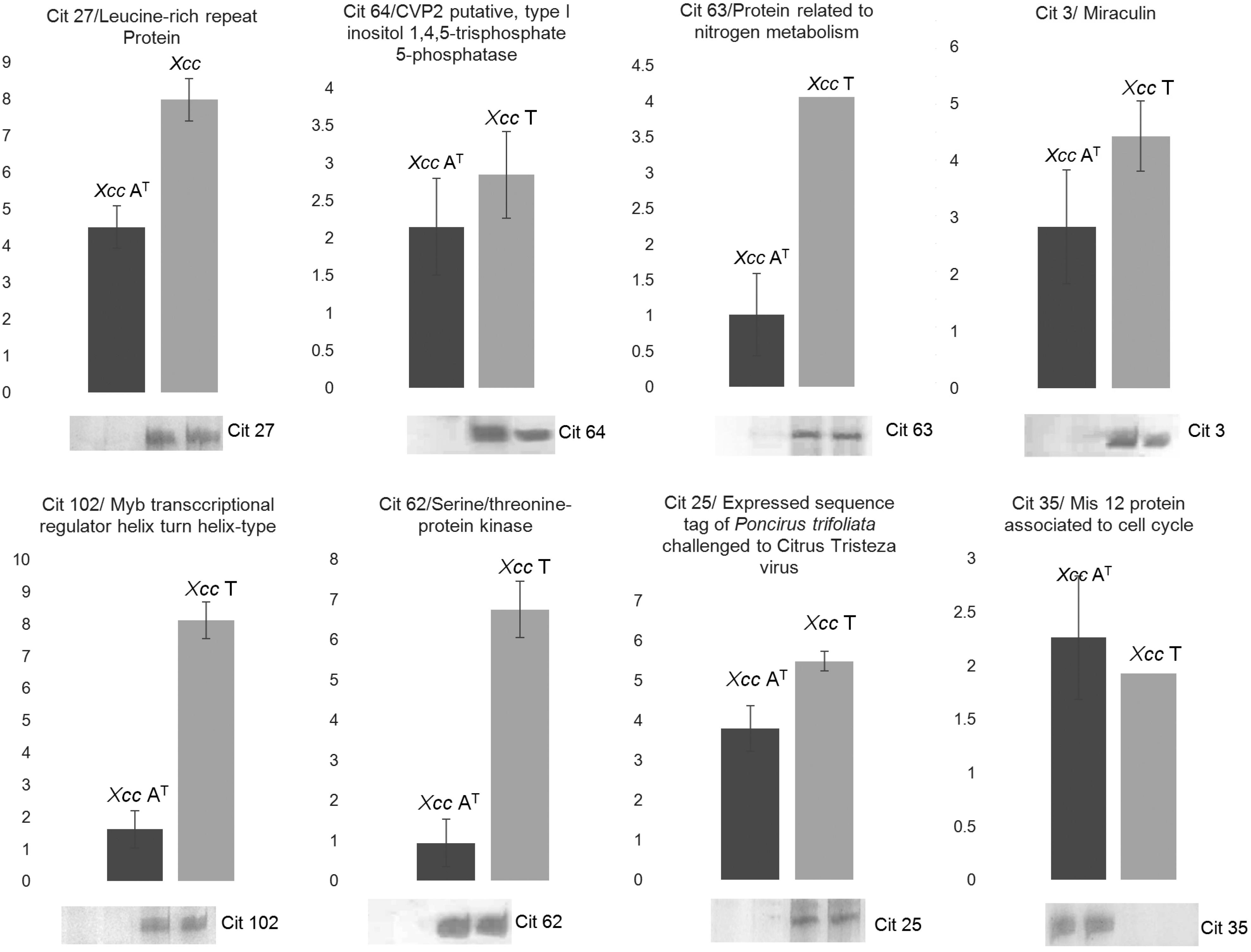
To find out what genes are involved in the interaction between C. limon and the new Xcc AT isolate, a transcript profile was attained through cDNA amplified fragment length polymorphism (cDNA-AFLP) technique, as a first approach to elucidate certain of the mechanisms involved in this specifically induced interaction. This study was carried out 48 h post-inoculation (hpi), comparing the gene profile induced by the avirulent Xcc AT isolate with those produced by the virulent strain (Xcc T) or mock inoculation with MgCl2. Out of 752 fragments derived from transcripts (TDFs) observed in silver-stained cDNA-AFLP gels, 121 TDFs were specifically induced or repressed by virulent (Xcc T) and avirulent (Xcc AT) isolates. Most of the up-regulated TDFs were observed when the plant was challenged with the virulent isolate Xcc T (47 %); the reverse was true for down-regulated TDFs, as their lowest number was recorded in this case (6 %) (Table 1). By contrast, in the interaction with the Xcc AT isolate, the numbers of up-regulated (26 %) and down-regulated (21 %) TDFs were similar (Table 1). A total of 62 differential TDFs sequences were successfully obtained. Table 1 shows the similarities of TDFs differentially expressed in response to either Xcc T or Xcc AT infection. The expression level of eight TDFs randomly selected from Table 1 was validated by qRT-PCR (Figure 3). As much as 18 % of the TDFs did not show any significant similarity to known proteins. The next most abundant group was composed of TDFs with significant similarity to resistance genes (15 %) and genes involved in plant metabolism (15 %). The other TDFs were identified as being similar to genes involved in transcription and translation (13 %), energy (10 %), pathway signaling (5 %), structural proteins (5 %) and transport mechanisms (2 %). Other TDF groups were linked with citrus EST (11 %) or uncharacterized proteins (8 %) in the database.
