ABSTRACT:
We studied Spodoptera frugiperda development using different food sources in the laboratory and field. Newly hatched larvae were fed soybean, cotton, maize, wheat, and oat leaves. An artificial diet was used as the control. Duration of pre-pupal, pupal, and larva-adult period, pupal weight, sex ratio, survival, larva feeding preferences, oviposition preferences, and nutritional quality of different hosts were evaluated. Insects fed on wheat showed the shortest larva-adult period. The insects fed on cotton and soybean had longer larval development cycles and pupae of lower weight. Feeding preference was evident for third instar larvae and did not differ between wheat, oat, maize, and soybean, which were the preferred hosts. Moths oviposited to a greater extent on the upper canopy of wheat than that of other plants in both the no-choice and free-choice tests. Treatments influenced insect growth, food consumption, and digestion when nutritional variables were analyzed. Thus, grasses were better hosts for S. frugiperda development. Cotton was the least preferred food, followed by soybean. The present study can improve our understanding of S. frugiperda in these different crops and help in developing management strategies. Even though S. frugiperda is considered to be polyphagous, this pest is closely associated with grasses (maize, wheat, oat) and has lower potential as a soybean or cotton feeder. Howerver, S. frugiperda food intake regulation appears to be triggered by a complex of different mechanisms. Thus, S. frugiperda can also damage soybean and cotton and adapt to them in the absence of preferred hosts.
Keywords:
agricultural entomology; feeding behavior; insect-plant interactions
Introduction
The fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), consists of two genetically differentiated strains. They are commonly referred as the rice-strain (R-strain) and the corn-strain (C-strain) (Quisenberry, 1991Quisenberry, S.S. 1991. Fall armyworm (Lepidoptera: Noctuidae) host strain reproductive compatibility. Florida Entomologist 74: 194–199.; Nagoshi and Meagher, 2004Nagoshi, R.N.; Meagher, R.L. 2004. Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environmental Entomology 33: 881–889.). This pest is polyphagous and is found in several countries such as Brazil, Argentina, and the USA (Prowell et al., 2004Prowell, D.P.; McMichael, M.; Silvain, J.F. 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America 97: 1034–1044.; Clark et al., 2007Clark, P.L.; Molina-Ochoa, J.; Martinelli, S.; Skoda, S.R.; Isenhour, D.J.; Lee, D.J.; Krumn, J.T.; Foster, J.E. 2007. Population variation of Spodoptera frugiperda (J. E. Smith) in the Western Hemisphere. Journal of Insect Science 7: 1–10.), causing economic losses in a variety of crops such as maize (Zea mays L.), soybean (Glycine max (L.) Merrill), cotton (Gossypium hirsutum L.), and beans (Phaseolus vulgaris L.) (Pogue, 2002Pogue, G.M. 2002. A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Memoirs of the American Entomological Society 43: 1–202.; Nagoshi, 2009Nagoshi, R.N. 2009. Can the amount of corn acreage predict fall armyworm (Lepidoptera: Noctuidae) infestation levels in nearby cotton? Journal of Economic Entomology 102: 210–218.; Bueno et al., 2011Bueno, R.C.O.F.; Bueno, A.F.; Moscardi, F.; Parra, J.R.; Hoffmann-Campo, C.B. 2011. Lepidopteran larvae consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest Management Science 67: 170–174.). Because of its wide host range, S. frugiperda is one of the most harmful pests threatening annual crops in tropical regions (Andrews, 1980Andrews, K.L. 1980. The whorlworm, Spodoptera frugiperda, in Central America and neighboring areas. Florida Entomologist 63: 456–467.; Cruz et al., 1999Cruz, I.; Figueiredo, M.L.C.; Oliveira, A.C.; Vasconcelos, C.A. 1999. Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminium saturation. International Journal of Pest Management 45: 293–296.). Control of this pest is challenging because possible host plants have different phenologies and are grown during different seasons of the year though in proximity to each other, which can facilitate movement of the pest between crops. This availability of different hosts might even result in the selection of insect populations with new food preferences due to different exposure of these insects to a variety of crops (Barros et al., 2010Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.). For example, although soybean is not one of the preferred hosts of S. frugiperda, it is one of the most abundant summer crops in Brazil, which may favor the establishment and environmental colonization of this crop by S. frugiperda (Andrews, 1980Andrews, K.L. 1980. The whorlworm, Spodoptera frugiperda, in Central America and neighboring areas. Florida Entomologist 63: 456–467.; Barros et al., 2010Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.).
Basic biological studies on the consumption and use of different food sources, in addition to those on the host preference of S. frugiperda, are important for addressing the effects of the nutritional composition of different crops on this pest (Scriber and Slansky, 1981Scriber, J.M.; Slansky, J.R.F. 1981. The nutritional ecology of immature insects. Annual Review of Entomology 26: 183–211.; Barros et al., 2010Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.). Recently, a number of studies on S. frugiperda biology have been carried out (Ball et al., 2006Ball, O.J.P.; Coudron, T.A.; Tappe, B.A.; Davies, E.; Trently, D.; Bush, L.P.; Gwinn, K.D.; Popay, A.J. 2006. Importance of host plant species, neotyphodium, endophyte isolate, and alkaloids on feeding by Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Journal of Economic Entomology 99: 1462–1473.; Storer et al., 2010Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. 2010. Discovery and characterization of field resistance to bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. Journal of Economic Entomology 103: 1031–1038.). Multiple effects regarding the use of a number of weeds, such as Ipomoea sp., or crops, such as rice, maize, and other grasses, as S. frugiperda hosts have been reported (Nabity et al., 2011Nabity, P.D.; Zangerl, A.R.; Berenbaum, M.R.; Delucia, E.H. 2011. Bioenergy crops Miscanthus × giganteus and Panicum virgatum reduce growth and survivorship of Spodoptera frugiperda (Lepidoptera: Noctuidae). Journal of Economic Entomology 104: 459–464.). However, to the best of our knowledge, this is the first study to compare biological characteristics of this pest when fed on different host species grown in different seasons of the year (summer and winter crops). This is crucial to an understanding of the survival, population increase, and infestation of this species throughout the year. These factors, including the moth's immigration and emigration, contribute to changes in the population dynamics of S. frugiperda in an agricultural landscape (Tisdale and Sappington, 2001Tisdale, R.A.; Sappington, T.W. 2001. Realized and potential fecundity, egg fertility, and longevity of laboratory-reared female beet armyworm (Lepidoptera: Noctuidae) under different adult diet regimes. Annals of the Entomological Society of America 94: 415–419.). Thus, this work studied the biology, food attractiveness, and oviposition preference of S. frugiperda and nutritional quality of soybean, cotton, maize, oat (Avena strigosa Schreb), wheat (Triticum aestivum L.), and artificial diet by conducting experiments under laboratory and field conditions in order to better understand this pest's feeding behavior.
Materials and Methods
Trials were performed under controlled laboratory conditions (25 ± 2 °C, 70 ± 10 % RH, and a photoperiod of 14h10 [L:D]) or in the field (23°12′28.6″ S 51°10′56.5″ W) (600 m of altitude), Summer, average temperature of 23 °C, maximum temperature of 28 °C, minimum temperature of 20 °C, 2 mm precipitation), in Londrina, PR, Brazil.
The hosts evaluated were soybean (‘BRS 284’ cultivar), cotton (‘FMT 701’ cultivar), maize (‘DKB390’ cultivar), oat (‘Embrapa 139’ cultivar), and wheat (‘BRS Pardela’ cultivar), which represent the most important crops used in the soybean production system during the summer and winter seasons in the south and middle west of Brazil. The soybean cultivar ‘BRS 284’ is a conventional early cultivar of indeterminate growth habit. It is highly productive and cropped in the states of Paraná, São Paulo, Santa Catarina, Mato Grosso do Sul, and parts of Minas Gerais and Goiás in Brazil. ‘FMT 701’ has high yields, hardiness, and stability as well as good fiber quality. Thus, it has recently emerged as one of the most commonly sown cotton varieties in the state of Mato Grosso, Brazil. Maize ‘DKB390’ has excellent quality of stem and root and is one of the most productive hybrids in the market. The oat cultivar ‘Embrapa 139’ has early maturity and moderate resistance to lodging and leaf rust. It is suitable for cultivation in the states of Rio Grande do Sul, Santa Catarina, Paraná and São Paulo, Brazil. The wheat cultivar ‘BRS Pardela’ has excellent baking quality, wide adaptation and good pest tolerance.
Spodoptera frugiperda colony
Spodoptera frugiperda specimens were obtained from insect colonies and maintained in a laboratory. S. frugiperda was originally collected and identified from maize plants (C-strain) in Rio Verde, Goiás (17º 47′ 53″ S, 50º 55′ 41″ W, 715 m of altitude), in 2007, and maintained in the laboratory for approximately 35 generations. They were reared under laboratory-controlled conditions (25 ± 2 °C, 70 ± 10 % RH, and a photoperiod of 14h10 [L:D]) and fed on an artificial diet as described by Greene et al. (1976)Greene, G.L.; Leppla, N.C.; Dickerson, W.A. 1976. Velvetbean caterpillar: a rearing procedure and artificial diet. Journal of Economic Entomology 69: 487–488..
Biology of S. frugiperda on different host plants
The experiment was conducted in a Biochemical Oxygen Demand (BOD) climate chamber under controlled laboratory conditions (25 ± 2 °C, 70 ± 10 % RH, and a photoperiod of 14h10 [L:D]) using a randomized block design with six treatments and six replications. Each replication was performed using 10 individual larvae (a total of 60 larvae per treatment = 6 replications of 10 larvae each). Blocks were considered as the different shelves of the BOD in order to control any possible temperature gradient that might have been inside the same BOD from its top to its bottom. For the treatments, leaves of soybean (‘BRS 284’ cultivar), cotton (‘FMT 701’ cultivar), maize (‘DKB390’ cultivar), oat (‘Embrapa 139’ cultivar), and wheat (‘BRS Pardela’ cultivar) were used, and an artificial diet described by Greene et al. (1976)Greene, G.L.; Leppla, N.C.; Dickerson, W.A. 1976. Velvetbean caterpillar: a rearing procedure and artificial diet. Journal of Economic Entomology 69: 487–488. was used as the control. These hosts were grown in a greenhouse in pots (capacity, 20 liters) filled with soil up to 15 cm from the top edge. They were sown at a density of five plants per pot.
