ABSTRACT:
The citrus scale insect Praelongorthezia praelonga (Douglas), a major pest of citrus and other economically important crops, has only two commonly documented natural enemies: an entomopathogenic strain of the fungus Colletotrichum nymphaeae (Pass.) Aa and several parasitoids. The entomopathogenic strain of C. nymphaeae, formerly recognized under the synonym C. gloeosporioides f. sp. ortheziidae, is under development for commercial application as a biological control agent in citrus in Brazil-the top exporter of citrus globally. The synonomy of C. gloeosporioides f. sp. ortheziidae with C. nymphaeae remains based on limited DNA sequence data and without morphological study. To qualify for future approval as a biological control agent by federal agencies in Brazil and the European Union, the circumscription of a microorganism must be explicit and without ambiguities. Herein, through morphological study and phylogenetic analysis of five DNA regions we clarify the circumscription and affinity of entomopathogenic C. nymphaeae and describe it as a new variety.
Keywords:
Biological control; Colletotrichum (Sordariomycetes: Glomerellaceae); entomopathogen
Introduction
Colletotrichum Corda (Ascomycota: Sordiariomycetes: Glomerallaceae) is a cosmopolitan and speciose genus of fungi comprised largely of plant symbionts (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
; Hanlin, 1998Hanlin, R.T. 1998. Combined Keys to Illustrated Genera of Ascomycetes. APS Press, St. Paul, MN, USA.). The host range within the genus is broad with both pathogenic and endophytic species reported from all major lineages of land plants (MacKenzie et al., 2009MacKenzie, S.J.; Peres, N.A.; Barquero, M.P.; Arauz, L.F.; Timmer, L.W. 2009. Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 99: 620-631. DOI: 10.1094/phyto-99-5-0620
https://doi.org/10.1094/phyto-99-5-0620...
; Manamgoda et al., 2013Manamgoda, D.; Udayanga, D.; Cai, L.; Chukeatirote, E.; Hyde, K. 2013. Endophytic Colletotrichum from tropical grasses with a new species C. endophytica. Fungal Diversity 61:107-115. DOI: 10.1007/s13225-013-0256-3
https://doi.org/10.1007/s13225-013-0256-...
; Photita et al., 2005Photita, W.; Taylor, P.W.J.; Ford, R.; Hyde, K.D.; Lumyong, S. 2005. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Diversity 18: 117-133.). Two Colletotrichum taxa are entomopathogenic: C. fiorinae and C. nymphaeae. Colletotrichum fiorinae was described from the hemlock scale insect Fiorna externa Ferris; however, C. nymphaeae is a widespread plant pathogen in which entomopathogencity is an exception and limited to strains isolated from the citrus scale insect Praelongorthezia praelonga (Douglas) (syn. Orthezia praelonga Douglas) (Hemiptera: Ortheziidae) (Marcelino et al., 2008Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. 2008. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353-374.).
Entomopathogenic C. nymphaeae is of particular interest because the citrus scale insect (P. praelonga), its primary host, is a serious pest of Citrus spp. and other major economic plants such as coffee, figs and ornamentals (Garcia-Roa, 1995Garcia-Roa, F.A. 1995. Management of Orthezia praelonga, A Citrus Pest = Manejo de Orthezia praelonga, una Plaga de los Cítricos. CO-BAC, Santafé de Bogotá, Bogotá, Colombia (in Spanish).). Citrus scale is considered a pest wherever it is found because of its elevated reproductive rate and highly polyphagous nature (Kondo et al., 2013Kondo, T.; Peronti, A.L.; Kozár, F.; Szita, É. 2013. The citrus orthezia, Praelongorthezia praelonga (Douglas) (Hemiptera: Ortheziidae), a potential invasive species. p. 301-319. In: CABI, Wallingford, UK. (CABI Invasive Species Series, 3).). The regularly occurring natural enemies of citrus scale are currently limited to C. nymphaeae and several parasitoids (Kondo et al., 2013Kondo, T.; Peronti, A.L.; Kozár, F.; Szita, É. 2013. The citrus orthezia, Praelongorthezia praelonga (Douglas) (Hemiptera: Ortheziidae), a potential invasive species. p. 301-319. In: CABI, Wallingford, UK. (CABI Invasive Species Series, 3).; Ramos et al., 2018Ramos, A.S.; Lemos, R.N.; Costa, V.A.; Peronti, A.L.; Silva, E.A.; Mondego, J.M.; Moreira, A.A. 2018. Hymenopteran parasitoids associated with scale insects (Hemiptera: Coccoidea) in tropical fruit trees in the eastern Amazon, Brazil. Florida Entomologist 101: 273-278.). Studies on entomopathogenic C. nymphaeae have focused on its utility and development as