Quantitative real time retrotranscription- PCR (RT-PCR) analysis of randomly selected transcript derived fragments (TDF) expressed diferentially during Xcc/ Citrus limon interaction. Relative expression of 8 randomly selected TDF (Cit 27, 64, 63, 3, 102, 62, 25 and 35) was quantified through quantitative real time RT-PCR using the 2−ΔΔCt method to validate the expression level observed in cDNA-AFLP analysis. All data were normalized using a plant cytochrome oxidase (COX) gene. The mean expression value was calculated for every TDF with three replications. The corresponding signal in a cDNA-AFLP analysis is included for each target.
Some TDFs differentially expressed in the interactions are involved in the same mechanisms; for example, genes encoding proteins of the light-harvesting complex of photosystem II (PSII) (Table 1, Cit 26, Cit 32 and Cit 89). It has been shown that the PSII system plays an important role in preventing the accumulation of reactive oxygen species (ROS) (Asada, 1999Asada, K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601-639.), and it also seems to participate in both compatible and incompatible plant pathogen interactions (Baldo et al., 2010Baldo, A.; Norelli, J.L.; Farrell, R.E.; Bassett, C.L.; Aldwinckle, H.S.; Malnoy, M. 2010. Identification of genes differentially expressed during interaction of resistant and susceptible apple cultivars (Malus × domestica) with Erwinia amylovora. BMC Plant Biology 10: 1.).
In the interaction with the virulent isolate, up-regulated genes involved in cell cycle, growth and differentiation (and related with hyperplasia and hypertrophy) were identified, as has been previously described (Cernadas et al., 2008Cernadas, R.A.; Camillo, L.R.; Benedetti, C.E. 2008. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Molecular Plant Pathology 9: 609-631.).
