ABSTRACT:
In acid soils, toxic aluminum ions inhibit plant root growth. In order to discriminate aluminum (Al) tolerance, trustful screening techniques are required. In this study, 20 wheat cultivars, showing different levels of Al tolerance, were evaluated in a short-term soil experiment to access their relative root length (RRL). Moreover, the alleles of two important genes (TaALMT1 and TaMATE1B) for Al tolerance in wheat were discriminated. Both of these genes encode membrane transporters responsible for the efflux of organic acids by the root apices that are thought to confer tolerance by chelating Al. Genotypes showing TaALMT1 alleles V and VI and an insertion at the TaMATE1B promoter were among the ones showing greater RRL. Mechanisms of Al tolerance, which are not associated with organic acid efflux, can be potentially present in two cultivars showing greater RRL among the ones carrying inferior TaALMT1 and TaMATE1B alleles. The RRL data were highly correlated with wheat performance in acid soil at three developmental stages, tillering (r = −0.93, p < 0.001), silking (r = −0.91, p < 0.001) and maturation (r = −0.90, p < 0.001), as well as with the classification index of aluminum toxicity in the field (r = −0.92, p < 0.001). Since the RRL was obtained after only six days of growth and it is highly correlated with plant performance in acid soil under field conditions, the short-term experiment detailed here is an efficient and rapid method for reliable screening of wheat Al tolerance.
Keywords:
Triticum aestivum; aluminum tolerance; citrate and malate transporters; short-term soil experiment
Introduction
Plants can come across toxic aluminum (Al) ions, especially the trivalent cation (Al3+), when grown on acid soils. The main effect of Al is the inhibition of root growth and, since roots are essential to nutrient and water uptake, a short root system can affect plant growth and productivity (Kochian et al., 2015Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhães, J.V. 2015. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology 66: 571-598.). In wheat, membrane transporters encoded by the TaALMT1 and TaMATE1B genes account for an important Al tolerance mechanism and are responsible for the efflux of malate and citrate, respectively (Sasaki et al., 2004Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. 2004. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal 37: 645-653.; Tovkach et al., 2013Tovkach, A.; Ryan, P.R.; Richardson, A.E.; Lewis, D.C.; Rathjen, T.M.; Ramesh, S.; Tyerman, S.D.; Delhaize, E. 2013. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology 161: 880-892.). Once released by the root tip, these organic acids form a complex with Al which reduces its toxicity. Polymorphisms at the promoter region of these genes are associated with the level of gene expression, organic acid efflux and Al tolerance (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.; Pereira et al., 2015Pereira, J.F.; Barichello, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Bonow, S.; Cargnin, A. 2015. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Molecular Breeding 35: 169.; Raman et al., 2008Raman, H.; Ryan, P.R.; Raman, R.; Stodart, B.J.; Zhang, K.; Martin, P; Wood, R.; Sasaki, T.; Yamamoto, Y.; Mackay, M.; Hebb, D.M.; Delhaize, E. 2008. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theoretical and Applied Genetics 116: 343-354.; Sasaki et al., 2006Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; Yamamoto, Y. 2006. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant and Cell Physiology 47: 1343-1354.; Tovkach et al., 2013Tovkach, A.; Ryan, P.R.; Richardson, A.E.; Lewis, D.C.; Rathjen, T.M.; Ramesh, S.; Tyerman, S.D.; Delhaize, E. 2013. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology 161: 880-892.).