a biological control agent of P. praelonga in citrus production (Teixeira et al., 2001Teixeira, M.A.; Bettiol, W.; Cesnik, R. 2001. Pathogenicity of Colletotrichum gloeosporioides, Orthezia praelonga pathogenicity agent, to citrus leaves, flowers and fruits = Patogenicidade de Colletotrichum gloeosporioides, agente de patogenicidade da Orthezia praelonga, para folhas, flores e frutos cítricos. Summa Phytopathologica 27: 352-357 (in Portuguese).; Teixeira et al., 2004Teixeira, M.A.; Bettiol, W.; Cesnik, R.; Vieira, R.F. 2004. Pathogenicity of the fungus Colletotrichum gloeosporioides, pathogen of Orthezia praelonga, to several fruits and to pumpkin seedlings. Revista Brasileira de Fruticultura 26: 356-358 (in Portuguese, with abstract in English).). Natural outbreaks of the fungus with high mortality of citrus scale are observed in conventional citrus groves; outbreaks appear to be density dependent with increased prevalence during rainy and warm weather (Mascarin et al., 2016Mascarin, G.M.; Guarín-Molina, J.H.; Arthurs, S.P.; Humber, R.A.; Andrade, R.M.; Demétrio, C.G.B.; Delalibera, I. 2016. Seasonal prevalence of the insect pathogenic fungus Colletotrichum nymphaeae in Brazilian citrus groves under different chemical pesticide regimes. Fungal Ecology 22: 43-45.).
In Brazil, C. nymphaeae on citrus scale is colloquially known as the salmão (salmon) fungus-a reference to the characteristic salmon pink color of the conidial masses on infected insects. The salmão fungus, previously C. gloeosporioides f. sp. ortheziidae, was transferred to C. nymphaeae based on a single locus and without a comparative morphological study (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
; Marcelino et al., 2008Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. 2008. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353-374.). The designation formae speciales (f. sp.) was dropped by this transfer; therefore, entomopathogenic isolates of C. nymphaeae are no longer distinguished by name from its plant pathogenic conspecifics. Because of its potential for biological control, the taxonomy and affinity of the salmão fungus require further study. In this study, through morphological and molecular studies, we take a polyphasic approach to clarifying the taxonomy of this important natural enemy of the citrus scale insect.
Materials and Methods
Fungal isolation and preservation
Two isolates of Colletotrichum sp., collected from the citrus scale insect, were purified in vitro on potatodextrose-agar and subsequently deposited in the Laboratório de Patologia e Controle Microbiano, Departamento de Entomologia e Acarologia of the Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo (USP/ESALQ). Isolate ESALQ 1393 was collected in Limeira, state of Sao Paulo, 8 Feb 2007, Sylvio Baggio s.n., and isolate ESALQ 1368 from Cordeirópolis, state of Sao Paulo, 13 July 2005, Luiz Fernando Padulla s.n. Both isolates were preserved in a lyophilized state and in sterile 10 % glycerol at −80 °C as metabolically inactive strains.
Morphological study
Morphological study and descriptions were made from observations of fungal structures mounted in water on a glass slide. Measurements and light photomicrographs were taken using an Olympus AX70 Provis compound light microscope and Cell ⋀A analysis image processing software as well as an Olympus SZX16 dissecting microscope. Herbarium acronyms followed those of the Index Herbariorum (Thiers, 2018Thiers, B. 2018. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Available at: http://sweetgum.nybg.org/science/ih/ [Accessed Nov 7, 2018]
http://sweetgum.nybg.org/science/ih/...
). Morphological characters were recorded from cultures grown on sabouraud dextrose agar (SDA), oatmeal agar (OA) (Crous et al., 2009Crous, P.W.; Verkley, G.J.M.; Groenewald, J.Z.; Samson, R.A. 2009. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands. (Fungal Biodiversity 269. CBS Laboratory Manual Series 1).), and synthetic nutrient-poor agar medium (SNA) (Nirenberg, 1976Nirenberg, H. 1976. Studies on morphological and biological differentiation in the Liseola section of the genus Fusarium = Untersuchungen Über die morphologische und biologische differenzierung in der Fusarium-section Liseola. Mitteilungen, Biologische Bundesanstalt fur Land und Forstwirtschaft 169: 1-117 (in German).) and maintained at 22 °C with a light regime of 18 h of darkness and 6 h of light. Figures were assembled with Adobe Photoshop CS4.