The trial was initiated when plants had 8 to 10 completely expanded leaves. Then, on a daily basis, one leaf from the top of the plants was exerted from each plant of all hosts studied. Leaf positioning varied from the first to the third leaf completely expanded depending on leaf availability. Then, plant leaves were cleaned by immersion in sodium hypochlorite (4 %), rinsed in distilled water for 3 to 5 s, and the excess water was removed with paper towels before being offered to the insects.
Initially, S. frugiperda eggs were isolated in waxed cups with different food sources, and temperature, humidity, and photoperiod were controlled until hatching. After that, first instar larvae were caged individually with plant leaves which were replaced on a daily basis to avoid excessive water loss. These insects were maintained in the same BOD (25 ± 2 °C, 70 ± 10 % RH, and for a photoperiod of 14h10 [L:D]) for daily assessment of the following biological variables: duration of pre-pupal (non-feeding stage between the larval and pupal period), pupal, larva-adult (total period from larva hatching to adult emergence) periods (days), pupal weight (g) measured 24 h after pupation, sex ratio, and survival (%).
Feeding preference of S. frugiperda among different host plants
Two experiments were performed under controlled laboratory conditions in a controlled environmental room (25 ± 2 °C, 70 ± 10 % RH, and a photoperiod of 14h10 [L:D]). Plants were grown under greenhouse conditions as described in the previous experiment (biology of S. frugiperda on different host plants). The trial was initiated when plants had 8 to 10 completely expanded leaves. Then, one leaf from the top of the plants (first completely expanded leaf) was extracted from each plant of all hosts studied to be used in the trials.
The first experiment was conducted with neonatal larvae and the second experiment with third instar larvae. A randomized block design was used for the experiments, with five treatments (leaves of soybean ‘BRS 284’, cotton ‘FMT 701’, maize ‘DKB390’, oat ‘Embrapa 139’, and wheat ‘BRS Pardela’) and 30 repetitions. For each replication, an acrylic petri dish (diameter, 15 cm) lined with filter paper moistened with distilled water was used, and leaf discs (diameter, 2 cm) of the center of soybean, cotton, and maize leaves and sections (50–70 g) of the middle region of wheat and oat leaves, similar to the weight of the leaf area of other tested crops, were used. The food source was distributed equidistantly to evaluate the feeding preferences of S. frugiperda. When the experiment was performed using first instar larvae, 24 larvae were released in the center of the petri dish. When the experiment was performed with third instar larvae, only 12 larvae were released. This difference in the number of insects was to better accommodate the size of the larva on the dish. Visual evaluations (counting) were performed after 60 min and also after 24 h of release, quantifying the number of insects that were feeding on the different hosts (insects that were above the leaves) (Botton et al., 1998Botton, M.; Carbonari, J.J.; Garcia, M.S.; Martins, J.F.S. 1998. Feeding preference and biology of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on rice and barnyardgrass. Anais da Sociedade Entomológica do Brasil 27: 207–212 (in Portuguese, with abstract in English).).
Oviposition preference of S. frugiperda among different host plants
Two experiments (free-choice and no-choice tests) were conducted to evaluate the oviposition preference of S. frugiperda adults for soybean, cotton, maize, oat, and wheat in the field without any environmental control. The plants were grown in pots (capacity, 20 liters) filled with soil up to 15 cm from the top edge. The host species (soybean, cotton, maize, oat, and wheat) were sown at a density of five plants per pot. The different host plants were sown on different dates in order to obtain the same growth stages for all plants. For standardization of the experiments, plants were used when they had 8 to 10 completely expanded leaves. The experiments were performed inside screened cages (5 × 4 × 2.5 m, length, width, and height) in a randomized block design, with five treatments (soybean, cotton, maize, oat, and wheat) and five replications. In the free-choice test each replication was 10 pots with five plants each (50 plants from each host) that were grouped (15 cm among pots) and equidistant from the other host plants inside the same screened cage (block with 250 plants, being 50 plants from each host). There was a total of five screened cages (five blocks) in the trial. In the no-choice test each replication was a screened cage with 50 pots (250 plants) of the same host. There were a total of 25 screened cages in the trial (five blocks and five hosts).
For both experiments, the moths were first reared in the laboratory on the artificial diet until the adult stage. After the start of oviposition in the laboratory (age, 3 d), the moths were released inside the cages at the beginning of scotophase in a density of 100 pairs (100 males and 100 females) per cage and maintained there for 3 d so that they could lay their eggs among the plants. Egg masses found 60 h after the release of the moths were removed and taken to the laboratory, where the number of eggs and egg position inside the plant (bottom, middle, and upper canopy) were evaluated. Canopy sectors were identified measuring plants of each species and equally dividing them into three parts. Therefore, the size of each part would be different for each host species. Even though some of the host species might have been small during oviposition canopy division data was still able to provide further insight into this biological parameter.
Nutritional quality of different host plants for S. frugiperda
The experiment was conducted in a BOD under controlled laboratory conditions (25 ± 2 °C temperature, 70 ± 10 % relative humidity, and a photoperiod of 14h10 [L:D]) in a completely randomized design with 30 replicates for each host plant. Plants were cultivated in a greenhouse and their leaves exerted and cleaned in sodium hypochlorite (4 %) to eliminate any possible contamination as described in the previous experiment (biology of S. frugiperda on different host plants).
First, S. frugiperda larvae were maintained on leaves of different hosts, soybean (BRS 284), cotton (FMT 701), maize (DKB 390), oat (Embrapa 139), and wheat (BRS Pardela) up to the third instar stage. We used the artificial diet described by Greene et al. (1976)Greene, G.L.; Leppla, N.C.; Dickerson, W.A. 1976. Velvetbean caterpillar: a rearing procedure and artificial diet. Journal of Economic Entomology 69: 487–488. as the control. Then, on reaching the third instar stage, 30 insects were weighed to obtain their initial weight, individually isolated in waxed cups and maintained in a climate chamber (BOD; 25 ± 2 °C, 70 ± 10 % RH, and a photoperiod of 14h10 [L:D]) for the treatments. The food (host leaves) provided was weighed daily on an analytical balance (Shimadzu/Toledo AY220) accurate to 0.0001 grams, and the remaining food was removed and stored. Feces were also removed and stored. Finally, on reaching the sixth instar, the larvae were weighed, killed by freezing, and subsequently dried in an oven, and the remaining food and feces were maintained at 55–60 °C for 72 h until reaching constant weight indicating complete dehydration.
The variables evaluated were as follows: initial weight of the third instar larvae (mg), larval weight (mg), food consumed (mg), feces weight (mg) and feeding time (days). At the same time, fresh and dry weights of 10 larvae were recorded to obtain the correction factor for initial dry weight, which was calculated from the average dry weight divided by the average fresh weight, and the value was multiplied by all the initial fresh weights of the larvae used in the experiment. The water loss of the hosts was calculated similarly to the water loss of the larvae. All weight values were converted to dry weight values for analysis.
Statistical analysis
Prior to analysis of variance (ANOVA), the experimental results were submitted for exploratory analysis to test the normality and independence of the residuals (Shapiro and Wilk, 1965Shapiro, S.S.; Wilk, M.B. 1965. An analysis of variance test for normality. Biometrika 52: 591–611.), homogeneity of variance of the errors of the treatments (Burr and Foster, 1972Burr, I.W.; Foster, L.A. 1972. A test for equality of variances. University of Purdue, West Lafayette, IN, USA.), and the non-additivity of the model (Tukey, 1949Tukey, J.W. 1949. One degree of freedon for non-additivity. Biometrics 5: 232–242.). The treatment mean values obtained by ANOVA were compared using Tukey's test (p ≤ 0.05) and in other cases (ANCOVA) with Tukey-Kramer test (p ≤ 0.05), with the SAS (Statistical Analysis System, version 9.2).
To evaluate the nutritional parameters, we used analysis of covariance (ANCOVA) to evaluate the effect of covariates on the response variables and estimate the growth, food consumption and conversion efficiency of the assimilation of food eaten and digested into biomass (in addition to weight gain), as proposed by Raubenheimer and Simpson (1992)Raubenheimer, D.; Simpson, S.J. 1992. Analysis of covariance: an alternative to nutritional indices. Entomologia Experimentalis et Applicata 62: 221–231.. The growth and consumption of larvae in the different treatments were obtained, respectively, by adjusting the final weight of the larvae and food consumption for the covariate feeding time. The efficiency in the conversion of ingested and digested food into biomass was calculated by the adjustment of final weight of the larvae, respectively, for the food consumed and digested (the amount of food consumed minus the amount of feces produced by the insects). Food assimilation was estimated after adjusting the amount of feces produced for the covariate amount of food consumed (Raubenheimer and Simpson, 1994Raubenheimer, D.; Simpson, S.J. 1994. The analysis of nutrient budgets. Functional Ecology 8: 783–791.).
The experimental design used in the majority of the trials was randomized blocks with the exception of the variables obtained in the study of the nutritional quality of different plant hosts for S. frugiperda that was installed under the completely randomized design.