Al tolerance in wheat is evaluated mainly in hydroponic solution (Shavrukov et al., 2012Shavrukov, Y.; Genc, Y.; Hayes, J. 2012. The use of hydroponics in abiotic stress tolerance research. p. 39-66. In: Asao, T., ed. Hydroponics: a standard methodology for plant biological researches. InTech, Rijeka, Croatia.) whereby seedlings are grown in both the presence and absence of Al and different measurements (relative root length, relative root weight, root regrowth and hematoxylin staining) are used as indicators of Al tolerance (Cai et al., 2008Cai, S.; Bai, G.H.; Zhang, D. 2008. Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theoretical and Applied Genetics 117: 49-56.; Delhaize et al., 1993Delhaize, E.; Ryan, P.R.; Randall, P.J. 1993. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiology 103: 695-702.; Pereira et al., 2010Pereira, J.F.; Zhou, G.; Delhaize, E.; Richardson, T.; Zhou, M.; Ryan, PR. 2010. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany 106: 205-214.; Polle et al., 1978Polle, E.; Konzak, C.F.; Kattrick, J.A. 1978. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Science 18: 823-827.; Taylor and Foy, 1985Taylor, G.J.; Foy, C.D. 1985. Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). I. Differential pH induced by winter cultivars in nutrient solutions. American Journal of Botany 72: 695-701.). However, the correlation with field trials is not usually described or confirmed. For instance, a recent report failed to confirm high correlation between wheat Al tolerance in the field and hydroponics (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.; Baier et al., 1995Baier, A.C.; Somers, D.J.; Gustafson, J.P. 1995. Aluminium tolerance in wheat: correlating hydroponic evaluations with field soil performances. Plant Breeding 114: 291-296.). Considering the importance of describing Al tolerance screening methods that are reliable, this study aimed to access the relative root length of wheat genotypes contrasting for acid soil tolerance using a short-term soil experiment and to correlate the initial root length with plant performance in acid soil under field conditions. Additionally, an association between the initial root length and the TaALMT1 and TaMATE1B alleles was sought. This association can reveal the putative additive effect of these genes or if other Al tolerance mechanisms can be found.
Materials and Methods
Twenty wheat cultivars were evaluated in this study representing four categories (sensitive, moderately sensitive, moderately tolerant and tolerant) of acid soil tolerance discriminated under field conditions (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.). Seeds from each genotype were germinated for 48 h at 23 °C in the dark and transferred to pots (five seeds in each pot) containing 450 g of limed or acid soil. The acid soil (dystrophic red latosol of clayey texture), with pH 4.2 and exchangeable aluminum representing 79 % of the effective cation exchange capacity, was collected at a depth of 0-20 cm in an experimental field located in the city of Passo Fundo, RS, Brazil (latitude −28.216979, longitude −52.408428, altitude 634 m). The limed soil corresponded to part of the acid soil whose acidity was neutralized which increased the pH to 6.2 and reduced the soluble aluminum to 0 %. Pots consisted of cylindrical PVC tubes (25 cm high × 5 cm diameter) that were cut longitudinally into two halves and re-connected using masking tape (Figure 1A and B). Each pot was placed inside a 200 mL plastic cup containing three holes (1 mm each) at the bottom. The plants were grown in a glasshouse under natural light for six days. The experiment was performed in Jan 2016 (summer) and the refrigeration system of the glasshouse was programmed to activate if the temperature was higher than 21 °C and to turn off when the temperature was lower than 18 °C. The pots were structured in completely randomized-block designs with two replications. They were watered up to the point where they reached 80 % of field capacity every two days. At the end of the experiment, the roots were carefully removed from the soil and washed (Figure 1C, D, E and F). Although the soil had a clayey texture, the removal and washing of the roots was performed gently and there was no visible damage. The length of the longest root of each plant was measured. The relative root length (RRL) was estimated as (root length in acid soil/root length in limed soil) x 100. Errors associated with deriving the RRL were calculated by SERRL= RRL [(SEx/x)2 + (SEy/y)2]1/2 where x represents the mean root length in the limed soil and y the mean root length in acid soil. The RRL from each of the 20 wheat cultivars was correlated (Pearson correlation coefficient) with the respective cultivar performance in an acid soil under field conditions, which has been previously described (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.). In the field experiments, the plants were evaluated at three developmental stages (tillering, silking and maturation) and, based on these scores, a classification index of aluminum toxicity in the field (CIATF) was also formulated. The field trials were conducted at a location near where the soil for the short-term experiment performed here was collected.