Molecular study
Genomic DNA was extracted from C. nymphaeae isolates from infected P. praelonga individuals collected from citrus orchards in Brazil. One isolate, ARSEF 4360, was obtained from the USDA-ARS Collection of Entomopathogenic Fungal Cultures (ARSEF) and ESALQ 1393 was obtained from the culture collection of the Laboratório de Patologia e Controle Microbiano, Universidade de São Paulo (USP/ESALQ). DNA from ESALQ 1393 and for ARSEF 4360 were extracted as described in Mascarin et al. (2016)Mascarin, G.M.; Guarín-Molina, J.H.; Arthurs, S.P.; Humber, R.A.; Andrade, R.M.; Demétrio, C.G.B.; Delalibera, I. 2016. Seasonal prevalence of the insect pathogenic fungus Colletotrichum nymphaeae in Brazilian citrus groves under different chemical pesticide regimes. Fungal Ecology 22: 43-45.; genomic DNA was obtained by grinding the mycelium with a plastic pestle inside a 1.5 mL Eppendorf tube. DNA was then isolated using a commercial plant DNA extraction kit using the standard protocol and eluted with 100 μL sterile deionized water.
Three loci were amplified: the entire nrDNA ITS region (ITS1-5.8S-ITS2), actin (ACT) and chitin synthase (CHS-1). The entire nrDNA ITS region (ITS1-5.8S-ITS2) was amplified using primer pairs ITS1F (Gardes and Bruns, 1993Gardes, M.; Bruns, T.D. 1993. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113-118. DOI: 10.1111/j.1365-294X.1993.tb00005.x
https://doi.org/10.1111/j.1365-294X.1993...
) + ITS4 (White et al., 1990White, T.J.; Bruns, T.; Lee, S.; Taylor. J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. p. 315-322. In: Innis, M.A.; Gelfand, D.H.; Sninksy, J.J.; White, T.J., eds. PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA, USA.), ACT and CHS-1 were amplified and sequenced using the primer pairs ACT-512F + ACT-783R and CHS-354R + CHS-79F (Carbone and Kohn, 1999Carbone, I.; Kohn, L.M. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553-556. DOI: 10.2307/3761358
https://doi.org/10.2307/3761358...
), respectively. Primers and primer sequences are listed in Table 1. PCR was performed under the following conditions for ITS: step 1) 1 min at 95 °C, 2) 45 s at 95 °C, 3) 40 s, 50.8 °C, 4) 90 s at 72 °C, 5) return to step 2 34 times, 6) a final step of 10 min at 72 °C; for both ACT and CHS-1: step 1) 3 min at 95 °C, 2) 15 s at 95 °C, 3) 20 s at 58 °C, 4) 1 min at 72 °C, 5) return to step 2 34 times, 6) final step of 5 min at 72 °C. Samples were kept at 10 °C until electrophoresis was performed on a 1 % agarose TAE gel and visualized under UV light. PCR products were cleaned using a PCR purification kit and sent for sequencing. Sequences were assembled with Sequencher v. 5.3 and aligned manually in MEGA5 (Tamura et al., 2011Tamura, K.; Peterson, D.; Peterson, N.; Stetcher, G.; Nei, M.; Kumar, S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-2739.).
Primer sequences and references for loci amplified in this study: ACT = actin, CHS-1= chitin synthase 1 and ITS = Internal transcribed spacer (ITS) situated between the small-subunit ribosomal RNA.
Using the newly obtained sequences as well as sequences from GenBank, including those generated by Damm et al. (2012)Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
, five datasets were produced: ITS and TUB2 datasets each having 77 sequences, and glyceraldehyde-3-phosphate dehydrogenase (GADPH), CHS-1 and ACT datasets each having 76 sequences. A total of three strains of C. nymphaeae isolated from P. praelonga were included in the molecular study: ESALQ 1368, ESALQ 1393, ARSEF 4360; however, only the last two, for which multiple loci were obtained, were included in the combined phylogenetic analysis. The taxa and isolates used in the study are shown in Table 2.
Host information, geographical origin and ITS, ACT, CHS-1, GADPH and TUB2 GenBank accession numbers of the taxa/strains included in the molecular study. Types are designated by an asterisk. Insect hosts are underlined.