The effects of the different treatments are qualitative with fixed effects and blocks and repetitions with random effect. The use of covariance analysis in this study had several functions: a) for assisting in the interpretation of experimental data, b) for controlling the error and increase the accuracy of the data analysis and c) for adjusting the treatment means as a function of the mean of the covariables, in terms of mean different covariates (secondary dependent variables). In this work the covariates used are not independent of the effects of the treatments and control of errors is partial. Where the effect of the covariate (model 2) and/or the interaction between the covariate and treatment (model 3) were significant, the effect of treatments was adjusted to the model including a covariate and the Tukey-Kramer mean comparison test was applied (model 2 and 3). Otherwise, it used the parallel model and the main effect of the covariate and/or treatment was considered, and the average by 5 % Tukey test (model 1) (Piubelli et al., 2005Piubelli, G.C.; Hoffmann-Campo, C.B.; Moscardi, F. ; Miyakubo, S.H.; Oliveira, M.C.N. 2005. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? Journal of Chemical Ecology 31: 1509–1525.). To meet these prerequisites, models of analysis of variance (ANOVA) and covariance (ANCOVA) were as follows:
Analysis of Variance (ANOVA): (model 1)
where:Yij is the dependent variable; ti a fixed effect of the treatment; bj the block with random effect; εij the randomized error effect with N = (0, σ2).
Analysis of Covariance for a covariate: (model 2)
where: Yij is an observation (dependent variable Y) of i treatment in j block; Xij an observation of covariate X in the plot receiving treatment i in j block; m, ti, bj and β are parameters, uncorrelated, and εij is the experimental error with N ≅ (0, σ2). .. the overall average for each covariate;
.. the overall average for each covariate;
Analysis of Covariance for the interactions between treatments and covariate: (model 3)
where: Yij is an observation (dependent variable Y) of i treatment in j block; Xij an observation of covariate X in the plot receiving treatment i in j block; .. the overall average for each covariate; m, tj, bj and β are parameters, uncorrelated; ij is the experimental error where N ≅ (0, σ2). is the effect of interaction between the treatment effect and the covariate, and ε
is the effect of interaction between the treatment effect and the covariate, and ε
Results
Biology of S. frugiperda on different host plants
The pre-pupal stage was the shortest for the insects fed on oat leaves, while the other treatments did not differ between themselves (dferror = 24, p < 0.0001). Similarly the pupal stage was shorter when the insects fed on grasses (maize, oat, and wheat). Larvae that fed on wheat, oat, and maize showed shorter larva-adult development time than those fed on cotton and soybean leaves (dferror = 25, p < 0.0001). Larva-adult period was 7.96, 7.38, and 5.99 days longer for cotton when compared to the larva-adult period for maize, oat, and wheat, respectively. Larva-adult period was 4.77, 4.19, and 2.80 days longer for soybean when compared to the larva-adult period for maize, oat, and wheat, respectively (Table 1).
Duration (mean ± standard error) of the pre-pupal and pupal stages, and larva-adult period of development (days) of Spodoptera frugiperda fed on different foods under controlled conditions (25 ± 2 °C, 70 ± 10 % relative humidity and photoperiod of 14h10 [L:D]).
Larva-adult survival (dferror = 25, p < 0.4872) and sex ratio (dferror = 25, p < 0.6252) did not differ between the host plants. The artificial diet supported the highest pupal weight among the treatments, followed by maize (which was similar to oat). The lowest pupal weight was for larvae fed on cotton leaves (Table 2).
Survival (%) (mean ± standard error), sex ratio, and pupal weight (grams) of Spodoptera frugiperda on different foods under controlled conditions (25 ± 2 °C, 70 ± 10 % relative humidity and photoperiod of 14h10 [L:D]).
Feeding preference of S. frugiperda among different host plants
In the first experiment (dferror = 15, p < 0.0001) using first instar larvae evaluated 60 min after release, the larvae exhibited an equal feeding preference for maize, soybean, cotton, and oat; wheat was the least attractive host. However, it is important to point out that little movement from the insects occurred, since most of the larvae were found in the middle of the plate (Table 3). First instar larvae were more attracted to wheat and soybean 24 h after release, while cotton was the least attractive food source (Table 3).
Feeding preferences of Spodoptera frugiperda (first instar n = 24 and third instar n = 12) at 60 min and 24 h in different hosts (25 ± 2 °C, 70 ± 10 % relative humidity and photoperiod of 14h10 [L:D]).
In the second experiment (dferror = 15, p < 0.0001), third instar larvae preferred maize, which is statistically similar to the preference for soybean and cotton, 60 min after being released into the cage (Table 3). Although not statistically different between hosts, the preference pattern of third instar larvae 24 h after release was similar to that seen in the first instars (Table 3).
Oviposition preference of S. frugiperda among different host plants
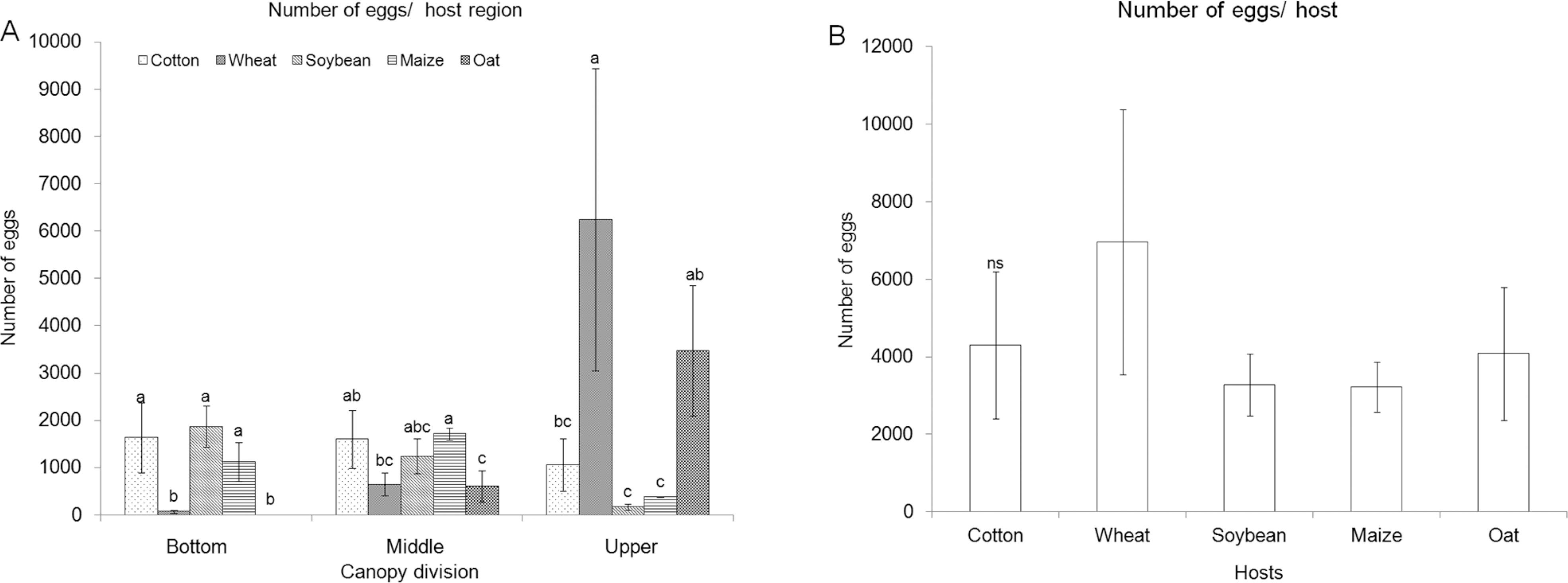
Oviposition preference of S. frugiperda in the free-choice test (Figure 1) was similar among the host plants at the bottom (dferror = 16, p = 0.2901) and middle (dferror = 16, p = 0.1927) canopies (Figure 1A). No eggs were laid in the upper canopy (dferror = 16, p = 0.0021) of oat plants, while the number of eggs in the upper regions of the other four hosts did not vary statistically (Figure 1A). The highest numbers of eggs over the entire plant were laid on wheat, with no difference in the number of eggs laid on entire plants of the other four host species (Figure 1B).
Number of eggs (Mean ± Standard Error) of Spodoptera frugiperda on different host region (A) and host species (B) in free-choice test. Means followed by the same letter do not differ for each portion of the canopy (bottom, middle and upper) or different hosts by Tukey test (p ≤ 0.05). nsAnalysis of variance (ANOVA) non-significant.
In the no-choice preference test (Figure 2), the number of S. frugiperda eggs found at the bottom (dferror = 16, p < 0.0001) and middle canopies (dferror = 16, p = 0.0033) of cotton, soybean, and maize was higher than the number of eggs found on the same canopy parts of the other host plants (Figure 2A). In the upper canopy (dferror = 16, p = 0.0002), the largest number of eggs was found on wheat. There was no difference in the total number of eggs found among the host plants (Figure 2B).
Number of eggs (Mean ± Standard Error) of Spodoptera frugiperda on different host region (A) and host species (B) in no-choice test. Means followed by the same letter do not differ for each portion of the canopy (bottom, middle and upper) by Tukey test (p ≤ 0.05). nsAnalysis of variance (ANOVA) not significant.
Nutritional quality of different host plants for S. frugiperda
Treatments differ (dferror = 118, p < 0.0001) for the initial weight variables of the larvae (third instar), final larval weight, amount of food consumed, feces weight, and feeding time (Table 4). Initial larval weight varied according to host crop, with the lightest larvae feeding on cotton, soybean and maize, and the heaviest on diet, wheat and oat (Table 4). Maize and soybean were consumed to a lesser extent by the insects; however, larvae that fed on maize took a shorter time to reach their sixth instar than did larvae on soybean and cotton (Table 4).
Initial weight of 3rd instar larvae (mg), weight of sixth larval instars (mg), food consumed (mg), feces weight (mg), feeding time (days), digested food (mg) (mean ± standard error) of Spodoptera frugiperda on different foods (25 ± 2 °C, 70 ± 10 % relative humidity and photoperiod of 14h10 [L:D]).
