ABSTRACT:
Cobia is one of the most promising warm water aquaculture species. In Brazil, cobia farming began in 2008 in the state of Rio de Janeiro from experimental scale facilities to regular near-shore farms based on fresh/frozen fish diets composed mostly of Sardinella sp. Despite the encouraging results achieved in the promotion of sustainable cobia farming, we advocate the replacement of fresh/frozen fish by a practical formulated feed. This experiment evaluated the zootechnical performance and environmental efficiency of moist and practical formulated feeds in early grow-out phases in the cycle of cobia nearshore cage culture. Four hundred and twenty juvenile cobia (151 ± 7 g) were fed with moist feed and practical formulated feed for 56 days. Biometrics were taken every two weeks and diets were analyzed for proximate composition, fatty acid composition and pellet quality. Although growth performance was equivalent between treatments, feed consumption and feed conversion ratios (FCR) were different (p < 0.05) and varied according to water temperature. Cobia fed moist feed exhibited an FCR two times higher than those fed formulated feed. Elevated settling speed and low floatability contributed to higher heterogeneity and lower efficiency of fish fed moist diet. Nitrogen excretion rate was reduced (64 %) and protein efficiency ratio elevated (27 %) within formulated diet groups in comparison to those fed moist diet (79 % and 15 %, respectively). The fatty acid profile of cobia muscle was similar across the groups. With no negative effects of diet substitution on production performance and improvement of environmental efficiency, this approach can be applied and advocated globally and contribute to the responsible intensification of sustainable marine fish culture.
Keywords:
aquaculture; environmental efficiency; protein efficiency ratio; nitrogen discharge; marine fish farming
Introduction
Cobia aquaculture started in Taiwan in the 90's, and underwent expansion after the development of massive fingerling production technology in 1997, spreading in the following years to other Asian countries and the western hemisphere (Benetti et al., 2010Benetti, D.D.; O'Hanlon, B.; Rivera, J.A.; Welch, A.W.; Maxey, C.; Orhun, M.R. 2010. Growth rates of cobia (Rachycentron canadum) cultured in open ocean submerged cages in the Caribbean. Aquaculture 302: 195-201.; Liao et al., 2004Liao, I.C.; Huang, T.-S.; Tsai, W.-S.; Hsueh, C.-M.; Chang, S.-L.; Leaño, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155-165.; Nhu et al., 2011Nhu, V.C.; Nguyen, H.Q.; Le, T.L.; Tran, M.T.; Sorgeloos, P.; Dierckens, K.; Reinertsen, H.; Kjørsvik, E.; Svennevig, N. 2011. Cobia Rachycentron canadum aquaculture in Vietnam: recent developments and prospects. Aquaculture 315: 20-25.). Although cobia cultured in offshore net cage systems generally rely on formulated feeds (Liao et al., 2004Liao, I.C.; Huang, T.-S.; Tsai, W.-S.; Hsueh, C.-M.; Chang, S.-L.; Leaño, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155-165.), most traditional near-shore cobia production is still based on rough fish, commonly referred to as trash fish (Petersen et al., 2015Petersen, E.H.; Glencross, B.D.; Van Tien, N.; Than, V.A.; Phuong, T.H. 2015. Recent changes in the bioeconomic of finfish mariculture in Vietnam. Journal of Aquaculture Research & Development 6: 1.).
Moist and fresh-frozen fish-based diets are still an important feed source for marine fish culture in Asian countries (Bunlipatanon et al., 2014Bunlipatanon, P.; Songseechan, N.; Kongkeo, H.; Abery, N.W.; Silva, S.S. 2014. Comparative efficacy of trash fish versus compounded commercial feeds in cage aquaculture of Asian seabass (Lates calcarifer) (Bloch) and tiger grouper (Epinephelus fuscoguttatus) (Forsskål). Aquaculture Research 45: 373-388.). However, substitution of formulated feeds has been encouraged, by virtue of its significant implication for the environment and the culture system, which includes the increasing of nutrient input, risk of contamination, rise of pathogen incidence, variations in nutritional quality and higher related feed conversion ratio (Kim et al., 2007Kim, J.H.; Gomez, D.K.; Choresca Jr, C.H.; Park, S.C. 2007. Detection of major bacterial and viral pathogens in trash fish used to feed cultured flounder in Korea. Aquaculture 272: 105-110.; Liao et al., 2004Liao, I.C.; Huang, T.-S.; Tsai, W.-S.; Hsueh, C.-M.; Chang, S.-L.; Leaño, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155-165.; New, 1996New, M. 1996. Responsible use of aquaculture feeds. Aquaculture Asia 1: 3-4.; Nhu et al., 2011Nhu, V.C.; Nguyen, H.Q.; Le, T.L.; Tran, M.T.; Sorgeloos, P.; Dierckens, K.; Reinertsen, H.; Kjørsvik, E.; Svennevig, N. 2011. Cobia Rachycentron canadum aquaculture in Vietnam: recent developments and prospects. Aquaculture 315: 20-25.)
In Brazil, cobia farming started in offshore net cages in the northeastern region, but prospered in the southern region in near-shore systems, encouraged by experimental ongrowing results (Sampaio et al., 2011Sampaio, L.A.; Moreira, C.B.; Miranda-Filho, K.C.; Rombenso, A.N. 2011. Culture of cobia Rachycentron canadum (L) in nearshore cages off the Brazilian coast. Aquaculture Research 42: 832-834.). In Rio de Janeiro, during the early grow-out period, cobia are fed moist diets based on a Sardinella sp. surplus generated by the trawl fisheries industry, which is a good nutritional quality resource with wide availability (Rombenso et al., 2016Rombenso, A.N.; Bowzer, J.C.; Moreira, C.B.; Sampaio, L.A. 2016. Culture of Caranx species [Horse-eye Jack Caranx latus (Agassiz), Blue Runner Caranx crysos (Mitchill), and Crevalle Jack Caranx hippos (Linnaeus)] in near-shore cages off the Brazilian coast during colder months. Aquaculture Research 47: 1687-1690.).
Despite the positive results achieved so far, the availability of a high quality cost-effective formulated aquafeed is paramount to guaranteeing a sustainable development allied to the economic feasibility of a near-shore cobia cage culture, but the issue is how to achieve a successful transition especially in view of the operational comprehension by producers of the benefits of practical formulated diets.