The individual ITS, GDPH, CHS-1, ACT, TUB2 matrices were exported in NEXUS format and converted to a combined data file in PAUP* v. 4.0.10b. The individual datasets were analyzed by maximum parsimony using a heuristic search with random addition sequence, TBR swapping and 1,000 heuristic replicates, saving no more than ten best trees per replicate, followed by a final search of saved trees. The phylograms of each dataset were visually inspected for topological congruence and then combined into a single dataset. The combined dataset was bootstrapped (2,000 replicates) using the same maximum parsimony search parameters. Following Damm et al. (2012)Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
, C. orchidophilum was used as the outgroup for the C. acutatum complex. A final phylogram with added bootstrap values was prepared using the Adobe Illustrator Professional. The combined dataset was deposited in TreeBase and can be accessed at http://purl.org/phylo/treebase/phylows/study/TB2:S18116
Results
Molecular study
The combined ITS+GDPH+CHS-1+ACT+TUB2 dataset included 1,661 characters of which 1,233 were constant, 50 were variable but not parsimony-informative, and 378 were parsimony-informative. A total of 600 equally most-parsimonious trees of 432 steps were recovered: a phylogram of one of these trees is shown in Figure 1. Isolates from the citrus scale insect are in a well-supported C. nymphaeae clade [97 % bootstrap support (bs)] (Figure 1). The combined dataset recovered two major clades for C. nymphaeae with respective bootstrap support values of 86 % and 89 %. The citrus scale insect isolates are in the same clade as isolate CBS 515.78, the type of C. nymphaeae designated as C. nymphaeae var. nymphaeae in Figure 1. In the beta-tubulin dataset, a single nucleotide mutation was present in all four entomopathogenic isolates of C. nymphaeae: this mutation is shared only by one other species, C. australe, which is also in the C. acutatum complex, but distantly related to C. nymphaeae (Table 3; Figure 1). Isolates of C. nymphaeae from the citrus scale insect differed from all other C. nymphaeae isolates by two nucleotide changes in the CHS-1 gene (Table 4). Insect pathogenic C. nymphaeae, colloquially referred to as the salmão fungus is described herein as a new variety of C. nymphaeae.