The analysis of covariance (ANCOVA) model showed that the initial weight (covariate) and different treatments showed no influence (Table 5) on the final weight of S. frugiperda (model 3). However, individual analyses of the treatments (model 2) demonstrated their influence on the final weight of the caterpillars which is the weight gain. According to the average obtained by ANCOVA, caterpillars fed on wheat, oat, and diet had the highest weight gain (Figure 3).
Analysis of covariance (ANCOVA) model of the effect of different treatments on the final weight adjustment by covariates caterpillar initial weight, final weight of the caterpillar covariates time and power consumption use by covariate feeding time, feces weight by covariate consumption, final weight of the caterpillar covariate digested food in Spodoptera frugiperda (25 ± 2 °C, 70 ± 10 % relative humidity and photoperiod of 14h10 [L:D]).
Analysis of covariance (ANCOVA) means of final weight of the caterpillar adjusted by initial weight of S. frugiperda in different foods. For statistical analysis see Table 5. Means followed by the same letter do not differ by Tukey test (p ≤ 0.05).
ANCOVA showed that there was no interactive effect of the treatments, feeding time (covariate) (Table 5), and consumption (covariate) (Table 5) on the final weight of larvae. Larvae growth and the efficiency of ingested food conversion are independent of the interaction between feeding time and consumption. Furthermore, feeding time (covariate) and treatments do not interfere in the amount of consumed food (Table 5), but the main effect of treatments and the feeding time influence the amount of food consumed when separately analyzed. This shows that larvae consumption depends on feeding time and consumed food, but not the interaction between them. The average from ANCOVA (Figure 4) illustrates the lowest consumption by larvae fed with soybean. It can be seen that using the covariate (model 2) gives a reduction in the residual mean square (QMR) (QMR = 27.952, 246) compared to model 1 (QMR = 33. 291, 405).
Analysis of covariance (ANCOVA) means of consumption adjusted by feeding time of S. frugiperda fed in different foods. For statistical analysis see Table 5. Means followed by the same letter do not differ by Tukey test (p ≤ 0.05).
The amount of feces produced by larvae was not dependent on the interaction between treatments and the amount of food consumed (Table 5). However, in the absence of interaction, the treatments and food consumed influenced the final weight of feces, also presenting reduction QMR (Table 5). Average weight of feces from ANCOVA (Figure 5) is inversely related to assimilation of consumed food. Larvae fed with soybean, cotton, and maize had the highest assimilation of consumed food since they produced the lowest weight of feces.
Analysis of covariance (ANCOVA) means of feces weight adjusted by consumption of S. frugiperda in different foods. For statistical analysis see Table 5. Means followed by the same letter do not differ by Tukey test (p ≤ 0.05).
Digested food (covariate) and treatments had no effect on the final weight of larvae (Table 5) with minimal reduction of QMR (Table 5), thus obtaining greater experimental precision. Independent analysis of digested food and treatments (plant species) revealed that the treatments altered larvae digestion of food consumed. Larvae fed with cotton, oat, and wheat had the greatest efficiency in digesting these foods compared with larvae fed with soybean and maize (Figure 6).
Analysis of covariance (ANCOVA) means of final weight of the caterpillar adjusted by digested food of S. frugiperda in different foods. For statistical analysis see Table 5. Means followed by the same letter do not differ by Tukey test (p ≤ 0.05).
Discussion
Cotton and soybean leaves were revealed to be less adequate hosts for the development of S. frugiperda when compared to the grasses studied, since the larvae that fed on both cotton and soybean were both adversely affected as they had a prolonged larva-adult period and reduced pupal weight. An extended duration of the larva-adult period is described as a compensatory action for a larva to recover when feeding on a low-quality host and still be able to pupate and achieve a greater weight. This may explain the differences in the treatments used in this study. In general, the development of insects depends on the quality of the food consumed in the first few instars, which may vary according to the host (Barros et al., 2010Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.). Moreover, the larvae have already been significantly affected by their host, at least in terms of weight, at the beginning of the experiment. The fact that the end weights are in the exact same order as the initial weights suggests that whatever host factors affect larval growth and development come into effect early in larval life.
The grass species evaluated in this study were the most suitable hosts for the growth of S. frugiperda. The low nutritional quality of cotton and soybean for S. frugiperda observed in this experiment in comparison with the grasses and artificial diet, resulted in the lower weight of the pupae and greater duration of the pupal stage. This insect preference and best performance for grasses has been previously demonstrated by other researchers. S. frugiperda preferred grasses such as maize, sorghum, and bermudagrass, which are C4 plants, as opposed to C3 plants such as cotton or soybean (Buntin, 1986Buntin, G.D. 1986. A review of plant response to fall armyworm, Spodoptera frugiperda (J.E. Smith), injury in selected field and forage crops. Florida Entomologist 69: 549–559.; Lewter et al., 2006Lewter, J.A.; Szalanski, A.L.; Nagoshi, R.N.; Meagher, R.L.; Owens, C.B.; Luttrellm, R.G. 2006. Genetic variation within and between strains of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Florida Entomologist 89: 63–68.; Nagoshi et al., 2007Nagoshi, R.N.; Adamczyk, J.J.; Meagher, J.; Gore, R.L.; Jackson, R. 2007. Using stable isotope analysis to examine fall armyworm (Lepidoptera: Noctuidae) host strains in a cotton habitat. Journal of Economic Entomology 100: 1569–1576.). This might be due to the composition and nutritional adequacy of these plants in relation to hosts from other botanical families (Pashley, 1986Pashley, D.P. 1986. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Annals of the Entomological Society of America 79: 898–904., 1988Pashley, D.P. 1988. Current status of fall armyworm host strains. Florida Entomologist 71: 227–234.; Barros et al., 2010Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.) with the consequence that, ultimately, insect foraging is an exercise in acquiring the best blend and balance of suite nutrients, including amino acids, carbohydrates, sterols, phospholipids, fatty acids, vitamins, minerals, trace elements, and water (Behmer, 2009Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.). In this regard, soybean leaves presented starch (carbohydrate) varying from 40 to over 120 mg dm−2; soluble carbohydrate from 30 to 50 mg dm−2, protein from 70 to 110 mg dm−2, and inorganic phosphate from 20 to 60 50 mg dm−2 (Mondal et al., 1978Mondal, M.H.; Brun, W.A.; Brenner, M.L. 1978. Effects of sink removal on photosynthesis and senescence in leaves of soybean (Glycine max L.) plants. Plant Physiology 61: 394–397.). Hall (1951)Hall, V.L. 1951. Biochemical composition of cotton leaves and their chemical defoliation as affected by environment. Plant Physiology 26: 677–686. reported the biochemical composition of cotton leaves for carbohydrate and nitrogen contents (percentage of dry weight). Carbohydrates varied from 0.56 to 2.42, 0.11 to 0.79, 1.51 to 12.80, and 3.71 to 5.27 for reducing sugar, non-reducing sugar, starch and dextrin, and hemi-cellulose carbohydrates, respectively. Total nitrogen varied from 2.27 to 3.47 (percentage of dry weight). Chen et al. (2009)Chen, Y.; Ni, X.; Buntin, G.D. 2009. Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. Journal of Chemical Ecology 35: 297–306., evaluating leaves of different maize germplasm lines, reported leaf chlorophyll (µmol m−2) from 522.56 to 610.37; total protein (mg g−1 of fresh weight of leaf tissue) from 5.74 to 11.12; amino acids (µmol g−1 of fresh weight of leaf tissue) from 6.43 to 11.0; glucose (µmol g−1 of fresh weight of leaf tissue) from 6.39 to 17.26; total nonstructural carbohydrate as the sum of glucose and starch (equivalents of glucose) (µmol g−1 of fresh weight of leaf tissue) from 24.25 to 27.94 and the relationship of protein/carbohydrate (w/w) from 1.27 to 2.69. However, nutrient availability to insects is frequently variable. Not only does this variation exist between different plant species but also within a species as a result of genotypic differences and environmental conditions (soil nutrient, light, and water level), as well as within a plant (young versus old leaves) (Behmer, 2009Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.). For example, Myzus persicae (Sulzer) and Macrosiphum euphorbiae (Thomas) (Hemiptera: Aphididae) on potato, Solanum tuberosum L., produced more offspring on younger potato leaves than on older leaves (Karley et al., 2002Karley, A.J.; Douglas, A.E.; Parker, W.E. 2002. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. Journal of Experimental Biology 205: 3009–3018.). Also, soybean leaf physiology, nutrient composition, and requirements change over time. Newly developing leaves depend on the phloem for nutrient intake and do not become exporters of photosynthate until the leaf is one-third to one-half expanded (Pate, 1980Pate, J.S. 1980. Transport and partitioning of nitrogenous solutes. Annual Review of Plant Physiology 31: 313–340.). Therefore, it seems unlikely that S. frugiperda would actively regulate the intake of all its required nutrients, because doing so would demand massive neurological effort and likely come at a great cost (Bernays, 2001Bernays, E.A. 2001. Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annual Review of Entomology 46: 703–727.). Thus, the key question would be which nutrients should this species regulate? Good candidates would be those that cannot be biosynthesized from scratch such as sterols, vitamins, many amino acids and also carbohydrates which, even though they can be biosynthesized, are often limiting (Behmer, 2009Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.).