Accordingly, this experiment aimed to evaluate the zootechnical performance and environmental efficiency of moist to practical formulated feed substitution in early grow-out phases of cobia near-shore cage culture, providing reliable subsidies to encourage farmers to adopt this switch.
Materials and Methods
Experimental structures and animals
Juvenile cobia weighing an average of 3 g were acquired from a commercial producer in Ilha Bela, São Paulo, Brazil (23°51′36.92″ S; 45°25′41.86″ W; 16m altitude) and transported to a marine fish facility in Angra dos Reis, in the state of Rio de Janeiro, Brazil (23°6′50.83″ S; 44°15′47.85″ W; sea level). Eight hundred fish were acclimated into three near-shore cages of 24 m−3 (6 × 2 × 2 m) at a stocking density below 1 kg m−3 for 3 months. During this period, fish were hand-fed until apparent satiation a non-specific cobia diet (45 % crude protein and 16 % lipid), two times a day.
Experimental diet production process and analysis
Practical Formulated feed (PFf) was formulated to meet or exceed cobia nutritional requirements (amino acids, fatty acids, vitamins and minerals) available in peer-reviewed scientific literature (Chou et al., 2001Chou, R.L.; Su, M.S.; Chen, H.Y. 2001. Optimal dietary protein and lipid levels for juvenile cobia (Rachycentron canadum). Aquaculture 193: 81-89.; Craig et al., 2006Craig, S.R.; Schwarz, M.H.; McLean, E. 2006. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics. Aquaculture 261: 384-391.; Fraser and Davies, 2009Fraser, T.W.K.; Davies, S.J. 2009. Nutritional requirements of cobia, Rachycentron canadum (Linnaeus): a review. Aquaculture Research 40: 1219-1234.) containing approximately 44.0 - crude protein, 8.0 - crude lipid, 11.0 - ashes, 36.0- non-nitrogenous extract, and g kg−1 on a dry matter basis. The PFf contained 500 g kg−1 of imported menhaden fish meal as a principal protein source and imported menhaden fish oil as a lipid source (Table 1). PFf was extruded with a 7.5 mm die at an animal feed factory in Cordeiro, in the state of Rio de Janeiro, Brazil (22°14′47.52″ S; 42°18′56.91″ W; 688 m altitude) following in-house extrusion protocols and then shipped and stored in a frozen chamber (-20 °C) throughout the feeding trial.
Formulation of experimental formulated diet and proximate composition and physical characteristics of experimental diets.
Moist feed (Mf) was manufactured every week on a grow-out site according to fish producer feed making protocol. Fresh sardines (72 %CP; 10 %CL) were ground, cooked, and mixed with a bran omnivorous fish feed (28 %CP; 4 %CL) in a 2:1 proportion and pelletized with a 7 mm die size on a meat grinder machine. After manufacturing, the moist feed was stocked in a freezer (-20 °C) and used on a weekly basis.
Proximate composition analysis
Diets and muscle were analyzed in triplicate for proximate composition (Table 1) according to the official methods of AOAC (2002)Association of Official Analytical Chemists - International [AOAC]. 2002. Official Methods of Analysis. 17ed. AOAC International, Gaithersburg, MD, USA.. Moisture content was determined by the dry method, and weighing samples were heated in an oven at 110 °C to constant weight. Ash content was determined by incinerating samples in a muffle furnace at 600 °C for 4 h. Crude protein (CP) was produced by Kjedhal methods and crude lipid (CL) was determined by the Folch et al. (1957)Folch, J.; Lees, M.; Sloane Stanley, G.H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry 226: 497-509. method. The non-nitrogenous extract (NNE) was calculated as the difference between total dry matter and the other nutrients (crude protein levels, crude lipid, ashes, and crude fiber). Crude energy content was determined by the following equation: .
Physical pellet characteristics
To evaluate the percentage of floating pellets (%FLT) samples of both diets were sieved through a 1mm mesh to remove all the bran. Next, 50 pellets were dumped from a height of ten centimeters into a 100 mL Becker filled with seawater (33 psu) at 25 °C. After one minute, the floating pellets were counted and then the FLT was calculated as follows:
The settling speed was evaluated based on Cromey et al. (2009)Cromey, C.J.; Nickell, T.D.; Treasurer, J.; Black, K.D.; Inall, M. 2009. Modelling the impact of cod (Gadus morhua L.) farming in the marine environment - CODMOD. Aquaculture 289: 42-53. with modifications. Briefly, 50 pellets were dumped individually into a graduated cylinder 48 cm high and 30 cm diameter filled with seawater (33 psu) at 25 °C. The settling speed was determined by recording the time each pellet took to reach the bottom.
Fatty acid analysis
Total lipids from diets and muscle were extracted using the methodology described by Folch et al. (1957)Folch, J.; Lees, M.; Sloane Stanley, G.H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry 226: 497-509.. Fatty acid (FA) was determined by gas chromatography (GC) coupled to a flame ionizer (FID) and auto-injector (Varian GC 3900). The oven temperature program was: 170 °C maintained for 1 min, from 170 °C to 240 °C at 2.5 °C min−1, maintained at 240 °C for 5 min. The temperature for both the detector and injector were kept at 250 °C and 260 °C, respectively, and a CP wax 52CB column was used, being 0.25 μm in thickness, 0.25 mm in internal diameter, and 30 m in length, with hydrogen as the carrier gas. The FA profile was determined by a time retention equation utilizing a known-time standard retention time (SUPELCO®, 37 Standard e Larodan Chemical Company - Mixture Me93 e Qualmix PUFA fish M - Menhaden Oil).
Production performance assay
A feeding trial was carried out at a marine fishing facility in Angra dos Reis, in the state of Rio de Janeiro, Brazil (23°6′50.83” S; 44°15′47.85” W; at sea level). A completely randomized experimental design in a factorial scheme with two treatments (moist and formulated diets) was used, each one having three replicates, in a 56 day feeding trial.