Wheat plants in the short-term soil experiment. A) Cylindrical PVC tubes, cut longitudinally into two halves and reconnected using masking tape, were placed inside a 200 mL plastic cup containing small holes at the bottom; B) After 6 days, one side of the pot was opened using a scalpel and the roots were carefully washed. The genotypes Tiacena 1 (C); Veadeiros (D); IAS 16 - Cruz Alta (E) and BH 1146 (F) are representatives of each class of acid soil tolerance (sensitive, moderately sensitive, moderately tolerant and tolerant, respectively) used in this study. In each picture, the plants on the left were grown in limed soil and the plants on the right were grown in acid soil.
The TaALMT1 alleles were discriminated by PCR using primers CCTGGTTTTCTTGATGGGGGCACA, TGCCCACCATCTCGCCGTCGCTCTCTCT, GCTCCTACCACTATGGTTGCG and CCAGGCCGACTTTGAGCGAG (Sasaki et al., 2006Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; Yamamoto, Y. 2006. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant and Cell Physiology 47: 1343-1354.). The following amplicon sizes were expected: 1190 bp (indicating allele I), 1221 bp (allele II), 1993 bp (allele III), 1470 bp (allele IV), 1750 bp (allele V), 1600 bp (allele VI) and 1395 bp (allele VII). To detect the presence of an insertion in the upstream region of the TaMATE1B gene, primers ATCCATCCTCCTTCCCTCAC and ATGAATGCTGTGTCCACCAA were used in PCR (Garcia-Oliveira et al., 2014Garcia-Oliveira, A.L.; Martins-Lopes, P; Tolrá, R.; Poschenrieder, C.; Tarquis, M.; Guedes-Pinto, H.; Benito, C. 2014. Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiologia Plantarum 152: 441-452.). In this case, the nonamplification of the 538 bp PCR fragment is related to the absence of the insertion in the TaMATE1B promoter. In this study the two TaMATE1B alleles are referred to as ‘ + ‘ for presence of the insertion and as ‘-’ for its absence. Amplification and electrophoresis on agarose gels were performed as described previously (Pereira et al., 2015Pereira, J.F.; Barichello, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Bonow, S.; Cargnin, A. 2015. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Molecular Breeding 35: 169.).
Results
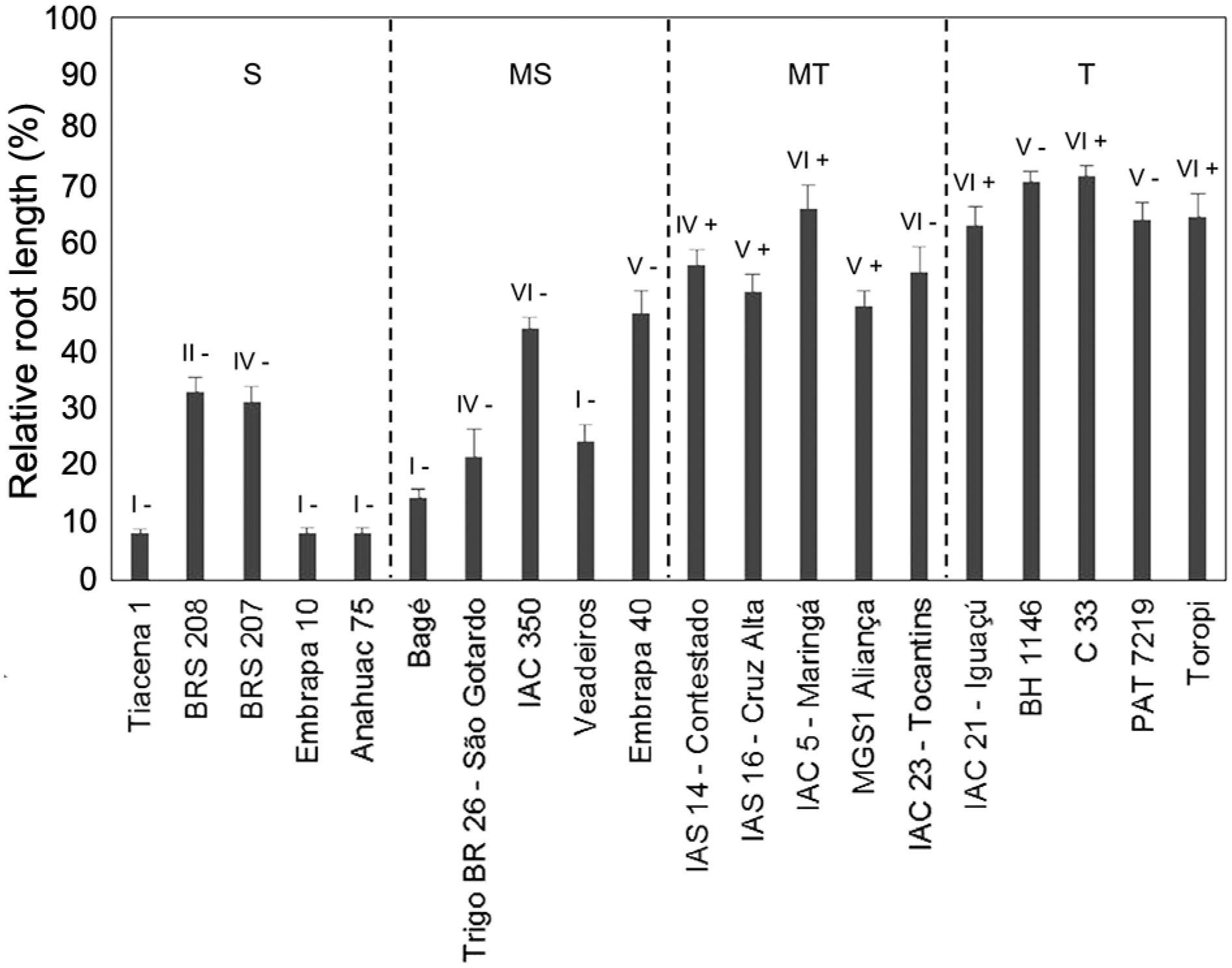
The short-term soil experiment clearly separated genotypes widely recognized as Al sensitive (Anahuac 75) or tolerant (BH 1146 and Toropi) (Figure 2). The lowest relative root length (RRL) was observed in three genotypes previously characterized as sensitive (Tiacena 1, Embrapa 10 and Anahuac 75) and the highest RRL were detected in two genotypes classified as tolerant (BH 1146 and C33). The average RRL was 18 %, 31 %, 55 % and 67 % considering all the sensitive, moderately sensitive, moderately tolerant and tolerant genotypes, respectively. This means that the average RRL among the five cultivars from each category was associated with their level of Al tolerance in the field.
Relative root length of 20 wheat cultivars in the shortterm soil experiment. The cultivars were representative of four classes of Al tolerance: sensitive (S), moderately sensitive (MS), moderately tolerant (MT) and tolerant (T). The bars represent the mean percentage of the relative root length ± SE. Numbers I to VI represent the TaALMT1 promoter alleles while the symbols “+” and “-” indicate the two alleles of the TaMATE1B gene.