Interestingly, one of the TDFs induced by Xcc T showed sequence similarity to an MYB transcription factor (Cit 102). Baldo et al. (2010)Baldo, A.; Norelli, J.L.; Farrell, R.E.; Bassett, C.L.; Aldwinckle, H.S.; Malnoy, M. 2010. Identification of genes differentially expressed during interaction of resistant and susceptible apple cultivars (Malus × domestica) with Erwinia amylovora. BMC Plant Biology 10: 1. also found a TDF homologue to MYB 11 in Malus domestica, which was overexpressed in susceptible cultivars after inoculation with Erwinia amylovora. In the same way, two MYB genes in Arabidopsis thaliana were specifically induced by Type 3 effector proteins, and they negatively regulated phenylpropanoid metabolism (Preston et al., 2004Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. 2004. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant Journal 40: 979-995.). They are widely distributed in plants and have been reported to be involved in the ABA-response and to have interactions with other transcription factors (Ambawat et al., 2013Ambawat, S.; Sharma, P.; Yadav, N.; Yadav, R. 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiololgy and Molecular Biology Plants 19: 307-321.). In this interaction, one TDF similar to a lipoxygenase (Cit 29) was also induced. Lipoxygenase (LOX) has been identified as generating highly cytotoxic compounds like unsaturated aldehydes and lipid hydroperoxides, which interact with transition metals to produce corrosive radicals (Brash, 1999Brash, A. 1999. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry 274: 23679-23682.). These compounds could produce damage in the membrane by an alternative way to the oxidative burst produced during hypersensitivity reactions (HR) of an incompatible interaction (Adam et al., 1989Adam, A.; Farkas, T.; Somlyai, G.; Hevesi, M.; Kiraly, Z. 1989. Consequence of O2 generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipid. Physiological and Molecular Plant Pathology 34: 13-26.; Porter et al., 1995Porter, N.A.; Caldwell, S.A.; Mills, K.A. 1995. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30: 277-290.; Mittler et al., 1996Mittler, R.; Shulaev, V.; Seskar, M.; Lam, E. 1996. Inhibition of programmed cell death in tobacco plants during a pathogeninduced hypersensitive response at low oxygen pressure. Plant Cell 8: 1991-2001.). However, the LOX mechanism has been proven to be associated with plant-pathogen interaction, and has been observed in both compatible and incompatible interactions (Jalloul et al., 2002Jalloul, A.; Montillet, J.L.; Assigbetsé, K.; Agnel, J.P.; Delannoy, E.; Triantaphylides, C.; Daniel, J.F.; Marmey, P.; Geiger, J.P.; Nicole, M. 2002. Lipid peroxidation in cotton: Xanthomonas interactions and the role of lipoxygenases during the hypersensitive reaction. Plant Journal 32: 1-12.). In concordance with Chiesa et al. (2013)Chiesa, M.; Siciliano, M.F.; Ornella, L.; Roeschlin, R.A.; Favaro, M.A.; Pino Delgado, N.; Sendín, L.N.; Orce, I.G.; Ploper, L.D.; Vojnov, A.A.; Gadea Vacas, J.; Filippone, M.P.; Castagnaro, A.P.; Marano, M.R. 2013. Characterization of a variant of Xanthomonas citri subsp. citri that triggers a host-specific defense response. Phytopathology 103: 555-564., we could say that it is possible that Xcc T inactivates ROS mechanism; nonetheless, the plant can activate another defense pathway, such as the LOX mechanism.