Seventy cobia with individual initial weight of 151 ± 7 g were stocked per cage (2 × 2 × 1.5 m) with a 40 mm mesh achieving a stocking density of 1.7 kg m−3. Fish were hand-fed two times a day until apparent satiation. Every two weeks, 40 fish per cage were anesthetized by 50 ppm clove oil for two min prior to weighing on a digital scale. Additionally, every sampling event consisted of three fish per tank being euthanized by clove oil overdose (150 ppm) and then eviscerated. Viscera and liver samples were collected and weighed for subsequent proximate composition analysis (crude protein, crude lipid, ashes and moist), using standard AOAC methods (AOAC, 2002Association of Official Analytical Chemists - International [AOAC]. 2002. Official Methods of Analysis. 17ed. AOAC International, Gaithersburg, MD, USA.).
Water temperature data were recorded daily at fixed intervals of three hours in an HDPE floating structure 1.5 m deep. At each sampling interval and at the end of the feeding trial the following production and environmental performance parameters were calculated:
The values of protein deposition on carcass and protein intake were used to calculate the protein efficiency ratio (PER) by the linear equation: , where: Y = carcass protein deposition (g) X, the protein intake (g), A, the straight inclination from linear regression representing the protein efficiency ratio in percentage terms (PER) and B, the constant representing the stretch interception point on the vertical axis (Sakomura and Rostagno, 2007Sakomura, N.K.; Rostagno, H.S. 2007. Research methods in monagastric nutrition = Métodos de Pesquisa em Nutrição de Monogástricos. Funep, Jaboticabal, SP, Brazil (in Portuguese).).
Feed cost of production
Feed cost production was calculated by: FCR x Feed price kg−1. Feed cost did not consider processing and storage costs as these were difficult to quantify. The labor provided to process the Mf is opposite to what generally happens in Asian countries (Bunlipatanon et al., 2014Bunlipatanon, P.; Songseechan, N.; Kongkeo, H.; Abery, N.W.; Silva, S.S. 2014. Comparative efficacy of trash fish versus compounded commercial feeds in cage aquaculture of Asian seabass (Lates calcarifer) (Bloch) and tiger grouper (Epinephelus fuscoguttatus) (Forsskål). Aquaculture Research 45: 373-388.). It is not provided by the farmers themselves and is an additional cost.
Nitrogen Budget
Nitrogen input in feed was calculated by the equation
Nitrogen retained (NR) was estimated by
Nitrogen lost on mortality (NM) was estimated by
Finally, Nitrogen excretion (NE) was calculated by the mass balance equation
Statistical analysis
Briefly, all data were tested for normality (Shapiro Wilk) and homogeneity of variance (Cochran). When necessary, data were converted for parametric testing (Zar, 2010Zar, J. 2010. Biostatistical Analysis. Prentice-Hall, Upper Saddle River, NJ, USA.). All data are shown as means and standard errors and were analyzed by one-way analysis of variance (ANOVA). When omnibus tests indicated significant post-hoc treatment effects, tests (Tukey´s, HSD) were used to determine the differences between the means. In all cases, an alpha level of 0.05 (p < 0.05) was used. Individual linear regression analyses were performed to investigate the relationship between abiotic factors and certain production metrics (e.g. feed conversation rate, weight gain, feed consumption and PER) using the PROC REG (p = 0.05) procedure from SAS/STAT software (Statistical Analysis System, 9.3 version).
Results
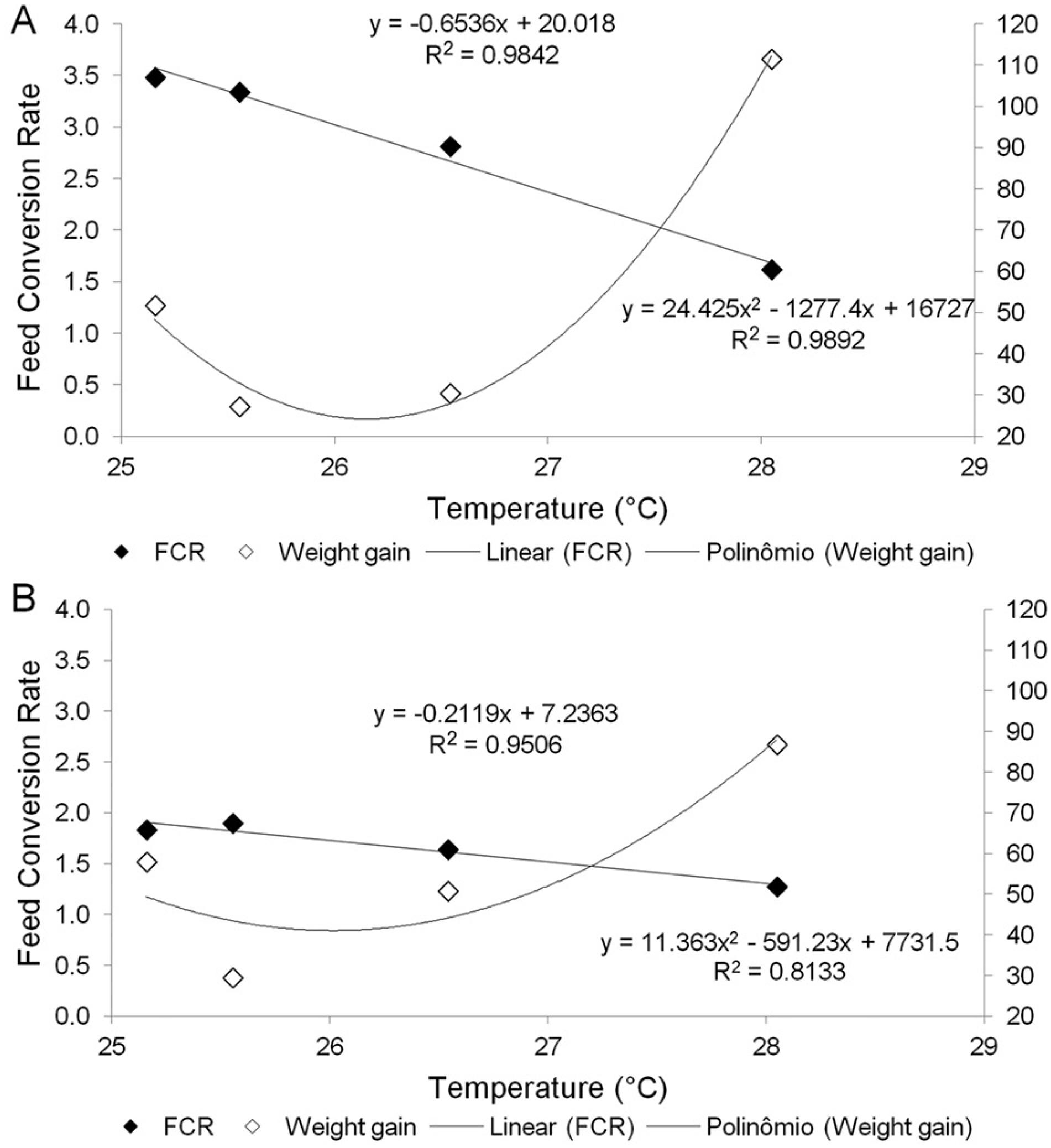
Juvenile cobia attained a final individual weight of 374.10 ± 33.15 g (grand mean ± SE) with final stocking densities lower than 5 kg m−3 under both treatments (Table 2). No differences between treatments were observed for final individual weight, WG, SGR, HSI and VSI (p > 0.005). Feed intake (27.87-7.35 g) and FCR (3.21-1.54) were different between treatments (p < 0.005; Table 2), being higher in the Mf group and varying according to water temperature (Figure 1A and B). In both treatments, FCR decreased as water temperature increased. For example, in the Mf group, FCR was 7.05 at 25 °C and 2.92 at 28 °C, whereas in the PFf group FCR declined from 1.87 at 25 °C to 1.22 at 28 °C. Additionally, negative correlation between feed consumption and temperature and positive correlation between weight gain and temperature were observed (Figure 1A and B).