Missing from this picture was the role that plant secondary metabolites, acting as allelochemicals, played in influencing nutrient regulation in insect herbivores adding even more complexity to this scenario (Behmer, 2009Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.). Several researchers have reported the deterrent, repellent, and stimulant properties of those chemicals present in plants (Berenbaum and Neal, 1985Berenbaum, M.; Neal, J.J. 1985. Synergism between myrysticin and xanthotoxin, a naturally co-occurring plant toxicant. Journal of Chemical Ecology 11: 1349–1358.; Ishaaya, 1986Ishaaya, I. 1986. Nutritional and allelochemic insect plant interactions relating to digestion and food intake: some examples. p. 191–223. In: Miller, J.R.; Miller, T.A. Insect-plant interactions. Springer, New York, NY, USA.; Norris and Kogan, 2005Norris, R.F.; Kogan, M. 2005. Ecology of interactions between weeds and arthropods. Annual Review of Entomology 50: 479–503.). Since the insect-plant coevolution model was proposed by Ehrlich and Raven (1964)Ehrlich, P.R.; Raven, P.H. 1964. Butterflies and plants: a study in coevolution. Evolution 18: 586–608., much emphasis has been placed on the toxicity of host-plant allelochemicals to explain this insect-host adaptation. Therefore, biochemical substances produced by plants under pest attack (Hoffmann Campo et al., 2001Hoffmann-Campo, C.B.; Harbone, J.B.; McAffery, A.R. 2001. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomologia Experimentalis et Applicata 98: 181–194.; Piubelli et al., 2005Piubelli, G.C.; Hoffmann-Campo, C.B.; Moscardi, F. ; Miyakubo, S.H.; Oliveira, M.C.N. 2005. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? Journal of Chemical Ecology 31: 1509–1525.) influence the growth, survival, and reproduction of insects (Fischer et al., 1990Fischer, D.C.; Kogan, M.; Paxton, J. 1990. Effect of glyceollin, a soybean phytoalexin, on feeding by three phytophagous beetles (Coleoptera: Coccinellidae and Chrysomelidae): dose versus response. Environmental Entomology 5: 1278–1282.) and would be the better way to understand insect-host performance (Veenstra et al., 1995Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.). A better understanding of the diversity of insect responses to these allelochemicals in their local ecological context represents a key challenge in developing durable pest control management (Després et al., 2007Després, L.; David, J.P.; Galett, C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology and Evolution 22: 298–307.). Consequently, host-plant adaptation could be viewed in terms of physiological, biochemical, and evolutional adaptation to host-plant allelochemicals (Caprio and Tabashnik, 1992Caprio, M.A.; Tabashnik, B.E. 1992. Evolution of resistance to plant defensive chemicals in insect. p. 179–215. In: Roitberg, B.D.; Isman, M.B., eds. Insect chemical ecology: an evolutionary approach. Chapman & Hall, New York, NY, USA.; Ahmad et al., 1986Ahmad, S.; Brattsten, L.B.; Mullin, C.A.; Yu, S.J. 1986. Enzymes involved in the metabolism of plant allelochemicals. p. 73–151. In: Brattsten, L.B.; Ahmad, S., eds. Molecular aspects of insect-plant associations. Plenum, New York, NY, USA.; Slansky, 1992Slansky, F. 1992. Allelochemical-nutrient interactions in herbivore nutritional ecology. p. 135–174. In: Rosenthal, G.A.; Berenbaum, M.R., eds. Herbivores: their interaction with secondary plant metabolites. Academic Press, San Diego, CA, USA.; Sorensen and Dearing, 2006Sorensen, J.S.; Dearing, M.D. 2006. Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. Journal of Chemical Ecology 32: 1181–1196.). The adaption mechanisms might include contact and ingestion avoidance of the plant containing the allelochemicals; excretion of the toxic food, sequestration or degradation of the toxins and target site mutation (Gould, 1984Gould, F. 1984. Mixed function oxidases and herbivore polyphagy: the devil`s advocate position. Ecological Entomology 9: 29–34.; Raffa, 1987Raffa, K.F. 1987. Maintenance of innate feeding preferences by a polyphagous insect despite ingestion of applied deleterious chemicals. Entomologia Experimentalis et Applicata 44: 221–227.; Sorensen and Dearing, 2006Sorensen, J.S.; Dearing, M.D. 2006. Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. Journal of Chemical Ecology 32: 1181–1196.; Wouters et al., 2014Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.). These adaptations to plant defense traits differ between pest species. Differential activity of multiple saponins (an additional group of compounds active against insects) was reported by Dowd et al. (2011)Dowd, P.F.; Berhow, M.A.; Johnson E.T. 2011. Differential activity of multiple saponins against omnivorous insects with varying feeding preferences. Journal of Chemical Ecology 37: 443–449. for Spodoptera spp. These authors noted activity of sapogenol B against S. frugiperda. This compound was inactive compared to some other alfafa saponins to S. littoralis (Adel et al., 2000Adel, M.M.; Sehnal, F.D.; Jurzysta, M. 2000. Effects of alfalfa saponins on the moth Spodoptera littoralis. Journal of Chemical Ecology 26: 1065–1078.).
In this context, benzoxazinoids are chemical defenses against herbivores which are produced by many members of the grass family including wheat and maize that were studied in our trials (Wouters et al., 2014Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.). These compounds are stored as stable glucosides in plant cells and require the activity of glucosidases to release the corresponding toxic aglucones (Wouters et al., 2014Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.). Among these compounds, DIMBOA and MBOA are considered to be the most important allelochemicals in the grass family (Veenstra et al., 1995Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.) which is reported to affect less S. frugiperda when compared to Spodoptera littoralis and Spodoptera exigua (Wouters et al., 2014Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.). It is due to the detoxification reported in S. frugiperda provided by the stereoselective reglucosylation of the ingested DIMBOA from the plant tissues of maize or wheat, for example (Wouters et al., 2014Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.). Glucosylation is an important detoxification pathway that stabilizes toxins and favors their excretion (Rojas et al., 1992Rojas, M.G.; Stipanovic, R.D.; Williams, H.J.; Vinson, S.B. 1992. Metabolism of gossypol by Heliothis virescens (F.) (Lepidoptera: Noctuidae). Environmental Entomology 21: 518–526.). This detoxification process reported for S. frugiperda might help to explain the results of our trials. Higher excretion was observed for treatments using hosts from the grass family (oat, wheat, and maize) as well as the artificial diet which may be a consequence of the detoxification process since these plants were also consumed more intensely (Figure 4 and 5). Consequently this results in higher consumption of toxic plant chemicals. Nevertheless, these treatments also resulted in heavier insects (Figure 6).
It is important to take into consideration that feeding preference rankings can be relatively rigid in a polyphagous species such as S. frugiperda favoring the evolution of such physiological detoxification mechanisms (Raffa, 1987Raffa, K.F. 1987. Maintenance of innate feeding preferences by a polyphagous insect despite ingestion of applied deleterious chemicals. Entomologia Experimentalis et Applicata 44: 221–227.). Therefore, the results presented herein indicated that S. frugiperda would be better physiologically or biochemically adapted to DIMBOA and MBOA than to the chemicals produced by soybean (phytoalexin glyceollin) and cotton (gossypol and some terpenoids). It is important to point out that this probably occurs when specialist insects perform better than generalist species (Veenstra et al., 1995Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.). Although S. frugiperda clearly prefers grasses (C4 plants), it is more of a generalist pest, being reported on more than 80 species in 23 families (Hardke et al., 2015Hardke, J.T.; Lorenz III, G.M.; Leonard, B.R. 2015. Fall Armyworm (Lepidoptera: Noctuidae) ecology in Southeastern Cotton. Journal of Integrated Pest Management 6: 10.).
Soybean has inducible phytoalexin glyceollin, an isoflavonoid produced in plants after pest attack or other sources of stress which play a role in insect resistance. This substance is toxic to herbivorous insects, acting as an effective antifeedant for certain pests protecting the plant by creating very toxic environments at the cellular level (Fischer et al., 1990Fischer, D.C.; Kogan, M.; Paxton, J. 1990. Effect of glyceollin, a soybean phytoalexin, on feeding by three phytophagous beetles (Coleoptera: Coccinellidae and Chrysomelidae): dose versus response. Environmental Entomology 5: 1278–1282.). Similarly, unique compounds present in cotton plants, such as high levels of gossypol found in the leaves, and certain terpenoids can affect the nutritional uptake of this food source and thereby also cause a reduction in pupal weight (Smith, 2005Smith, C.M. 2005. Antibiosis: adverse effects of resistance on arthropod biology. p. 65–99. In: Smith, C.M., ed. Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecht, Holland.; Stipanovic et al., 2006Stipanovic, R.D.; Lopez, J.D.; Dowd, M.K.; Puckhaber, L.S.; Duke, S.E. 2006. Effect of racemic and (+) and (-) gossypol on the survival and development of Helicoverpa zea larvae. Journal of Chemical Ecology 32: 959–968.). This soybean and cotton allelochemical effects were also observed in our results. Cotton triggered S. frugiperda pupae with lower weight (Table 2). In addition, antifeedant effects of soybean allelochemicals help to explain the lower amount of food consumed by S. frugiperda on this host (Figure 4). Thus, S. frugiperda, would likely show preferences for plants on which they have the best detoxification adaptation (Thompson, 1988Thompson, J.N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomologia Experimentalis et Applicata 47: 3–14.; Via, 1990Via, S. 1990. Ecological genetics and host adaptation in herbivorous insects: the experimental study of evolution in natural and agricultural systems. Annual Review of Entomology 35: 421–446.) which might be the stereoselective reglucosylation of the ingested DIMBOA from the plant tissues of maize, oat, and wheat, for example.