Five TaALMT1 alleles and two TaMATE1B alleles were detected (Figure 2). For TaALMT1, the alleles V and VI were the most frequent ones while, for TaMATE1B, the absence of the insertion was detected in more genotypes than its presence. Among the ten sensitive or moderately sensitive cultivars, six showed TaALMT1 alleles I or II and, among the ten cultivars classified as moderately tolerant or tolerant, nine presented TaALMT1 promoter V or VI. The insertion in the TaMATE1B promoter was detected only in genotypes classified as moderately tolerant or tolerant. When combining the alleles from both genes, eight haplotypes were detected with the allele TaMATE1B(+) not being detected along with TaALMT1 alleles I and II (Figure 2). The TaMATE1B(+) allele appears to be advantageous for genotypes carrying the TaALMT1 allele IV since two genotypes (BRS 207 and Trigo BR 26 - São Gotardo) showing the TaALMT1 allele but lacking the insertion had RRL lower than one genotype showing allele IV and the insertion (IAS 14 - Contestado). However, no clear advantage was observed for the presence of the insertion upstream of the TaMATE1B coding region for cultivars carrying TaALMT1 alleles V and VI, as the highest relative root length was observed in cultivars carrying alleles V or VI with or without TaMATE1B(+). Among the cultivars carrying TaALMT1 alleles I or II and TaMATE1B(-), the highest relative root length was observed for BRS 208 (33 %) and Veadeiros (25 %).
The RRL data obtained after six days of growth in the short-term soil experiment was correlated with the wheat acid soil tolerance in the field using previously published data (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.). The initial RRL was highly correlated with the wheat tolerance to acid soil in all the developmental stages (r = −0.93, p < 0.001 in tillering, r = −0.91, p < 0.001 in silking and r = −0.90, p < 0.001 in maturation) and with the classification index of aluminum toxicity in the field - CIATF (r = −0.92, p < 0.001) (Figure 3). That index is based on the overall average plant performance in the three developmental stages over the 2 year period. A negative correlation was obtained because the field scores were high for sensitive plants and low for the tolerant ones while, in the analysis reported here, Al sensitive genotypes showed lower RRL than tolerant materials.
Correlation of the relative root length (RRL), based on root growth after six days in a short-term soil experiment, with the wheat tolerance to acid soil under field conditions. The RRL obtained here was correlated with the acid soil tolerance in three developmental stages (tillering, silking and maturation) and with the classification index of aluminum toxicity in the field (CIATF) obtained previously (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.).
Discussion
In acid soils, toxic Al decreases root cell division and elongation (Kochian et al., 2015Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhães, J.V. 2015. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology 66: 571-598.) and the different phenotyping methods for Al tolerance are based on the small root growth caused by Al. In wheat, although some studies have used soil experiments in glasshouse or growth chambers (Pereira et al., 2010Pereira, J.F.; Zhou, G.; Delhaize, E.; Richardson, T.; Zhou, M.; Ryan, PR. 2010. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany 106: 205-214.; Pereira et al., 2015Pereira, J.F.; Barichello, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Bonow, S.; Cargnin, A. 2015. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Molecular Breeding 35: 169.; Tang et al., 2003Tang, C.; Nuruzzaman, M.; Rengel, Z. 2003. Screening wheat genotypes for tolerance of soil acidity. Australian Journal of Agricultural Research 54: 445-452.; Zhou et al., 2013Zhou, G.; Delhaize, E.; Zhou, M.; Ryan, PR. 2013. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Annals of Botany 112: 603-612.), most of the reports assessing Al stress response have used hydroponic culture. The advantages in using hydroponics are the isolation of the Al stress, greater control of environmental conditions and easy observation of the roots. However, this methodology may not necessarily reflect plant performance in the field. For instance, only a moderate correlation (r = 0.56, p < 0.001) was obtained by comparing the wheat Al tolerance in hydroponics and the acid soil tolerance in field conditions (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.). Here, the initial root growth of wheat under a short-term soil experiment was highly correlated (r = −0.92, p < 0.001) with plant performance in acid soil under field conditions (Figure 3). The importance of using rapid screening methods with high correlation with field screening data is that the results not only discriminate Al tolerance of the genotypes but also reflect their real performance. In addition, since field screening is laborious, the use of reliable and fast screening methods is important to save time and resources especially the ones related to personnel.