Linear and quadratic polynomial regression between feed conversion ratio (FCR) in dry matter basis, weight gain (g), and water temperature for dietary treatments moist diet (A) and formulated diet (B).
Production performance and muscle proximate composition of juvenile Rachycentron canadum L. fed experimental diets in near-shore cage system. Values are presented as LS-means ± standard error. Means with different letter are significantly different (p < 0.05).
PFf sinking speeds were slower (1.05 ± 0.2 cm s−1) than Mf (6.60 ± 1.2 cm s−1), whereas the floatability rate was higher (100 ± 0 %) when compared to the other tested diet (6 ± 1 %; Table 1).
Fish fed PFf exhibited higher PER (27 %, on dry matter basis) compared to those fed Mf (15 %; Figure 2A and B).
Linear regression between protein deposition and protein intake of juvenile cobia fed moist diet (A) and formulated diet (B).
Fillet fatty acid profile mirrored dietary fatty acid composition (Table 3 and Table 4). Similar levels of saturated fatty acids (SFAs), polyunsaturated fatty acids (PUFAs), Arachidonic acid (ARA), n-6 and n-3 fatty acids were observed between treatments. cobia fed formulated feeds exhibited higher levels of monounsaturated fatty acids (MUFAs), 20:3n-3, Eicosapentanoic acid (EPA) and 22:5n-3 in the fillet, whereas those fed moist feeds presented significantly higher levels of docosahexaenoic acid (DHA).
Dietary fatty acid composition (g 100 g−1 fatty acid methyl esters). Values are presented as LS-means ± standard error (SE) triplicate fatty acid samples.
Fillet fatty acid composition (g 100 g−1 fatty acid methyl esters) of Rachycentron canadum L fed moist and practical formulated feeds. Values are presented as LS-means ± standard error triplicate fatty acid samples and p values resulting from oneway ANOVA test are also provided; means with common letter are not significantly different (p < 0.05).
Similarly, the nitrogen budget, presented in Table 5, was lower for the PFf group (67 %) in comparison to the Mf group (82 %). Nitrogen excretions in Mf (2.755 ± 0.369 mg g d−1) were higher (p < 0.005) than PFf (1.497 ± 0.093 mg g d−1).
Nitrogen budget of juvenile Rachycentron canadum L fed experimental diets. Means with different letter are significantly different (p < 0.05).
Discussion
A recent survey conducted in central Vietnam stressed that fish farmers choose to use trash fish for aquaculture due to the low cost, production performance, lack of alternative feeds and purchase convenience (Huntington and Hasan, 2009Huntington, T.C.; Hasan, M.R. 2009 Fish as feed inputs for aquaculture - practices, sustainability and implications: a global synthesis. p. 1-61. In: Hasan, M.R.; Halwart, M., eds. Fish as feed inputs for aquaculture - practice, sustainability and implications: a global synthesis. FAO, Rome, Italy. (FAO Fisheries and Aquaculture Technical Paper, 518).).
Focused on production performance, their survival ratio was elevated, higher than 94 % for both treatments, which is consistent with other studies (Benetti et al., 2010Benetti, D.D.; O'Hanlon, B.; Rivera, J.A.; Welch, A.W.; Maxey, C.; Orhun, M.R. 2010. Growth rates of cobia (Rachycentron canadum) cultured in open ocean submerged cages in the Caribbean. Aquaculture 302: 195-201.; Liao et al., 2004Liao, I.C.; Huang, T.-S.; Tsai, W.-S.; Hsueh, C.-M.; Chang, S.-L.; Leaño, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155-165.; Sampaio et al., 2011Sampaio, L.A.; Moreira, C.B.; Miranda-Filho, K.C.; Rombenso, A.N. 2011. Culture of cobia Rachycentron canadum (L) in nearshore cages off the Brazilian coast. Aquaculture Research 42: 832-834.; Weirich et al., 2010Weirich, C.R.; Wills, P.S.; Baptiste, R.M.; Riche, M.A. 2010. Production Characteristics and body composition of juvenile Cobia fed three different commercial diets in recirculating aquaculture systems. North American Journal of Aquaculture 72: 43-49.). Under both treatments, stocking density remained below this 5 kg m−3 rate reported to yield optimum growth for cobia (Benetti et al., 2010Benetti, D.D.; O'Hanlon, B.; Rivera, J.A.; Welch, A.W.; Maxey, C.; Orhun, M.R. 2010. Growth rates of cobia (Rachycentron canadum) cultured in open ocean submerged cages in the Caribbean. Aquaculture 302: 195-201.; Weirich et al., 2010Weirich, C.R.; Wills, P.S.; Baptiste, R.M.; Riche, M.A. 2010. Production Characteristics and body composition of juvenile Cobia fed three different commercial diets in recirculating aquaculture systems. North American Journal of Aquaculture 72: 43-49.).