One of 9,139 equally most-parsimonious phylograms from a maximum parsimony analysis of ITS + GADPH + CHS-1 + ACT + TUB2 combined sequence data from 73 isolates within the Colletotrichum acutatum species complex. Thickened branches lead to nodes with bootstrap support values ≥ 70 % (2000 replicates).
Beta-tubulin gene sequence segment showing single nucleotide mutation present in all four isolates of C. nymphaeae var. entomophilum and shared by only one other taxon (C. australe) within the C. acutatum complex.
CHS-1 gene nucleotide differences between C. nymphaeae var. nymphaeae and C. numphaeae var. entomophilum.
Taxonomy
Collectrichum nymphaeae var. entomophilum A.A. Wynns & I. Delalibera, var. nov.
MycoBank 823559 Figure 2A-H
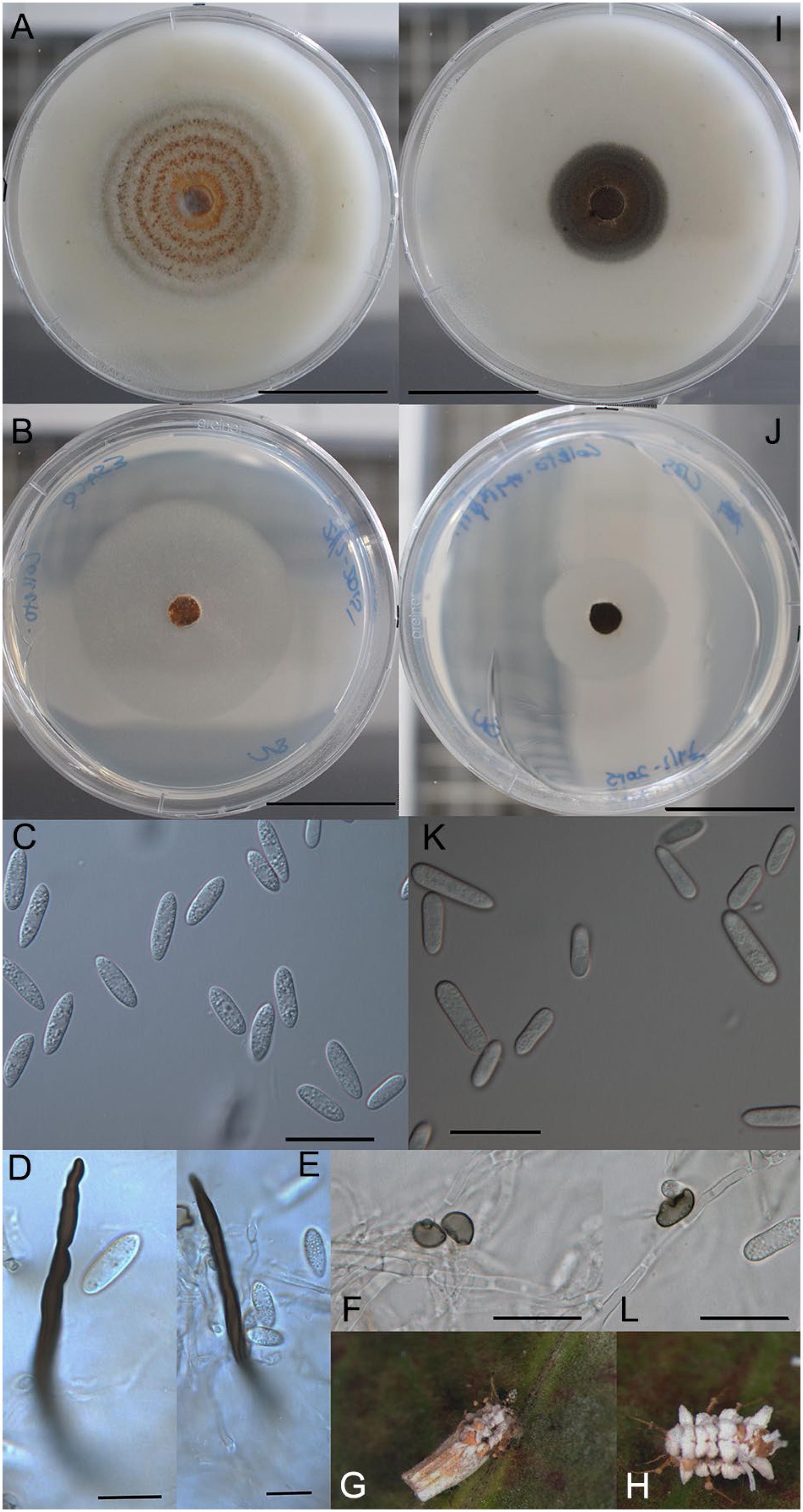
A - H) Colletotrichum nymphaeae var. entomophilum (ESALQ 1393). A) culture on OA after eight days. B) culture on SNA after seven days. C) conidia. D - E) setae. F) appressorium. G - H) infected, sporulating, Praelongortheziidae praelonga cadavers. I - L) Plant pathogenic C. nymphaeae var. nymphaeae (CBS 515.78). I) culture on OA after seven days. J) culture on SNA after seven days. K) conidia. L) appressoria. Scale bars: A-J = 20 μm; C-F, K-L = 10 μm.
= C. gloeosporioides f. sp. ortheziidae (Marcelino et al., 2008Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. 2008. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353-374.)
Etymology: The varietal epithet, which means insect loving, indicates its predilection for insects.
Type: Limeira, state of São Paulo, isolated from a Praelongorthezia praelonga (Hemiptera: Ortheziidae) cadaver, 8 Feb 2007, Silvio Baggio s.n. Holotype, ESA 142963 permanently preserved in a metabolically inactive state as a dried culture in microscope slides of ESALQ 1393-H deposited in the herbarium of Escola Superior de Agricultura “Luiz de Queiroz” from the Universidade de São Paulo (USP/ESALQ/ESA - http://splink.cria.org.br/manager/detail?setlang=pt&resource=ESA).
Ex-holotype cultures are stored in lyophilized inactive state as ESALQ 1393-H in the Collection of Entomopathogenic Fungi, Laboratório de Patologia e Controle Microbiano, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo (USP/ESALQ), Piracicaba, state of São Paulo, and as CG1414 in the Invertebrate-Associated Fungal Collection of Embrapa (CG), Embrapa Genetic Resources and Biotechnology, DF-70770-970, Brazil (http://www.wfcc.info/ccinfo/collection/by_id/712).