It is unlikely though, that glucosylation alone can completely explain S. frugiperda feeding behavior. According to the geometrical framework proposed by Raubenheimer and Simpson (1993)Raubenheimer, D.; Simpson, S.J. 1993. The geometry of compensatory feeding in the locust. Animal Behaviour 45: 953–964. insects grow best when they ingest equal amounts of protein and carbohydrate and reach the optimal mixture of these nutrients. The relation of protein/carbohydrate (P/C) (w/w) of maize leaves might vary from 1.27 to 2.69 while these values can vary from 0.41 to 1.57 in soybean leaves. Soybean leaves have P/C values closer to the artificial diet used in our trials (Greene et al., 1976Greene, G.L.; Leppla, N.C.; Dickerson, W.A. 1976. Velvetbean caterpillar: a rearing procedure and artificial diet. Journal of Economic Entomology 69: 487–488.) when compared to maize leaves. Nevertheless, maize and the other grasses showed they were more appropriate hosts than soybean and cotton leaves. It indicates that other factors besides leaf nutrients may be regulating S. frugiperda intake. It is important to take into consideration that the insect can reach its intake target by regulating the amount of an individual plant part that is eaten, feeding from a range of different plants or, more likely, though a combination of these two mechanisms (Simpson and Raubenheimer, 2000Simpson, S.J.; Raubenheimer, D. 2000. The hungry locust. Advances in the Study of Behavior 29: 1–44.). In this context, S. frugiperda larvae used in our trial were confined to a single food source, which might not occur in nature. Also, on a more general note, protein-carbohydrate regulation can sometimes break down in species (Behmer, 2009Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.). For example, Spodoptera littoralis (Boisduval, 1833) (Lepidoptera: Noctuidae) and Manduca sexta (Linnaeus, 1763) (Lepidoptera: Sphingidae) abandon protein-carbohydrate regulation in response to extreme macronutrient dilution (Lee et al., 2003Lee, K.P.; Raubenheimer, D.; Behmer, S.T.; Simpson, S.J. 2003. A correlation between macronutrient balancing and insect hostplant range: evidence from the specialist caterpillar Spodoptera exempta (Walker). Journal of Insect Physiology 49: 1161–1171.; Thompson and Redak, 2005Thompson, S.N.; Redak, R.A. 2005. Feeding behaviour and nutrient selection in an insect Manduca sexta L. and alterations induced by parasitism. Journal of Comparative Physiology A 191: 909–923.) or to changes in the protein quality of one of their available foods (Lee, 2007Lee, K.P. 2007. The interactive effects of protein quality and macronutrient imbalance on nutrient balancing in an insect herbivore. Journal of Experimental Biology 219: 3236–3244.).
In this discussion, it is certain that the amount and quality of available food exert a direct influence on host preference and affect the rate of growth, development time, body weight, and survival of lepidopterans, including S. frugiperda (Nation, 2002Nation, J.L. 2002. Insect Physiology and Biochemistry. Boca Raton: CRC Press, FL, USA.; Golizadeh et al., 2009Golizadeh, A.; Kamali, K.; Fathipour, Y.; Abbasipour, H. 2009. Life table of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) on five cultivated brassicaceous host plants. Journal of Agricultural Science and Technology 11: 115–124.). Therefore, in order to analyze the factors that affect the development of insects, it is necessary to know the parameters that help establish nutritional comparisons between different hosts (Scriber and Slansky, 1981Scriber, J.M.; Slansky, J.R.F. 1981. The nutritional ecology of immature insects. Annual Review of Entomology 26: 183–211.). A short period of feeding, high larval and pupal weights and low mortality rates in insects results in a suitable growth rate (Soo Hoo and Fraenkel, 1966Soo Hoo, C.F.; Fraenkel, G. 1966. The selection of food plants in a polyphagous insect, Prodenia eridania (Cramer). Journal of Insect Physiology 12: 693–709.) which was observed in our trials with maize, oat and wheat as hosts. Thus, the results of the nutritional experiments on S. frugiperda demonstrated that, in general, growth, weight gain, and efficiency in the conversion of ingested food were influenced by the different host plants. The insects were fed on soybean and cotton leaves and even used compensatory strategies such as extension of the feeding period and increase in food intake; however, they did not achieve the weight of the insects fed on the other host plants evaluated. This ratifies the importance of the presence of a number of deterrent allelochemicals that are impacting insect development. However, herbivorous insects possess a number of these detoxification enzymes, such as mixed-function oxidase (MFO) and general esterases, in their midguts that metabolize plant allelochemicals (Veenstra et al., 1995Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.). Thus, a possible explanation is that the allelochemicals of grasses (DIMBOA and MBOA) induce more efficient MFO activity in S. frugiperda than the allelochemicals of soybean (phytoalexin glyceollin) and cotton (gossypol and some terpenoids).
It is also important to consider alternative explanations to understand the best adaptation of S. frugiperda for grasses which include the importance of ecological factors in determining host use (Veenstra et al., 1995Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.). In this way, S. frugiperda would likely to evolve a preference for host plants on which larvae experience the low rates of predation (Bernays and Graham, 1988Bernays, E.A.; Graham, M. 1988. On the evolution of host specificity in phytophagous arthropods. Ecology 69: 886–892.) or for hosts that are abundant locally (Fox and Morrow, 1981Fox, I.R.; Morrow, P.A. 1981. Specialization: species property or local phenomenon. Science 211: 887–893.). Grasses, and more specifically maize, certainly offer S. frugiperda larvae better shelter than soybean or cotton. As larvae hatch from the eggs they find shelter on maize whorl, a site that makes it more difficult for predation and chemical control to succeed (Sparks, 1979Sparks, A.N. 1979. A review of the biology of the fall armyworm. The Florida Entomologist 62: 83–87.; Prowell et al., 2004Prowell, D.P.; McMichael, M.; Silvain, J.F. 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America 97: 1034–1044.). Despite all these possible different explanations, it seems that none of them alone can explain S. frugiperda intake regulation. On the one hand, as previously mentioned, protein-carbohydrate regulation in nutrient intake, even though important, cannot explain S. frugiperda preferences for grasses. On the other hand, Raffa (1987)Raffa, K.F. 1987. Maintenance of innate feeding preferences by a polyphagous insect despite ingestion of applied deleterious chemicals. Entomologia Experimentalis et Applicata 44: 221–227. noted the maintenance of S. frugiperda feeding preferences for grasses despite the ingestion of deleterious chemicals applied on those plants. It is a clear indication that all possible explanations herein discussed can be impacting S. frugiperda feeding in a more complex food intake regulation. From ecological and behavioral perspectives, there are two explanations for differential host specificity observed in our trials. Either the S. frugiperda females exhibits differences in ovipositional specificity or differential immature survival occurs after random egg laying. It is important to consider that the S. frugiperda oviposited more on wheat in a free-choice oviposition test (Figure 1). However, moths oviposited on all hosts in the no-choice test (Figure 2). This indicates that the moths oviposit on available substrates in the absence of the preferred host, thus ensuring survival of the species. Egg masses oviposited on the screen of the cages during the evaluation period were also found but not counted. This behavior had previously been reported by Luginbill (1928)Luginbill, P. 1928. The Fall Armyworm. United States Departament of Agriculture, Washington, DC, USA. (Technical Bulletin, 34). and later by Sparks (1979)Sparks, A.N. 1979. A review of the biology of the fall armyworm. The Florida Entomologist 62: 83–87., who noted that large populations of S. frugiperda can lay their eggs in non-host plants and objects. This non-specific oviposition behavior of S. frugiperda had also been reported by Rojas et al. (2003)Rojas, J.C.; Virgen, A.; Cruz-Lopez, L. 2003. Chemical and tactile cues influencing oviposition of a generalist moth, Spodoptera frugiperda (Lepidoptera: Noctuidae). Environmental Entomology 32: 1386–1392., who found several moths laying their eggs on striated surfaces, indicating that short distances (a physical factor) have greater influence than plant volatiles on oviposition location choice.
Prowell et al. (2004)Prowell, D.P.; McMichael, M.; Silvain, J.F. 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America 97: 1034–1044. offered an alternative explanation: if larval survival differences in each habitat maintain differential host use, differences are not likely to be due to nutritional factors. Nutritional studies herein described indicated that S. frugiperda develops at least as well on soybean as on wheat, maize or oat. Larvae had similar survival on tested hosts (Table 2), similar weight when fed on soybean and maize (Figures 3 and 6), and also similar pupal weight when fed on soybean and wheat, indicating that S. frugiperda host specificity to grasses might also be due to ecological or behavioral characteristics of the insect species.
For lepidopteran species, larval preference is commonly associated with adult choice for oviposition (Singer et al., 1994Singer, M.C.; Thomas, C.D.; Billington, H.L.; Parmesan, C. 1994. Correlates of speed of evolution of host preference in a set of twelve populations of the butterfly Euphydryas editha. Ecoscience. 1: 107–114.; Leal and Zucoloto, 2008Leal, T.B.S.; Zucoloto, F.S. 2008. Selection of artificial hosts for oviposition by wild Anastrepha obliqua (Macquart) (Diptera: Tephritidae): influence of adult food and effect of experience. Revista Brasileira de Entomologia 52: 467–471.). Because of this behavior, many studies have investigated the relationship between the host preference of adult females and the growth of their offspring (Damman and Feeney, 1988Damman, H.; Feeney, P. 1988. Mechanisms and consequences of selective oviposition by the zebra swallowtail butterfly. Animal Behavior 36: 563–573.; Nylin and Janz, 1993Nylin, S.; Janz, N. 1993. Oviposition preference and larval performance in Polygonia c-album (Lepidoptera: Nymphalidae): the choice between bad and worse. Ecological Entomology 18: 394–398.; Singer et al., 1994Singer, M.C.; Thomas, C.D.; Billington, H.L.; Parmesan, C. 1994. Correlates of speed of evolution of host preference in a set of twelve populations of the butterfly Euphydryas editha. Ecoscience. 1: 107–114.), known as the “mother-knows-best hypothesis” (Gripenberg et al., 2010Gripenberg, S.; Mayhew, P.J.; Parnell, M.K.; Roslim, T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecology Letters 13: 383–393.). With regard to the feeding preference experiment, more larvae were found on crop plants 24 h after larval release than at 60 min after release. This difference was more notable in the trial using larvae from the first instar. It may be explained by the need of the larvae soon after hatching to establish a feeding site for survival (Zalucki et al., 2002Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. 2002. Ecology and behavior of first ínstar larval Lepidoptera. Annual Review of Entomology 47: 361–93.). A larger number of first instar larvae were found on wheat leaves 24 h after their release. Similarly, in the free-choice test, the preferred host was wheat. This shows that the “mother-knows-best hypothesis” (Gripenberg et al., 2010Gripenberg, S.; Mayhew, P.J.; Parnell, M.K.; Roslim, T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecology Letters 13: 383–393.) could be associated with S. frugiperda because of its preference for wheat, as demonstrated during its larval development in this study and previous studies (Ali and Luttrell, 1990Ali, A.; Luttrell, R.G. 1990. Survival of fall armyworm (Lepidoptera: Noctuide) immatures on cotton. Florida Entomologist 73: 459–465.). This can probably be a possible explanation for the positive correlation observed in the current study between the host preference of adult females and performance of their offspring mainly when observing oviposition in the free-choice test and feeding preference of the larvae (first instar, 24 h after the start of the test) which had wheat as the most favorable host. However, although a positive correlation is expected, it does not always occur, which is explained by Thompson (1988)Thompson, J.N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomologia Experimentalis et Applicata 47: 3–14. as a result of changes in ecological conditions and site selection pressure where these insects are introduced which highlights the importance of research projects such as the current study.