In this same location and under similar conditions Moreira et al. (2015)Moreira, C.B.; Rombenso, A.N.; Candiotto, F.B.; Tsuzuki, M.Y. 2015. Feeding frequency affects growth of juvenile cobia Rachycentron canadum cultured in near-shore cages. Boletim do Instituto de Pesca 42: 219-226. observed an SGR of 2.3 % d−1 and FCR of 2.3:1 in a 15-day period in juvenile cobia (15 - 30 g) fed nonspecific commercial feed (45 %CP; 16 %CL) at an average temperature of 24 °C. Additionally, at the culture site Sampaio et al. (2011)Sampaio, L.A.; Moreira, C.B.; Miranda-Filho, K.C.; Rombenso, A.N. 2011. Culture of cobia Rachycentron canadum (L) in nearshore cages off the Brazilian coast. Aquaculture Research 42: 832-834., reported an SGR of 1.5 % d−1 and FCR of 5:1 in cage-bred cobia within the weight range of 184-600 g, sardine fed at an average temperature of 24 °C.
Despite the different sizes, feed types and time intervals, the available literature indicates comparable SGR and higher FCR for nonspecific and sardine-based feeds in comparison to PFf.
Decreased FCR associated with increasing temperature was reported by Sampaio et al. (2011)Sampaio, L.A.; Moreira, C.B.; Miranda-Filho, K.C.; Rombenso, A.N. 2011. Culture of cobia Rachycentron canadum (L) in nearshore cages off the Brazilian coast. Aquaculture Research 42: 832-834. with a minimum value (1.65:1) recorded during summer (highest average temperature of 28 °C), which corroborates the present findings. Warmer water temperatures (31-33 °C) are reported to optimize growth and FCR of juvenile Cobia up to 200 g (Sun and Chen, 2014Sun, L.; Chen, H. 2014. Effects of water temperature and fish size on growth and bioenergetics of cobia (Rachycentron canadum). Aquaculture 426-427: 172-180.; Sun et al., 2006aSun, L.; Chen, H.; Huang, L. 2006a. Effect of temperature on growth and energy budget of juvenile cobia (Rachycentron canadum). Aquaculture 261: 872-878., bSun, L.; Chen, H.; Huang, L.; Wang, Z. 2006b. Growth, faecal production, nitrogenous excretion and energy budget of juvenile cobia (Rachycentron canadum) relative to feed type and ration level. Aquaculture 259: 211-221.).
Wills et al. (2013)Wills, P.S.; Weirich, C.R.; Baptiste, R.M.; Riche, M.A. 2013. Evaluation of commercial marine fish feeds for production of juvenile Cobia in recirculating aquaculture systems. North American Journal of Aquaculture 75: 178-185. testing three different commercial feeds for marine fish that would be suitable for grow out of juvenile Cobia: A (50 % CP:15 % CL); B (45 % CP:16 % CL) and C (44 % CP:15 %CL) reported a protein efficiency ratio of 25 ± 0; 23 ± 1 and 14 ± 1 % consistent with that registered in the PFf group (26 %) and the Mf group (15 %).
Feed type and feeding frequency affect directly the amount of lost feed during feeding events and are of great importance to polluting potential. As a general rule, moist and trash fish-based diets result in higher environmental impact when compared to pelletized and extruded diets due to greater loss of nutrients and reduced water stability (Islam, 2005Islam, M.S. 2005. Nitrogen and phosphorus budget in coastal and marine cage aquaculture and impacts of effluent loading on ecosystem: review and analysis towards model development. Marine Pollution Bulletin 50: 48-61.; Wu, 1995Wu, R.S.S. 1995. The environmental impact of marine fish culture: towards a sustainable future. Marine Pollution Bulletin 31: 159-166.). Mf presented poor water stability quickly disintegrating once in contact with water. In terms of production, this could result in reduced feed efficiency, raising FCR. Mf also exhibited faster sinking speed, which could also have influenced the great heterogeneity of fish size. The short time of pellet in water column favors dominant fish to access feed and consequently develop more.
In agreement, Costa-Bomfim et al. (2014)Costa-Bomfim, C.N.; Pessoa, W.V.N.; Oliveira, R.L.M.; Farias, J.L.; Domingues, E.C.; Hamilton, S.; Cavalli, R.O. 2014. The effect of feeding frequency on growth performance of juvenile cobia, Rachycentron canadum (Linnaeus, 1766). Journal of Applied Ichthyology 30: 135-139. reported the aggressive behavior of juvenile cobia during feeding events. On the other hand, PFf presented better water stability and a slow sinking rate increased feed opportunity and homogeneity in this treatment. Similarly, Moreira et al. (2015)Moreira, C.B.; Rombenso, A.N.; Candiotto, F.B.; Tsuzuki, M.Y. 2015. Feeding frequency affects growth of juvenile cobia Rachycentron canadum cultured in near-shore cages. Boletim do Instituto de Pesca 42: 219-226. highlighted that higher feed availability reduced feeding competition and increased homogeneity in experimental units.
Economically, feed represents the most expensive variable cost component in carnivorous fish production, and using an adequately formulated diet is critical to keeping cost down and minimizing metabolic waste products (Craig et al., 2006Craig, S.R.; Schwarz, M.H.; McLean, E. 2006. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics. Aquaculture 261: 384-391.). PFf has a current stipulated cost of US$ 1.60 kg−1 and Mf US$ 0.32 kg−1, but in terms of cost of feed per kilogram of fish this difference drops considerably, the price being US$ 2.55 ± 0.44 and 2.12 ± 0.80 kg−1, respectively. Despite lower prices, high water content (51 %) has disadvantages in terms of transport, handling, storage, expiration date and logistics of Mf and must be taken into account.