As expected, many of the TDFs expressed differentially during the interaction between C. limon and Xcc AT were found to be related in some way to resistance and plant defense response. Xcc AT induced a TDF similar to plant receptor-like serine threonine kinase (RLSTK) (Cit 34). This receptor recognizes molecules of pathogens and activates signals triggering plant defense responses; such is the case of rice bacterial blight resistance gene product XA21 (Song et al., 1995Song, W.Y.; Wang, G.L.; Chen, L.; Kim, K.S.; Holstein, T.; Wang, B.; Zhai, Z.; Zhu, L.H.; Fauquet, C.; Ronald, P.C. 1995. The rice disease resistance gene Xa-21 encoded a receptor kinase-like protein. Science 270: 1804-1806.) and FLS2, a flagellin perception gene in A. thaliana (Gómez-Gómez and Boller, 2000Gómez-Gómez, L.; Boller, T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Molecular Cell 5: 1003-1011.). Another of the TDFs induced by Xcc AT was Cit 11, which shows similarity to the enzyme aminocyclopropanecarboxylate oxidase (ACC oxidase), which in turn catalyzes the cleavage of 1-aminocyclopropane-1-carboxylic acid (ACC) into ethylene. It is widely known that ethylene, together with other compounds such as salicylic acid, phytoalexins or reactive oxygen species (ROS), forms part of the complex plant defense mechanism (Joshi and Nayak, 2011Joshi, R.K.; Nayak, S. 2011. Functional characterization and signal transduction ability of nucleotide-binding site-leucine-rich repeat resistance genes in plants. Genetic and Molecular Research 10: 2637-2652.). ACC oxidase induction could indicate that ethylene increases with the interaction between C. limon and this avirulent isolate. The activation of this gene in citrus plants was previously described in sweet orange Pera inoculated with X. axonopodis pv. aurantifolii (Xaa) pathotype C, which does not produce canker in this genotype. Xaa induces ethylene related genes like ACC oxidases, as well as ethylene receptors, ethylene-induced esterase and ethylene response factors 48 hpi, and these responses are associated to disease resistance (Cernadas et al., 2008Cernadas, R.A.; Camillo, L.R.; Benedetti, C.E. 2008. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Molecular Plant Pathology 9: 609-631.).
Interestingly, Xcc AT induced the expression of a TDF similar to a PRP38 splicing factor (Cit 108). Recently, new findings have provided insights into the roles of alternative splicing in the regulation of expression of plant defense genes (Marone et al., 2013Marone, D.; Russo, M.; Laido, G.; Leonardis, A.M.; Mastrangelo, A.M. 2013. Plant Nucleotide Binding Site-Leucine-Rich Repeat (NBS-LRR) genes: active guardians in host defense responses. International Journal of Molecular Science 14: 7302-7326.). This factor has been generally associated with abiotic stresses such as cold and salinity (Forment et al., 2005Forment, J.; Gadea, J.; Huerta, L. 2005. Development of a citrus genome-wide EST collection and cDNA microarray as resources for genomic studies. Plant Molecular Biology 57: 375-391.; Iida et al., 2004Iida, K.; Seki, M.; Sakurai, T.; Satou, M.; Akiyama, K.; Toyoda, T.; Konagaya, A.; Shinozaki, K. 2004. Genome-wide analysis of alternative pre-mRNA splicing in Arabidopsis thaliana based on full-length cDNA sequences. Nucleic Acids Research 32: 5096-5103.); however, this is the first report of this gene in association with a biotic factor.
On the other hand, in the interaction between C. limon and Xcc AT a TDF similar to a gene coding for SNARE proteins (Cit 90) was suppressed. One of the primary roles of SNARE proteins is to mediate fusion between cellular transport vesicles and the cellular plasma membrane for exocytosis. A study using inhibitors of vesicle trafficking has shown that this inhibition delays pustule development induced by Xcc in sweet orange Pera leaves (Cernadas et al., 2008Cernadas, R.A.; Camillo, L.R.; Benedetti, C.E. 2008. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Molecular Plant Pathology 9: 609-631.), so the suppression of this gene would be consistent with the lack of symptoms in Xcc AT inoculated leaves.
In conclusion, unlike the virulent type, the avirulent isolate Xcc AT did not prevent the development of physical barriers, such as starch accumulation or cell wall thickening, both events associated with plant defense mechanisms. Differences in gene expression patterns of infected plants, together with the close genetic relationship between these two isolates, could serve as the basis for developing different hypotheses related to the resistance or susceptibility mechanisms responsible for the different infection patterns that these two isolates exhibit. However, a high-throughput sequencing approach will be needed to get a more complete picture of differences in the plant molecular response to both Xcc isolates.
Acknowledgments
This project was partially supported by the EEAOC (Obispo Colombres Agro-Industrial Experimental Station) Citrus Program. We especially thank Dr. Welin (EEAOC, Argentina) and Miss Adriana Manes (English teacher) for the critical reading of this manuscript and for reviewing the English version of the manuscript.