Colonies on OA 10.5 cm at seven days, a low cottony mycelium with concentric alternating white, salmon and grey rings (Figure 2A); salmon color from numerous small acervuli oozing conidia; culture in reverse pale greyish-brown with a greyish-salmon center. On SNA 9 cm at seven days, mycelium uniform, low, white, opaque (Figure 2B); colorless in reverse; acervuli few, colorless. Vegetative hyphae 1.1-8.0 μm, average 3.2 μm. Conidiomata acervular. Conidiogenous cells hyaline, 4.3 – 18.2 × 2.2 – 4.1 μm, ave. 11.3 × 3.2 μm. Conidia hyaline, pale orange in mass, 1-celled, 6.4 – 16.0 × 3.2 – 5.4 μm, ave. 10.9 × 4.2 μm (n = 41), L/W ratio 2.6, cylindrical to ellipsoidal with ends broadly or acutely rounded (Figure 2C). Appressoria reniform, brown, smooth-walled, margin entire, 5.9 – 9.8 × 3.9 – 7.7 μm (Figure 2F). Setae on OA rare, brown, thick-walled, irregular margin, tip acute, 68.5 – 73.1 × 3.4 – 3.7 μm (Figure 2D and E).
Host: Praelongorthezia praelonga. Figure 2G-H.
Diagnosis: Collectrichum nymphaeae var. entomophilum is distinguished morphologically from C. nymphaeae var. nymphaeae by its smaller conidia 6.4 – 16 × 3.2 – 5.4 μm, ave. 10.9 × 4.2 μm and the presence of setae (Table 5). The average length and width of conidia reported for C. nymphaeae is 16.1 × 4.9 μm with the exception of one strain, CBS 526.77, which was reported as having conidia measuring 9 – 13 × 3 – 4.5 μm (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
). In addition to morphological differences, C. nymphaeae var. entomophilum is distinct from all other isolates of C. nymphaeae by a single nucleotide mutation in the beta-tubulin gene (Table 3), two nucleotide mutations in the CHS-1 gene (Table 4) and by its insect, rather than plant host preference.
Colony characteristics on oatmeal agar (OA) and synthetic nutrient-poor agar medium (SNA) at seven days and morphological measurements of SNA cultures for C. nymphaeae var. entomophilum and C. nymphaeae var. nymphaeae. Information not provided is indicated by a dash (-).
Additional notes. Colletotrichum nymphaeae var. entomophilum more closely matches the description of Gloeosporium (Colletotrichum) nymphaeae Hemmi and Kawase, isolated from leaves of the Nymphaea in Japan (Hemmi and Kawase, 1954Hemmi, T.; Kawase, Y. 1954. On a new anthracnose of water lily caused by Gloeosporium nymphaeae sp. Bulletin of Naniwa University 4: 1-6.), than the description of C. nymphaeae sensuDamm et al. (2012)Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
. Hemmi and Kawase report dark brown thick-walled setae, 19 – 76 μm long and conidia ranging from 9 – 17 × 3 – 6 μm. Unfortunately, the specimens cited by Hemmi and Kawasi (1954)Hemmi, T.; Kawase, Y. 1954. On a new anthracnose of water lily caused by Gloeosporium nymphaeae sp. Bulletin of Naniwa University 4: 1-6. cannot be found and no living cultures exist. According to Damm et al. (2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
), C. nymphaeae as currently circumscribed is probably not the same organism described by Hemmi and Kawasi primarily because setae have never been observed in C. nymphaeae, neither by Damm et al. (2012)Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
nor by van der Aa (1978)van der Aa, H.A. 1978. A leaf spot of Nymphaea alba in the Netherlands. Netherlands Journal of Plant Pathology 84: 109-115.; however, Batista and Bezerra (1966)Batista, A.C.; Bezerra J.L. 1966. On the parasitism of Colletotrichum gloeosporioides and other fungi in Orthezia praelonga Douglas. Brotéria 35: 68-74 (in Portuguese, with abstract in English) described abundant setae in citrus orthezia insects infected by a “special strain” of C. gloeosporioides. Batista and Bezerra (1966)Batista, A.C.; Bezerra J.L. 1966. On the parasitism of Colletotrichum gloeosporioides and other fungi in Orthezia praelonga Douglas. Brotéria 35: 68-74 (in Portuguese, with abstract in English) did not cite specimens in their study; however, the special strain they discovered and reported for the first time was likely C. nymphaeae var. entomophilum.
Additional specimens were examined at Cordeirópolis, São Paulo, 13 July 2005, L.F. Padulla s.n., ESALQ 1368, and Rio de Janeiro, state of Rio de Janeiro, Feb 1994, C.F. Robbs ARSEF 4360.