Cobia, in common with other carnivorous fish, have a limited capacity for synthesizing physiologically important n-3 LC-PUFAs (long-chain polyunsaturated fatty acids), such as ecosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), to satisfy their physiological demand, requiring dietary supplementation (Sargent et al., 1999Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. 1999. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179: 217-229.; Tocher, 2010Tocher, D.R. 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717-732.; Trushenski et al., 2012Trushenski, J.; Schwarz, M.; Bergman, A.; Rombenso, A.; Delbos, B. 2012. DHA is essential, EPA appears largely expendable, in meeting the n - 3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326-329: 81-89.). Although PFf contained a reduced level of DHA compared to the recommended levels of 8-12 g kg−1 fish fed this dietary treatment exhibited similar performance, which is probably due to the elevated content of EPA in formulated feed that was bioconverted to DHA satisfying the nutritional and physiological demand of fish. Corroborating this, EPA content in fillet of fish fed formulated feeds was not as high as at dietary levels, but still higher than those fed the moist feed.
In terms of fillet fatty acid composition, the dietary fatty acid composition showed consistency with the literature available (Asdari et al., 2011Asdari, R.; Aliyu-Paiko, M.; Hashim, R.; Ramachandran, S. 2011. Effects of different dietary lipid sources in the diet for Pangasius hypophthalmus (Sauvage, 1878) juvenile on growth performance, nutrient utilization, body indices and muscle and liver fatty acid composition. Aquaculture Nutrition 17: 44-53.; Tocher, 2010Tocher, D.R. 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717-732.; Turchini et al., 2009Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. 2009. Fish oil replacement in finfish nutrition. Reviews in Aquaculture 1: 10-57.; Zakeri et al., 2011Zakeri, M.; Kochanian, P.; Marammazi, J.G.; Yavari, V.; Savari, A.; Haghi, M. 2011. Effects of dietary n-3 HUFA concentrations on spawning performance and fatty acids composition of broodstock, eggs and larvae in yellowfin sea bream, Acanthopagrus latus. Aquaculture 310: 388-394.). Although no differences in the fillet fatty acid profile of both treatments were observed for SFAs, PUFAs, ARA, n-6 and n-3 fatty acids, statistical differences were seen in the fatty acid signature of each diet. These findings are consistent with previous lipid and fatty acid research in cobia (Trushenski et al., 2012Trushenski, J.; Schwarz, M.; Bergman, A.; Rombenso, A.; Delbos, B. 2012. DHA is essential, EPA appears largely expendable, in meeting the n - 3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326-329: 81-89.; Woitel et al., 2014Woitel, F.R.; Trushenski, J.T.; Schwarz, M.H.; Jahncke, M.L. 2014. More judicious use of fish oil in Cobia feeds. I. Assessing the relative merits of alternative lipids. North American Journal of Aquaculture 76: 222-231.).
Increased nitrogen levels in coastal waters due to anthropogenic sources are a worldwide concern, generally resulting in algal blooms and nutrient enrichment or eutrophication. Marine cage aquaculture operations are a recognized source of nitrogenous discharge released both in the form of particulate matter (uneaten feed and feces containing undigested feed that passes through the digestive tract of fish) and dissolved metabolic wastes including ammonia and urea (Price et al., 2015Price, C.; Black, K.D.; Hargrave, B.T.; Morris Jr, J.A. 2015. Marine cage culture and the environment: effects on water quality and primary production. Aquaculture Environment Interactions 6: 151-174.).
Nitrogen excretion levels recorded are lower in the PFf group (67 %) and higher in the Mf group (82 %) than those registered by Alston et al. (2005), who reported 79 % of the nitrogen fed to the fish was released into the water in a marine cage culture of Lutjanus analis and R. canadum.
Water temperature seems to affect cobia nitrogen excretion as well as growth (Sun and Chen, 2014Sun, L.; Chen, H. 2014. Effects of water temperature and fish size on growth and bioenergetics of cobia (Rachycentron canadum). Aquaculture 426-427: 172-180.). Furthermore, the same authors attested to a relatively constant nitrogen budget in elevated temperatures (2733 °C) with more than 68 % of feed nitrogen being lost in excretion. Nitrogen excretion in the Mf fed group was higher even at lower temperatures whereas the PFf group remained close to these results.
Sun et al. (2006bSun, L.; Chen, H.; Huang, L.; Wang, Z. 2006b. Growth, faecal production, nitrogenous excretion and energy budget of juvenile cobia (Rachycentron canadum) relative to feed type and ration level. Aquaculture 259: 211-221.) reported that feeding efficiency and fecal production are significantly affected by the feed type, observing higher nitrogen excretion in groups fed with in natura sardines compared to commercial diets, highlighting the great eutrophication potential of in natura diets, the same pattern as was observed in this study on the moist diet composed principally of raw ingredients.
Currently, the limited supply of trash fish as the main feed source for cobia grow-out has become a major constraint for cobia culture in Vietnam and other countries. Also, due to the increase in pressure on natural stocks of small pelagic fish used in aquaculture feed production, fishmeal utilization must be discouraged in order to reach environmental sustainability (Naylor et al., 2000Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017-1024.).
In the Brazilian market, due to the paucity of attractiveness of the ingredients, previous experiences with a practical formulated feed introduction did not succeed (Moreira et al., 2015Moreira, C.B.; Rombenso, A.N.; Candiotto, F.B.; Tsuzuki, M.Y. 2015. Feeding frequency affects growth of juvenile cobia Rachycentron canadum cultured in near-shore cages. Boletim do Instituto de Pesca 42: 219-226.). Thus we opted for the use of imported premium fishmeal as the principal protein source.
Several studies indicate a future positive outlook and potential for growth for the Latin American aquaculture industry, especially for the continued growth of cage culture for salmonids and warm water species including cobia, and projections concerning future market availability and the price of fishmeal and fish oil within the region are that supplies will remain tight and prices high. As in Europe, there is a need to reduce the dependence of the aquaculture sector on fishmeal and fish oil through the use of alternative, locally available feed ingredient sources, the production of which can keep pace with the growth and specific requirements of the aquaculture sector within the region.