References
- Adam, A.; Farkas, T.; Somlyai, G.; Hevesi, M.; Kiraly, Z. 1989. Consequence of O2 generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipid. Physiological and Molecular Plant Pathology 34: 13-26.
- Ambawat, S.; Sharma, P.; Yadav, N.; Yadav, R. 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiololgy and Molecular Biology Plants 19: 307-321.
- Asada, K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601-639.
- Baldo, A.; Norelli, J.L.; Farrell, R.E.; Bassett, C.L.; Aldwinckle, H.S.; Malnoy, M. 2010. Identification of genes differentially expressed during interaction of resistant and susceptible apple cultivars (Malus × domestica) with Erwinia amylovora BMC Plant Biology 10: 1.
- Bertani, R.P.; Perera, M.F.; Arias, M.E.; Luque, C.; Funes, C.; González, V.; Cuenya, M.I.; Ploper, D.L.; Castagnaro, A.P. 2014. A study of the sugarcane yellow leaf disease in Argentina. Plant Disease 98: 1036-1042.
- Brash, A. 1999. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry 274: 23679-23682.
- Brunings, A.M.; Gabriel, D.W. 2003. Xanthomonas citri: breaking the surface. Molecular Plant Pathology 4: 141-157.
- Cernadas, R.A.; Camillo, L.R.; Benedetti, C.E. 2008. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii Molecular Plant Pathology 9: 609-631.
- Chiesa, M.; Siciliano, M.F.; Ornella, L.; Roeschlin, R.A.; Favaro, M.A.; Pino Delgado, N.; Sendín, L.N.; Orce, I.G.; Ploper, L.D.; Vojnov, A.A.; Gadea Vacas, J.; Filippone, M.P.; Castagnaro, A.P.; Marano, M.R. 2013. Characterization of a variant of Xanthomonas citri subsp. citri that triggers a host-specific defense response. Phytopathology 103: 555-564.
- D' Ambrogio de Argüeso, A. 1986. Manual of Vegetal Histology Technics. Ed. Hemisferio Sur, Buenos Aires, Argentina.
- Forment, J.; Gadea, J.; Huerta, L. 2005. Development of a citrus genome-wide EST collection and cDNA microarray as resources for genomic studies. Plant Molecular Biology 57: 375-391.
- Graham, J.H.; Gottwald, T.R.; Cubero J.; Achor, D.S. 2004. Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Molecular Plant Pathology 5: 1-15.
- Gómez-Gómez, L.; Boller, T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis Molecular Cell 5: 1003-1011.
- Gottig, N.; Garavaglia, B.S.; Daurelio, L.D.; Valentine, A.; Gehring, C.; Orellano, E.G.; Ottado, J. 2008. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proceedings of the National Academy of Sciences of the United States of America 105: 18631-18636.
- Gottwald, T.; Graham, J.; Bock, C.; Bonnc, G.; Civerolo, E.; Irey, M.; Leite, R.; McColluma, G.; Parker, P.; Ramallo, J.; Riley, T.; Schubert, T.; Stein, B.; Taylor, E. 2009. The epidemiological significance of post-packinghouse survival of Xanthomonas citri subsp. citri for dissemination of Asiatic citrus canker via infected fruit. Crop Protection 28: 508-524.
- Gottwald, T.R.; Graham, J.H.; Schubert, T.S. 2002. Citrus canker: the pathogen and its impact. Plant Health Progress. DOI: 10.1094/PHP-2002-0812-01-RV.
» https://doi.org/10.1094/PHP-2002-0812-01-RV - Gottwald, T.R. 1993. Differential host range reaction of Citrus and citrus relatives to citrus canker and citrus bacterial spot determined by leaf mesophyll susceptibility. Plant Disease 77: 1004.