A good correlation between short-term soil experiment and acid soil tolerance in field conditions has been reported previously for barley and wheat (Gallardo et al., 1999Gallardo, F.; Borie, F.; Alvear, M.; von Baer, E. 1999. Evaluation of aluminum tolerance of three barley cultivars by two shortterm screening methods and field experiments. Soil Science and Plant Nutrition 45: 713-719.; Tang et al., 2003Tang, C.; Nuruzzaman, M.; Rengel, Z. 2003. Screening wheat genotypes for tolerance of soil acidity. Australian Journal of Agricultural Research 54: 445-452.). In these reports, the plants were also grown for six days. However, the methodologies used in these studies were different in, for example, the origin of the genotypes, the amount of soil used per pot and the proportion of exchangeable aluminum in each soil. Here, except for Anahuac 75, all other wheat cultivars were developed in Brazil. This is an important difference because Brazilian wheat cultivars are internationally recognized as important Al tolerance sources and Brazil is considered as one of the countries where Al tolerant genes may have originated independently (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.; Hu et al., 2008Hu, S.W.; Bai, G.H.; Carver, B.F.; Zhang, D.D. 2008. Diverse origins of aluminum-resistance sources in wheat. Theoretical and Applied Genetics 118: 29-41.; Raman et al., 2008Raman, H.; Ryan, P.R.; Raman, R.; Stodart, B.J.; Zhang, K.; Martin, P; Wood, R.; Sasaki, T.; Yamamoto, Y.; Mackay, M.; Hebb, D.M.; Delhaize, E. 2008. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theoretical and Applied Genetics 116: 343-354.). Moreover, the soil used here presented the highest proportion of exchangeable aluminum in comparison to previous studies (Gallardo et al., 1999Gallardo, F.; Borie, F.; Alvear, M.; von Baer, E. 1999. Evaluation of aluminum tolerance of three barley cultivars by two shortterm screening methods and field experiments. Soil Science and Plant Nutrition 45: 713-719.; Tang et al., 2003Tang, C.; Nuruzzaman, M.; Rengel, Z. 2003. Screening wheat genotypes for tolerance of soil acidity. Australian Journal of Agricultural Research 54: 445-452.). Nevertheless, all these reports evidenced that short-term soil experiments and plant performance in field conditions are correlated if the same soil is used in both screenings. This aspect should be considered for wheat breeding programs targeting improvements in acid soil tolerance. For instance, acid soils are common in the Brazilian Cerrado (savanna) (Lopes et al., 2012Lopes, A.S.; Guilherme, L.R.G.; Ramos, S.J. 2012. The saga of the agricultural development of the Brazilian Cerrado. Electronic International Fertilizer Correspondent 32: 29-56.) and this region is thought to be the new frontier for increasing wheat production in Brazil. For this reason, the short-term soil experiment, as the one detailed here, could be used by wheat breeders interested in increasing wheat production in the Cerrado region. Under that scenario, it would be interesting to evaluate the RRL from wheat plants in short-term experiments using soil collected from the Cerrado. As drought is also an important stress in the Cerrado region during the wheat season (Poersch-Bortolon et al., 2016Poersch-Bortolon, L.B.; Pereira, J.F.; Nhani Junior, A.; Gonzáles, H.H.S.; Torres, G.A.M.; Consoli, L.; Arenhart, R.A.; Bodanese-Zanettini, M.H.; Margis-Pinheiro, M. 2016. Gene expression analysis reveals important pathways for drought response in leaves and roots of a wheat cultivar adapted to rainfed cropping in the Cerrado biome. Genetics and Molecular Biology 39: 629-645.), the deeper root growth presented by Al tolerant genotypes could lead to improved drought tolerance, because deeper roots can potentially access water in deep subsoil during the dry season. In fact, a deeper root system can better extract not only water but also nutrients (Lynch and Wojciechowski, 2015Lynch, J.P; Wojciechowski, T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66: 2199-2210.; Wasson et al., 2012Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. 2012. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63: 3485-3498.).