Differences in oviposition preference observed in the free-choice and no-choice tests might be due to flexibility of the host range presented by S. frugiperda which increases long term evolutionary survival of this species. Moths when they have no-choice for oviposition laid eggs in plant parts that had no eggs in the free-choice test as was observed on the top of oat plants thus ratifying the great host flexibility of the species. The absence of eggs on the top of oat plants in the free-choice test might be related to moth's fixation on the top of the tiny plants on windy days but it clearly needs further study to better understand this phenomena.
Despite the host suitability of grasses for S. frugiperda development, as previously mentioned, high survival rate (%) of larvae was recorded for soybean and cotton, as well as grasses. Thus, S. frugiperda also has the potential to survive and multiply in soybean and cotton, and therefore population damage can also occur in these economically important plants (Pitre and Hogg, 1983Pitre, H.N.; Hogg, D.B. 1983. Development of the fall armyworm on cotton, soybean and corn. Journal of the Georgia Entomological Society 18: 182–187.; Bueno et al., 2010Bueno, R.C.O.F.; Carneiro, T.R.; Bueno, A.F.; Pratissoli, D.; Fernandes, O.A.; Vieira, S.S. 2010. Parasitism capacity of Telenomus remus Nixon (Hymenoptera: Scelionidae) on Spodoptera frugiperda (Smith) (Lepidoptera:Noctuidae) eggs. Brazilian Archives of Biology and Technology 53: 133–139.). Moreover, this survival can help increase the population of S. frugiperda in subsequent grass crops grown after soybean or cotton. As a consequence, S. frugiperda long-term evolutionary survival also increases with its flexible host range. Moreover, S. frugiperda used in our trials was originally collected in maize, corresponding to the maize strain. Other S. frugiperda strains might lead to different results which still need to be studied in future research projects.
Migration of S. frugiperda among crops can have a serious impact on the management of this pest, especially considering that insecticides and mainly Bt plants can be used simultaneously on adjacent crops in the same landscape. Comparable selection pressure can occur across crops increasing overall selection pressure for resistance (Roush, 1989Roush, R.T. 1989. Designing resistance management programs: how can you choose? Pesticide Science 26: 423–441.; Hernández-Martínez et al., 2009Hernández-Martínez, P.; Ferré, J.; Escriche, B. 2009. Broad-spectrum cross-resistance in Spodoptera exigua from selection with a marginally toxic Cry protein. Pest Management Science 65: 645–650.). Furthermore, the coexistence of different crops in the agro-ecosystem, especially soybean and maize, can trigger new feeding preferences in the absence of the main host (Andrews, 1980Andrews, K.L. 1980. The whorlworm, Spodoptera frugiperda, in Central America and neighboring areas. Florida Entomologist 63: 456–467.) despite the fact that S. frugiperda food intake regulation appears to be triggered by a complex of different mechanisms including plant allelochemicals and the existence of its corresponding insect adaptation, and nutrient regulation. Even the alternative explanation of sheltering and predation impact cannot be excluded.
Acknowledgments
The authors would like to thank Embrapa Soybean, the Coordination for the Improvement of Higher Level Personnel (CAPES), and the Brazilian National Council for Scientific and Technological Development (CNPq), grant number 301420/2012-2, for funds that supported this research. Thanks are also extended to the Editage Editor for the revision in English of this manuscript. This paper was approved for publication by the Editorial Board of Embrapa Soybean.
References
- Adel, M.M.; Sehnal, F.D.; Jurzysta, M. 2000. Effects of alfalfa saponins on the moth Spodoptera littoralis Journal of Chemical Ecology 26: 1065–1078.
- Ahmad, S.; Brattsten, L.B.; Mullin, C.A.; Yu, S.J. 1986. Enzymes involved in the metabolism of plant allelochemicals. p. 73–151. In: Brattsten, L.B.; Ahmad, S., eds. Molecular aspects of insect-plant associations. Plenum, New York, NY, USA.
- Ali, A.; Luttrell, R.G. 1990. Survival of fall armyworm (Lepidoptera: Noctuide) immatures on cotton. Florida Entomologist 73: 459–465.
- Andrews, K.L. 1980. The whorlworm, Spodoptera frugiperda, in Central America and neighboring areas. Florida Entomologist 63: 456–467.
- Ball, O.J.P.; Coudron, T.A.; Tappe, B.A.; Davies, E.; Trently, D.; Bush, L.P.; Gwinn, K.D.; Popay, A.J. 2006. Importance of host plant species, neotyphodium, endophyte isolate, and alkaloids on feeding by Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Journal of Economic Entomology 99: 1462–1473.
- Barros, E.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.
- Behmer, S.T. 2009. Insect herbivore nutrient regulation. Annual Review of Entomology 54: 165–187.
- Berenbaum, M.; Neal, J.J. 1985. Synergism between myrysticin and xanthotoxin, a naturally co-occurring plant toxicant. Journal of Chemical Ecology 11: 1349–1358.
- Bernays, E.A. 2001. Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annual Review of Entomology 46: 703–727.
- Bernays, E.A.; Graham, M. 1988. On the evolution of host specificity in phytophagous arthropods. Ecology 69: 886–892.
- Botton, M.; Carbonari, J.J.; Garcia, M.S.; Martins, J.F.S. 1998. Feeding preference and biology of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on rice and barnyardgrass. Anais da Sociedade Entomológica do Brasil 27: 207–212 (in Portuguese, with abstract in English).
- Bueno, R.C.O.F.; Bueno, A.F.; Moscardi, F.; Parra, J.R.; Hoffmann-Campo, C.B. 2011. Lepidopteran larvae consumption of soybean foliage: basis for developing multiple-species economic thresholds for pest management decisions. Pest Management Science 67: 170–174.
- Bueno, R.C.O.F.; Carneiro, T.R.; Bueno, A.F.; Pratissoli, D.; Fernandes, O.A.; Vieira, S.S. 2010. Parasitism capacity of Telenomus remus Nixon (Hymenoptera: Scelionidae) on Spodoptera frugiperda (Smith) (Lepidoptera:Noctuidae) eggs. Brazilian Archives of Biology and Technology 53: 133–139.
- Buntin, G.D. 1986. A review of plant response to fall armyworm, Spodoptera frugiperda (J.E. Smith), injury in selected field and forage crops. Florida Entomologist 69: 549–559.
- Burr, I.W.; Foster, L.A. 1972. A test for equality of variances. University of Purdue, West Lafayette, IN, USA.
- Caprio, M.A.; Tabashnik, B.E. 1992. Evolution of resistance to plant defensive chemicals in insect. p. 179–215. In: Roitberg, B.D.; Isman, M.B., eds. Insect chemical ecology: an evolutionary approach. Chapman & Hall, New York, NY, USA.
- Chen, Y.; Ni, X.; Buntin, G.D. 2009. Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. Journal of Chemical Ecology 35: 297–306.
- Clark, P.L.; Molina-Ochoa, J.; Martinelli, S.; Skoda, S.R.; Isenhour, D.J.; Lee, D.J.; Krumn, J.T.; Foster, J.E. 2007. Population variation of Spodoptera frugiperda (J. E. Smith) in the Western Hemisphere. Journal of Insect Science 7: 1–10.
- Cruz, I.; Figueiredo, M.L.C.; Oliveira, A.C.; Vasconcelos, C.A. 1999. Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminium saturation. International Journal of Pest Management 45: 293–296.
- Damman, H.; Feeney, P. 1988. Mechanisms and consequences of selective oviposition by the zebra swallowtail butterfly. Animal Behavior 36: 563–573.
- Després, L.; David, J.P.; Galett, C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology and Evolution 22: 298–307.
- Dowd, P.F.; Berhow, M.A.; Johnson E.T. 2011. Differential activity of multiple saponins against omnivorous insects with varying feeding preferences. Journal of Chemical Ecology 37: 443–449.
- Ehrlich, P.R.; Raven, P.H. 1964. Butterflies and plants: a study in coevolution. Evolution 18: 586–608.
- Fischer, D.C.; Kogan, M.; Paxton, J. 1990. Effect of glyceollin, a soybean phytoalexin, on feeding by three phytophagous beetles (Coleoptera: Coccinellidae and Chrysomelidae): dose versus response. Environmental Entomology 5: 1278–1282.
- Fox, I.R.; Morrow, P.A. 1981. Specialization: species property or local phenomenon. Science 211: 887–893.
- Golizadeh, A.; Kamali, K.; Fathipour, Y.; Abbasipour, H. 2009. Life table of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) on five cultivated brassicaceous host plants. Journal of Agricultural Science and Technology 11: 115–124.
- Gould, F. 1984. Mixed function oxidases and herbivore polyphagy: the devil`s advocate position. Ecological Entomology 9: 29–34.