- Iida, K.; Seki, M.; Sakurai, T.; Satou, M.; Akiyama, K.; Toyoda, T.; Konagaya, A.; Shinozaki, K. 2004. Genome-wide analysis of alternative pre-mRNA splicing in Arabidopsis thaliana based on full-length cDNA sequences. Nucleic Acids Research 32: 5096-5103.
- Jalloul, A.; Montillet, J.L.; Assigbetsé, K.; Agnel, J.P.; Delannoy, E.; Triantaphylides, C.; Daniel, J.F.; Marmey, P.; Geiger, J.P.; Nicole, M. 2002. Lipid peroxidation in cotton: Xanthomonas interactions and the role of lipoxygenases during the hypersensitive reaction. Plant Journal 32: 1-12.
- Joshi, R.K.; Nayak, S. 2011. Functional characterization and signal transduction ability of nucleotide-binding site-leucine-rich repeat resistance genes in plants. Genetic and Molecular Research 10: 2637-2652.
- Li, W.; Hartung, J.S.; Levy, L. 2005. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. Journal of Microbiological Methods 66: 104-115.
- Livak, K.J.; Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408.
- Marone, D.; Russo, M.; Laido, G.; Leonardis, A.M.; Mastrangelo, A.M. 2013. Plant Nucleotide Binding Site-Leucine-Rich Repeat (NBS-LRR) genes: active guardians in host defense responses. International Journal of Molecular Science 14: 7302-7326.
- Mittler, R.; Shulaev, V.; Seskar, M.; Lam, E. 1996. Inhibition of programmed cell death in tobacco plants during a pathogeninduced hypersensitive response at low oxygen pressure. Plant Cell 8: 1991-2001.
- Pardo, E.M.; Grellet, C.F.; Salazar, S.M.; Castagnaro, A.P.; Ricci, J.C.; Arias, M.E. 2012. Histopathology of the resistance to Colletotrichum gloeosporioides of wild strawberries and species related to commercial strawberry. Australian Journal of Crop Science 6: 1147-1153.
- Porter, N.A.; Caldwell, S.A.; Mills, K.A. 1995. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30: 277-290.
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. 2004. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant Journal 40: 979-995.
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual. 2ed. Cold Spring Harbor Laboratory Press, New York, NY, USA.
- Sendín, L.N.; Filippone, M.P.; Orce, I.G.; Rigano, L.; Enrique, R.; Peña, L.; Vojnov, A.A.; Marano, M.R.; Castagnaro, A.P. 2011. Transient expression of pepper Bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathology 61: 648-657.
- Song, W.Y.; Wang, G.L.; Chen, L.; Kim, K.S.; Holstein, T.; Wang, B.; Zhai, Z.; Zhu, L.H.; Fauquet, C.; Ronald, P.C. 1995. The rice disease resistance gene Xa-21 encoded a receptor kinase-like protein. Science 270: 1804-1806.
- Van Sluys, M.; Monteiro-Vitorello, C.B.; Camargo, L.E.; Menck, C.F.M.; Silva, A.C.R.; Ferro, J.A.; Oliveira, M.C.; Setubal, J.C.; Kitajima, J.P.; Simpson, A.J. 2002. Comparative genomic analysis of plant-associated bacteria. Annual Review of Phytopathology 40: 169-189.
- Vojnov, A.A.; Amaral, A.M.; Dow, J.M.; Maxwell, J.; Castagnaro, A.P.; Marano, M.R. 2010. Bacteria causing important diseases of citrus utilise distinct modes of pathogenesis to attack a common host. Applied Microbiology and Biotechnology 87: 467-477.
- Vuylsteke, M.; Peleman, J.D.; van Eijk, M.J.T. 2007. AFLP-based transcript profiling (cDNA-AFLP) for genome-wide expression analysis. Nature Protocols 2: 1399-1413.
Edited by
Publication Dates
-
Publication in this collection
Nov-Dec 2016
History
-
Received
29 Oct 2015 -
Accepted
25 Feb 2016