In this study, the genotypes carrying TaALMT1 alleles V and VI showed the highest RRL while low RRL was observed for genotypes with TaALMT1 alleles I and II (Figure 2). This is in agreement with previous reports and could be explained by the higher gene expression, higher malate efflux and, consequently, greater Al tolerance commonly found in genotypes carrying alleles V and VI while the opposite is usually true for genotypes with alleles I and II (Aguilera et al., 2016Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.; Pereira et al., 2015Pereira, J.F.; Barichello, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Bonow, S.; Cargnin, A. 2015. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Molecular Breeding 35: 169.; Raman et al., 2008Raman, H.; Ryan, P.R.; Raman, R.; Stodart, B.J.; Zhang, K.; Martin, P; Wood, R.; Sasaki, T.; Yamamoto, Y.; Mackay, M.; Hebb, D.M.; Delhaize, E. 2008. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theoretical and Applied Genetics 116: 343-354.; Sasaki et al., 2006Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; Yamamoto, Y. 2006. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant and Cell Physiology 47: 1343-1354.). Nevertheless, two cultivars (BRS 208 and Veadeiros) showed the greatest RRL among those carrying inferior TaALMT1 and TaMATE1B alleles and they should be further evaluated for potential mechanisms to cope with Al other than organic acid efflux. Although the insertion in the TaMATE1B promoter showed no clear advantage when detected in genotypes carrying TaALMT1 alleles V and VI, it appears to be beneficial when associated with TaALMT1 allele IV. This allele shows lower repeat of blocks in the promoter and it is associated with lower TaALMT1 expression than alleles V and VI (Sasaki et al., 2006Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; Yamamoto, Y. 2006. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant and Cell Physiology 47: 1343-1354.). This suggests that this result corroborates a recent report showing that the insertion in the TaMATE1B promoter is advantageous if it is associated with an inferior TaALMT1 allele but not with a superior TaALMT1 allele (Han et al., 2016Han, C.; Zhang, P; Ryan, PR.; Rathjen, T.M.; Yan, Z.; Delhaize, E. 2016. Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theoretical and Applied Genetics 129: 729-739.).
In conclusion, this study provides evidence that the superior TaMATE1B allele appears to be advantageous if associated with an inferior TaALMT1 allele and that a mechanism, not related to organic acid efflux, is possibly present in BRS 208 and Veadeiros. Additionally, a high correlation between initial root length and wheat tolerance to acid soil in field conditions was observed. The short-term soil experiment detailed here can be useful to projects aiming for improvements in the wheat root system when grown in acid soils.
Acknowledgements
I am thankful to Embrapa (Brazilian Agricultural Research Corporation) for financial support (project 02.11.08.001.00.00). I also appreciate the cooperation of Ricardo C. Leão and his team for providing the soil, Dr. Sirio Wiethölter and his team for the soil analysis, and Dr. Luciano Consoli for providing the seeds. I am also grateful to Dr. Sirio Wiethölter and Dr. Gilberto R. Cunha (Embrapa Wheat) and to Jéssica R. Ferreira for valuable discussions.
References
- Aguilera, J.G.; Minozzo, J.A.; Barichello, D.; Fogaça, C.M.; Silva Júnior, J.P.; Consoli, L.; Pereira, J.F. 2016. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theoretical and Applied Genetics 129: 1317-1331.
- Baier, A.C.; Somers, D.J.; Gustafson, J.P. 1995. Aluminium tolerance in wheat: correlating hydroponic evaluations with field soil performances. Plant Breeding 114: 291-296.
- Cai, S.; Bai, G.H.; Zhang, D. 2008. Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theoretical and Applied Genetics 117: 49-56.
- Delhaize, E.; Ryan, P.R.; Randall, P.J. 1993. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiology 103: 695-702.
- Gallardo, F.; Borie, F.; Alvear, M.; von Baer, E. 1999. Evaluation of aluminum tolerance of three barley cultivars by two shortterm screening methods and field experiments. Soil Science and Plant Nutrition 45: 713-719.