Colletotrichum nymphaeae var. nymphaeae
(Pass.) Aa, Netherlands Journal of Plant Pathology, Supplement 1 84 (3): 110, Fig. 20. 1978; ≡Ascochyta nymphaeae Pass., Hedwigia 16:120, 1877. Lectotype, Italy, Parma, from the leaf of Nymphaea alba (Nymphaeaceae), summer 1875, G. Passerini, 176820 (K, non vidi); Epitype, Netherlands, Ubbergen, Oude Waal near Nijmegen, from the leaf spots of Nymphaeae alba, 7 Aug 1978, G. van der Velde (CBS H-20787), ex-epitype culture CBS 515.78! = van der Aa No. 657. Figure 2I-L
Colonies on OA 3.6 cm in seven days, a low felty mycelium with white, floccose center (Figure 2I-J); acervuli salmon colored; culture in reverse dark brownish-grey. On SNA 5.0 mm in seven days, flat with a diffuse but well-defined irregular margin, hyaline to whitish; acervuli scattered, colorless. Vegetative hyphae 1.1 – 3.2 μm, septate, branched. Conidiogenous cells hyaline, 13.3 – 20.9 × 3.2 – 3.8 μm, ave. 17.1 × 3.6 μm. Conidia hyaline, pale orange in mass, 1-celled, 12.3 – 24 (-32.7) × 3.7 – 6.9 μm, ave. 17.2 × 5.0 μm (n = 31), L/W ratio 3.4, cylindrical, frequently narrowly obovate (Figure 2K). Appressoria reniform to elongate with crenulate margins, brown, 7.6 – 10.5 × 4.8 – 9.8 μm (Figure 2L). Setae not observed.
Discussion
Delineating taxa and resolving the relationships within Colletotrichum is a challenge because of high sequence homogeneity and morphological uniformity within the genus. For this reason, multiple genes are required not just for elucidating the relationships between taxa but also for species identification. Using DNA sequence data from five gene regions: GDPH+TUB2+ITS+ACT+CHS-1, we found strong support for including the insect pathogenic isolates of C. nymphaeae (formerly C. gloeosporioides f. sp. ortheziidae in the species C. nymphaeae (97 % bs) (Figure 1). On the basis of morphological and molecular characters we recognize the insect pathogenic isolates of C. nymphaeae as a new variety and designate the name C. nymphaeae var. entomophilum. A single nucleotide mutation in the GADPH gene, two mutations in CHS-1, and the presence of setae readily distinguishes this new variety from its closest relatives. Once additional informative markers are found for Colletotrichum, it may be possible to better resolve the relationships within C. nymphaeae and determine if C. nymphaeae var. entomophilum should continue to be recognized as a variety or as a distinct species. Until then, the circumscription of C. nymphaeae sensuDamm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
, should be modified to include setae. Setae are present in C. nymphaeae var. entomophilum but have so far not been observed in plant symbiont isolates of C. nymphaeae. In order to clarify their taxonomic utility, further studies should be conducted to confirm the presence of setae in situ and to elucidate any effects that sub-culturing or other environmental conditions might have on the production of setae in vitro.
Both entomopathogenic Colletotrichum taxa, C. nymphaeae var. entomophilum and C. fiorniae, display remarkably diverse lifestyle strategies with the ability to live as insect pathogens, plant pathogens and endophytes. At least 50 phytopathogenic isolates of C. fiorinae have been identified by DNA sequencing (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
). Additionally, C. fiorinae has been isolated from fruit rot and detected as an endophyte in 28 species of plants in the region of epizootic outbreaks on the hemlock scale insect (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
; Marcelino et al., 2009Marcelino, J.A.; Gouli, S.; Parker, B.L.; Skinner, M.; Schwarzberg, L.; Giordano, R. 2009. Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. Journal of Insect Science 9: 25. DOI: 10.1673/031.009.2501
https://doi.org/10.1673/031.009.2501...
). Colletotrichum nymphaeae var. entomophilum has also been recovered as an endophyte but only in plants inoculated with the fungus (Marcelino et al., 2009Marcelino, J.A.; Gouli, S.; Parker, B.L.; Skinner, M.; Schwarzberg, L.; Giordano, R. 2009. Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa. Journal of Insect Science 9: 25. DOI: 10.1673/031.009.2501
https://doi.org/10.1673/031.009.2501...