With the expected wide adoption of cobia pelleted and extruded feeds, the next step in improvements to the sustainable expansion of cobia culture in Brazil, embraces lowering fishmeal and fish oil content by adopting alternative proteins and lipid sources, including soybean meal and oil, rapeseed oil, palm oil, poultry-by meal and fat, pork lard, beef tallow among other animal or plant ingredients, with proven suitability without production nor nutritional impairment (Betancor et al., 2016Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. 2016. Replacement of marine fish oil with de novo Omega-3 Oils From Transgenic Camelina sativa in feeds for gilthead sea bream (Sparus aurata L.). Lipids 51: 1171-1191.; Rombenso et al., 2017Rombenso, A.N.; Trushenski, J.T.; Schwarz, M.H. 2017. Beef tallow is suitable as a primary lipid source in juvenile Florida pompano feeds. Aquaculture: DOI: 10.1111/anu.12502
https://doi.org/10.1111/anu.12502...
; Suarez et al., 2013Suarez, J.A.; Tudela, C.; Davis, D.; Daugherty, Z.; Taynor, M.; Glass, L.; Hoenig, R.; Buentello, A.; Benetti, D.D. 2013. Replacement of fish meal by a novel non-GM variety of soybean meal in cobia, Rachycentron canadum: ingredient nutrient digestibility and growth performance. Aquaculture 416-417: 328-333.; Trushenski et al., 2012Trushenski, J.; Schwarz, M.; Bergman, A.; Rombenso, A.; Delbos, B. 2012. DHA is essential, EPA appears largely expendable, in meeting the n - 3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326-329: 81-89.; Watson et al., 2014Watson, A.M.; Buentello, A.; Place, A.R. 2014. Partial replacement of fishmeal, poultry by-product meal and soy protein concentrate with two non-genetically modified soybean cultivars in diets for juvenile cobia, Rachycentron canadum. Aquaculture 434: 129-136.; Woitel et al., 2014Woitel, F.R.; Trushenski, J.T.; Schwarz, M.H.; Jahncke, M.L. 2014. More judicious use of fish oil in Cobia feeds. I. Assessing the relative merits of alternative lipids. North American Journal of Aquaculture 76: 222-231.).
This study successfully substituted moist with formulated feed without any impairment in production performance. Formulated diet was better used, less wasted, and promoted less eutrophication compared to the moist feed. Further research is encouraged to investigate ways to incorporate the surplus from the fisheries into formulated extruded diets as fish meal and fish oil, or as silage among other possibilities. This approach would be beneficial for transforming this highly nutritional resource into a high-quality feed assisting with the available, satisfactory and affordable marine finfish feed constraint.
This approach can be applied and advocated globally and thereby contribute to the responsible intensification of marine fish culture.
Acknowledgments
Thanks are due to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors appreciate the support of Mr. Kazuo Tonack (Maricultura Costa Verde, Angra dos Reis, Brazil), as also Mr. Marcelo Lacerda and Julio Magno Ramos from the Depto. de Aquicultura e Pesca de Angra dos Reis, Mr. André Araujo and Paulo Marcio Costa from the Fundação Instituto de Pesca do Estado do Rio de Janeiro Costa Verde local office, Mr. Pablo Lopez and Ms. Alioune Diarra PROFIFISH S.A. representatives and Mr. Antonio Carlos Erthal, owner of North Central animal feeds.
References
- Asdari, R.; Aliyu-Paiko, M.; Hashim, R.; Ramachandran, S. 2011. Effects of different dietary lipid sources in the diet for Pangasius hypophthalmus (Sauvage, 1878) juvenile on growth performance, nutrient utilization, body indices and muscle and liver fatty acid composition. Aquaculture Nutrition 17: 44-53.
- Association of Official Analytical Chemists - International [AOAC]. 2002. Official Methods of Analysis. 17ed. AOAC International, Gaithersburg, MD, USA.
- Benetti, D.D.; O'Hanlon, B.; Rivera, J.A.; Welch, A.W.; Maxey, C.; Orhun, M.R. 2010. Growth rates of cobia (Rachycentron canadum) cultured in open ocean submerged cages in the Caribbean. Aquaculture 302: 195-201.
- Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. 2016. Replacement of marine fish oil with de novo Omega-3 Oils From Transgenic Camelina sativa in feeds for gilthead sea bream (Sparus aurata L.). Lipids 51: 1171-1191.
- Bunlipatanon, P.; Songseechan, N.; Kongkeo, H.; Abery, N.W.; Silva, S.S. 2014. Comparative efficacy of trash fish versus compounded commercial feeds in cage aquaculture of Asian seabass (Lates calcarifer) (Bloch) and tiger grouper (Epinephelus fuscoguttatus) (Forsskål). Aquaculture Research 45: 373-388.
- Chou, R.L.; Su, M.S.; Chen, H.Y. 2001. Optimal dietary protein and lipid levels for juvenile cobia (Rachycentron canadum). Aquaculture 193: 81-89.
- Costa-Bomfim, C.N.; Pessoa, W.V.N.; Oliveira, R.L.M.; Farias, J.L.; Domingues, E.C.; Hamilton, S.; Cavalli, R.O. 2014. The effect of feeding frequency on growth performance of juvenile cobia, Rachycentron canadum (Linnaeus, 1766). Journal of Applied Ichthyology 30: 135-139.
- Craig, S.R.; Schwarz, M.H.; McLean, E. 2006. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics. Aquaculture 261: 384-391.
- Cromey, C.J.; Nickell, T.D.; Treasurer, J.; Black, K.D.; Inall, M. 2009. Modelling the impact of cod (Gadus morhua L.) farming in the marine environment - CODMOD. Aquaculture 289: 42-53.
- Folch, J.; Lees, M.; Sloane Stanley, G.H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry 226: 497-509.
- Fraser, T.W.K.; Davies, S.J. 2009. Nutritional requirements of cobia, Rachycentron canadum (Linnaeus): a review. Aquaculture Research 40: 1219-1234.
- Huntington, T.C.; Hasan, M.R. 2009 Fish as feed inputs for aquaculture - practices, sustainability and implications: a global synthesis. p. 1-61. In: Hasan, M.R.; Halwart, M., eds. Fish as feed inputs for aquaculture - practice, sustainability and implications: a global synthesis. FAO, Rome, Italy. (FAO Fisheries and Aquaculture Technical Paper, 518).