- Garcia-Oliveira, A.L.; Martins-Lopes, P; Tolrá, R.; Poschenrieder, C.; Tarquis, M.; Guedes-Pinto, H.; Benito, C. 2014. Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiologia Plantarum 152: 441-452.
- Han, C.; Zhang, P; Ryan, PR.; Rathjen, T.M.; Yan, Z.; Delhaize, E. 2016. Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theoretical and Applied Genetics 129: 729-739.
- Hu, S.W.; Bai, G.H.; Carver, B.F.; Zhang, D.D. 2008. Diverse origins of aluminum-resistance sources in wheat. Theoretical and Applied Genetics 118: 29-41.
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhães, J.V. 2015. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology 66: 571-598.
- Lopes, A.S.; Guilherme, L.R.G.; Ramos, S.J. 2012. The saga of the agricultural development of the Brazilian Cerrado. Electronic International Fertilizer Correspondent 32: 29-56.
- Lynch, J.P; Wojciechowski, T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66: 2199-2210.
- Pereira, J.F.; Barichello, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Bonow, S.; Cargnin, A. 2015. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Molecular Breeding 35: 169.
- Pereira, J.F.; Zhou, G.; Delhaize, E.; Richardson, T.; Zhou, M.; Ryan, PR. 2010. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany 106: 205-214.
- Polle, E.; Konzak, C.F.; Kattrick, J.A. 1978. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Science 18: 823-827.
- Poersch-Bortolon, L.B.; Pereira, J.F.; Nhani Junior, A.; Gonzáles, H.H.S.; Torres, G.A.M.; Consoli, L.; Arenhart, R.A.; Bodanese-Zanettini, M.H.; Margis-Pinheiro, M. 2016. Gene expression analysis reveals important pathways for drought response in leaves and roots of a wheat cultivar adapted to rainfed cropping in the Cerrado biome. Genetics and Molecular Biology 39: 629-645.
- Raman, H.; Ryan, P.R.; Raman, R.; Stodart, B.J.; Zhang, K.; Martin, P; Wood, R.; Sasaki, T.; Yamamoto, Y.; Mackay, M.; Hebb, D.M.; Delhaize, E. 2008. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theoretical and Applied Genetics 116: 343-354.
- Sasaki, T.; Ryan, P.R.; Delhaize, E.; Hebb, D.M.; Ogihara, Y.; Kawaura, K.; Noda, K.; Kojima, T.; Toyoda, A.; Matsumoto, H.; Yamamoto, Y. 2006. Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant and Cell Physiology 47: 1343-1354.
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. 2004. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal 37: 645-653.
- Shavrukov, Y.; Genc, Y.; Hayes, J. 2012. The use of hydroponics in abiotic stress tolerance research. p. 39-66. In: Asao, T., ed. Hydroponics: a standard methodology for plant biological researches. InTech, Rijeka, Croatia.
- Tang, C.; Nuruzzaman, M.; Rengel, Z. 2003. Screening wheat genotypes for tolerance of soil acidity. Australian Journal of Agricultural Research 54: 445-452.
- Taylor, G.J.; Foy, C.D. 1985. Mechanisms of aluminum tolerance in Triticum aestivum L. (wheat). I. Differential pH induced by winter cultivars in nutrient solutions. American Journal of Botany 72: 695-701.
- Tovkach, A.; Ryan, P.R.; Richardson, A.E.; Lewis, D.C.; Rathjen, T.M.; Ramesh, S.; Tyerman, S.D.; Delhaize, E. 2013. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology 161: 880-892.
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. 2012. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63: 3485-3498.
- Zhou, G.; Delhaize, E.; Zhou, M.; Ryan, PR. 2013. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Annals of Botany 112: 603-612.
Edited by
Publication Dates
-
Publication in this collection
Jan-Feb 2018
History
-
Received
19 Oct 2016 -
Accepted
20 Dec 2016