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. 1976. Velvetbean caterpillar: a rearing procedure and artificial diet. Journal of Economic Entomology 69: 487–488.
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.K.; Roslim, T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecology Letters 13: 383–393.
- Hall, V.L. 1951. Biochemical composition of cotton leaves and their chemical defoliation as affected by environment. Plant Physiology 26: 677–686.
- Hardke, J.T.; Lorenz III, G.M.; Leonard, B.R. 2015. Fall Armyworm (Lepidoptera: Noctuidae) ecology in Southeastern Cotton. Journal of Integrated Pest Management 6: 10.
- Hernández-Martínez, P.; Ferré, J.; Escriche, B. 2009. Broad-spectrum cross-resistance in Spodoptera exigua from selection with a marginally toxic Cry protein. Pest Management Science 65: 645–650.
- Hoffmann-Campo, C.B.; Harbone, J.B.; McAffery, A.R. 2001. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomologia Experimentalis et Applicata 98: 181–194.
- Ishaaya, I. 1986. Nutritional and allelochemic insect plant interactions relating to digestion and food intake: some examples. p. 191–223. In: Miller, J.R.; Miller, T.A. Insect-plant interactions. Springer, New York, NY, USA.
- Karley, A.J.; Douglas, A.E.; Parker, W.E. 2002. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. Journal of Experimental Biology 205: 3009–3018.
- Leal, T.B.S.; Zucoloto, F.S. 2008. Selection of artificial hosts for oviposition by wild Anastrepha obliqua (Macquart) (Diptera: Tephritidae): influence of adult food and effect of experience. Revista Brasileira de Entomologia 52: 467–471.
- Lee, K.P. 2007. The interactive effects of protein quality and macronutrient imbalance on nutrient balancing in an insect herbivore. Journal of Experimental Biology 219: 3236–3244.
- Lee, K.P.; Raubenheimer, D.; Behmer, S.T.; Simpson, S.J. 2003. A correlation between macronutrient balancing and insect hostplant range: evidence from the specialist caterpillar Spodoptera exempta (Walker). Journal of Insect Physiology 49: 1161–1171.
- Lewter, J.A.; Szalanski, A.L.; Nagoshi, R.N.; Meagher, R.L.; Owens, C.B.; Luttrellm, R.G. 2006. Genetic variation within and between strains of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Florida Entomologist 89: 63–68.
- Luginbill, P. 1928. The Fall Armyworm. United States Departament of Agriculture, Washington, DC, USA. (Technical Bulletin, 34).
- Mondal, M.H.; Brun, W.A.; Brenner, M.L. 1978. Effects of sink removal on photosynthesis and senescence in leaves of soybean (Glycine max L.) plants. Plant Physiology 61: 394–397.
- Nabity, P.D.; Zangerl, A.R.; Berenbaum, M.R.; Delucia, E.H. 2011. Bioenergy crops Miscanthus × giganteus and Panicum virgatum reduce growth and survivorship of Spodoptera frugiperda (Lepidoptera: Noctuidae). Journal of Economic Entomology 104: 459–464.
- Nagoshi, R.N. 2009. Can the amount of corn acreage predict fall armyworm (Lepidoptera: Noctuidae) infestation levels in nearby cotton? Journal of Economic Entomology 102: 210–218.
- Nagoshi, R.N.; Meagher, R.L. 2004. Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turf grass habitats. Environmental Entomology 33: 881–889.
- Nagoshi, R.N.; Adamczyk, J.J.; Meagher, J.; Gore, R.L.; Jackson, R. 2007. Using stable isotope analysis to examine fall armyworm (Lepidoptera: Noctuidae) host strains in a cotton habitat. Journal of Economic Entomology 100: 1569–1576.
- Nation, J.L. 2002. Insect Physiology and Biochemistry. Boca Raton: CRC Press, FL, USA.
- Norris, R.F.; Kogan, M. 2005. Ecology of interactions between weeds and arthropods. Annual Review of Entomology 50: 479–503.
- Nylin, S.; Janz, N. 1993. Oviposition preference and larval performance in Polygonia c-album (Lepidoptera: Nymphalidae): the choice between bad and worse. Ecological Entomology 18: 394–398.
- Pashley, D.P. 1986. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Annals of the Entomological Society of America 79: 898–904.
- Pashley, D.P. 1988. Current status of fall armyworm host strains. Florida Entomologist 71: 227–234.
- Pate, J.S. 1980. Transport and partitioning of nitrogenous solutes. Annual Review of Plant Physiology 31: 313–340.
- Pitre, H.N.; Hogg, D.B. 1983. Development of the fall armyworm on cotton, soybean and corn. Journal of the Georgia Entomological Society 18: 182–187.
- Piubelli, G.C.; Hoffmann-Campo, C.B.; Moscardi, F. ; Miyakubo, S.H.; Oliveira, M.C.N. 2005. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? Journal of Chemical Ecology 31: 1509–1525.
- Pogue, G.M. 2002. A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Memoirs of the American Entomological Society 43: 1–202.
- Prowell, D.P.; McMichael, M.; Silvain, J.F. 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America 97: 1034–1044.
- Quisenberry, S.S. 1991. Fall armyworm (Lepidoptera: Noctuidae) host strain reproductive compatibility. Florida Entomologist 74: 194–199.
- Raffa, K.F. 1987. Maintenance of innate feeding preferences by a polyphagous insect despite ingestion of applied deleterious chemicals. Entomologia Experimentalis et Applicata 44: 221–227.
- Raubenheimer, D.; Simpson, S.J. 1992. Analysis of covariance: an alternative to nutritional indices. Entomologia Experimentalis et Applicata 62: 221–231.
- Raubenheimer, D.; Simpson, S.J. 1993. The geometry of compensatory feeding in the locust. Animal Behaviour 45: 953–964.
- Raubenheimer, D.; Simpson, S.J. 1994. The analysis of nutrient budgets. Functional Ecology 8: 783–791.
- Rojas, J.C.; Virgen, A.; Cruz-Lopez, L. 2003. Chemical and tactile cues influencing oviposition of a generalist moth, Spodoptera frugiperda (Lepidoptera: Noctuidae). Environmental Entomology 32: 1386–1392.
- Rojas, M.G.; Stipanovic, R.D.; Williams, H.J.; Vinson, S.B. 1992. Metabolism of gossypol by Heliothis virescens (F.) (Lepidoptera: Noctuidae). Environmental Entomology 21: 518–526.
- Roush, R.T. 1989. Designing resistance management programs: how can you choose? Pesticide Science 26: 423–441.
- Scriber, J.M.; Slansky, J.R.F. 1981. The nutritional ecology of immature insects. Annual Review of Entomology 26: 183–211.
- Shapiro, S.S.; Wilk, M.B. 1965. An analysis of variance test for normality. Biometrika 52: 591–611.
- Simpson, S.J.; Raubenheimer, D. 2000. The hungry locust. Advances in the Study of Behavior 29: 1–44.
- Singer, M.C.; Thomas, C.D.; Billington, H.L.; Parmesan, C. 1994. Correlates of speed of evolution of host preference in a set of twelve populations of the butterfly Euphydryas editha Ecoscience. 1: 107–114.
- Slansky, F. 1992. Allelochemical-nutrient interactions in herbivore nutritional ecology. p. 135–174. In: Rosenthal, G.A.; Berenbaum, M.R., eds. Herbivores: their interaction with secondary plant metabolites. Academic Press, San Diego, CA, USA.
- Smith, C.M. 2005. Antibiosis: adverse effects of resistance on arthropod biology. p. 65–99. In: Smith, C.M., ed. Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecht, Holland.
- Soo Hoo, C.F.; Fraenkel, G. 1966. The selection of food plants in a polyphagous insect, Prodenia eridania (Cramer). Journal of Insect Physiology 12: 693–709.
- Sorensen, J.S.; Dearing, M.D. 2006. Efflux transporters as a novel herbivore countermechanism to plant chemical defenses. Journal of Chemical Ecology 32: 1181–1196.
- Sparks, A.N. 1979. A review of the biology of the fall armyworm. The Florida Entomologist 62: 83–87.
- Stipanovic, R.D.; Lopez, J.D.; Dowd, M.K.; Puckhaber, L.S.; Duke, S.E. 2006. Effect of racemic and (+) and (-) gossypol on the survival and development of Helicoverpa zea larvae. Journal of Chemical Ecology 32: 959–968.
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. 2010. Discovery and characterization of field resistance to bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. Journal of Economic Entomology 103: 1031–1038.
- Thompson, J.N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomologia Experimentalis et Applicata 47: 3–14.
- Thompson, S.N.; Redak, R.A. 2005. Feeding behaviour and nutrient selection in an insect Manduca sexta L. and alterations induced by parasitism. Journal of Comparative Physiology A 191: 909–923.
- Tisdale, R.A.; Sappington, T.W. 2001. Realized and potential fecundity, egg fertility, and longevity of laboratory-reared female beet armyworm (Lepidoptera: Noctuidae) under different adult diet regimes. Annals of the Entomological Society of America 94: 415–419.
- Tukey, J.W. 1949. One degree of freedon for non-additivity. Biometrics 5: 232–242.
- Veenstra, K.H.; Pashley, D.P.; Ottea, J.A. 1995. Host-plant adaptation in fall armyworm host strains: comparasion of food consumption, utilization, and detoxication enzyme activities. Annals of the Entomological Society of America 88: 80–91.
- Via, S. 1990. Ecological genetics and host adaptation in herbivorous insects: the experimental study of evolution in natural and agricultural systems. Annual Review of Entomology 35: 421–446.
- Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–11506.
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. 2002. Ecology and behavior of first ínstar larval Lepidoptera. Annual Review of Entomology 47: 361–93.
Edited by
Publication Dates
-
Publication in this collection
Jan-Feb 2017
History
-
Received
16 Apr 2015 -
Accepted
17 Mar 2016