- Islam, M.S. 2005. Nitrogen and phosphorus budget in coastal and marine cage aquaculture and impacts of effluent loading on ecosystem: review and analysis towards model development. Marine Pollution Bulletin 50: 48-61.
- Kim, J.H.; Gomez, D.K.; Choresca Jr, C.H.; Park, S.C. 2007. Detection of major bacterial and viral pathogens in trash fish used to feed cultured flounder in Korea. Aquaculture 272: 105-110.
- Liao, I.C.; Huang, T.-S.; Tsai, W.-S.; Hsueh, C.-M.; Chang, S.-L.; Leaño, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture 237: 155-165.
- Moreira, C.B.; Rombenso, A.N.; Candiotto, F.B.; Tsuzuki, M.Y. 2015. Feeding frequency affects growth of juvenile cobia Rachycentron canadum cultured in near-shore cages. Boletim do Instituto de Pesca 42: 219-226.
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017-1024.
- New, M. 1996. Responsible use of aquaculture feeds. Aquaculture Asia 1: 3-4.
- Nhu, V.C.; Nguyen, H.Q.; Le, T.L.; Tran, M.T.; Sorgeloos, P.; Dierckens, K.; Reinertsen, H.; Kjørsvik, E.; Svennevig, N. 2011. Cobia Rachycentron canadum aquaculture in Vietnam: recent developments and prospects. Aquaculture 315: 20-25.
- Petersen, E.H.; Glencross, B.D.; Van Tien, N.; Than, V.A.; Phuong, T.H. 2015. Recent changes in the bioeconomic of finfish mariculture in Vietnam. Journal of Aquaculture Research & Development 6: 1.
- Price, C.; Black, K.D.; Hargrave, B.T.; Morris Jr, J.A. 2015. Marine cage culture and the environment: effects on water quality and primary production. Aquaculture Environment Interactions 6: 151-174.
- Rombenso, A.N.; Bowzer, J.C.; Moreira, C.B.; Sampaio, L.A. 2016. Culture of Caranx species [Horse-eye Jack Caranx latus (Agassiz), Blue Runner Caranx crysos (Mitchill), and Crevalle Jack Caranx hippos (Linnaeus)] in near-shore cages off the Brazilian coast during colder months. Aquaculture Research 47: 1687-1690.
- Rombenso, A.N.; Trushenski, J.T.; Schwarz, M.H. 2017. Beef tallow is suitable as a primary lipid source in juvenile Florida pompano feeds. Aquaculture: DOI: 10.1111/anu.12502
» https://doi.org/10.1111/anu.12502 - Sakomura, N.K.; Rostagno, H.S. 2007. Research methods in monagastric nutrition = Métodos de Pesquisa em Nutrição de Monogástricos. Funep, Jaboticabal, SP, Brazil (in Portuguese).
- Sampaio, L.A.; Moreira, C.B.; Miranda-Filho, K.C.; Rombenso, A.N. 2011. Culture of cobia Rachycentron canadum (L) in nearshore cages off the Brazilian coast. Aquaculture Research 42: 832-834.
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. 1999. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179: 217-229.
- Suarez, J.A.; Tudela, C.; Davis, D.; Daugherty, Z.; Taynor, M.; Glass, L.; Hoenig, R.; Buentello, A.; Benetti, D.D. 2013. Replacement of fish meal by a novel non-GM variety of soybean meal in cobia, Rachycentron canadum: ingredient nutrient digestibility and growth performance. Aquaculture 416-417: 328-333.
- Sun, L.; Chen, H. 2014. Effects of water temperature and fish size on growth and bioenergetics of cobia (Rachycentron canadum). Aquaculture 426-427: 172-180.
- Sun, L.; Chen, H.; Huang, L. 2006a. Effect of temperature on growth and energy budget of juvenile cobia (Rachycentron canadum). Aquaculture 261: 872-878.
- Sun, L.; Chen, H.; Huang, L.; Wang, Z. 2006b. Growth, faecal production, nitrogenous excretion and energy budget of juvenile cobia (Rachycentron canadum) relative to feed type and ration level. Aquaculture 259: 211-221.
- Tocher, D.R. 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717-732.
- Trushenski, J.; Schwarz, M.; Bergman, A.; Rombenso, A.; Delbos, B. 2012. DHA is essential, EPA appears largely expendable, in meeting the n - 3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326-329: 81-89.
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. 2009. Fish oil replacement in finfish nutrition. Reviews in Aquaculture 1: 10-57.
- Watson, A.M.; Buentello, A.; Place, A.R. 2014. Partial replacement of fishmeal, poultry by-product meal and soy protein concentrate with two non-genetically modified soybean cultivars in diets for juvenile cobia, Rachycentron canadum. Aquaculture 434: 129-136.
- Weirich, C.R.; Wills, P.S.; Baptiste, R.M.; Riche, M.A. 2010. Production Characteristics and body composition of juvenile Cobia fed three different commercial diets in recirculating aquaculture systems. North American Journal of Aquaculture 72: 43-49.
- Wills, P.S.; Weirich, C.R.; Baptiste, R.M.; Riche, M.A. 2013. Evaluation of commercial marine fish feeds for production of juvenile Cobia in recirculating aquaculture systems. North American Journal of Aquaculture 75: 178-185.
- Woitel, F.R.; Trushenski, J.T.; Schwarz, M.H.; Jahncke, M.L. 2014. More judicious use of fish oil in Cobia feeds. I. Assessing the relative merits of alternative lipids. North American Journal of Aquaculture 76: 222-231.
- Wu, R.S.S. 1995. The environmental impact of marine fish culture: towards a sustainable future. Marine Pollution Bulletin 31: 159-166.
- Zakeri, M.; Kochanian, P.; Marammazi, J.G.; Yavari, V.; Savari, A.; Haghi, M. 2011. Effects of dietary n-3 HUFA concentrations on spawning performance and fatty acids composition of broodstock, eggs and larvae in yellowfin sea bream, Acanthopagrus latus. Aquaculture 310: 388-394.
- Zar, J. 2010. Biostatistical Analysis. Prentice-Hall, Upper Saddle River, NJ, USA.
Edited by
Publication Dates
-
Publication in this collection
Mar-Apr 2019
History
-
Received
20 June 2017 -
Accepted
17 Nov 2017