). The occurrence of C. nymphaeae var. entomophilum in nature, either as an endophyte or on additional insect hosts has not been studied. This is an area of research that merits prompt attention given that endophytic entomopathogens may serve as defensive mutualists when living in plant tissue (Bultman and Faeth, 2002Bultman, T.L.; Faeth, S.H. 2002. Endophytic fungi and interactions among host plants, herbivores, and natural enemies. p. 89-123. In: Tscharntke, T.; Hawkins, B.A., eds. Multitrophic level interactions. Cambridge University Press, Cambridge, England.; Crouch et al., 2014Crouch, J.; O'Connell, R.; Gan, P.; Buiate, E.; Torres, M.F.; Beirn, L.; Shirasu, K.; Vaillancourt, L. 2014. The genomics of Colletotrichum. p. 69-102. In: Dean, R.A.; Lichens-Park, A.; Kole, C., eds. Genomics of plant-associated fungi: monocot pathogens. Springer, Heidelberg, Germany. DOI: 10.1007/978-3-662-44053-7_3
https://doi.org/10.1007/978-3-662-44053-...
; Redman et al., 2002Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. 2002. Thermotolerance generated by plant/fungal symbiosis. Science 298: 1581. DOI: 10.1126/science.1078055
https://doi.org/10.1126/science.1078055...
). For example, if C. nymphaeae var. entomophilum occurs endophytically in nature, it may potentially play a larger role in controlling citrus scale and other sap-sucking insects than previously realized.
The significance of the varied lifestyle strategies of C. fiorniae and C. nymphaeae var. entomophilum has previously been downplayed because the insect hosts of these fungi are plant sucking insects (Damm et al., 2012Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
https://doi.org/10.3114/sim0010...
). A close relationship between insect pathogenic fungi and grass endosymbionts has previously been shown (Spatafora et al., 2007Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; White Jr., J.F. 2007. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Molecular Ecology 16: 1701-1711.). Comparative genomic analyses showed that insect pathogenic Metarhizium spp. are more closely related to endophytes and plant pathogens compared to animal pathogens (Gao et al., 2011Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G.; Peng, G. 2011. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. Plos Genetics 7: e1001264. DOI: 10.1371/journal.pgen.1001264
https://doi.org/10.1371/journal.pgen.100...
). The evolutionary transition from plant pathogen to endophyte in Colletotrichum is thought to occur relatively easily; for example, in at least one species of Colletotrichum, a single-locus mutation converts the fungus from a symptomatic plant pathogen to an endophyte. However, transitioning from plant pathogen or endophyte to a pathogen of plant sucking insects may represent a more significant and complex physiological shift than is assumed with genes co-opted, evolved or acquired by horizontal gene transfer from a plant-associated fungus (Barelli et al., 2016Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. 2016. Fungi with multifunctional lifestyles: endophytic insect pathogenic fungi. Plant Molecular Biology 90: 657-664.). Gene expression in Colletotrichum is highly dependent on plant signaling (Crouch et al., 2014Crouch, J.; O'Connell, R.; Gan, P.; Buiate, E.; Torres, M.F.; Beirn, L.; Shirasu, K.; Vaillancourt, L. 2014. The genomics of Colletotrichum. p. 69-102. In: Dean, R.A.; Lichens-Park, A.; Kole, C., eds. Genomics of plant-associated fungi: monocot pathogens. Springer, Heidelberg, Germany. DOI: 10.1007/978-3-662-44053-7_3
https://doi.org/10.1007/978-3-662-44053-...
; O'Connell et al., 2012O'Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; Altmüller, J. 2012. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44: 1060-1065.). In vitro signaling has been shown to be markedly different from in planta signaling even from the point of spore germination to the ultimate necrotrophic phase characteristic of plant pathogens (O'Connell et al., 2012O'Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; Altmüller, J. 2012. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44: 1060-1065.). Transcriptomic studies comparing gene expression of the entomopathogenic Colletotrichum taxa in planta, in insecta and in vitro may provide insight into the mechanisms behind inter-kingdom host shifts or the potential of a dual life style as insect pathogens and endophytes; in turn, these insights may help to determine how much or little weight should be given to host preferences for delimiting Colletotrichum taxa.
Much remains to be known about C. nymphaeae var. entomophilum. For example, how it is dispersed, its insect host-range, if it occurs endophytically both in nature and in citrus orchards and if endophytic growth affects the fitness of the citrus scale insect. Improved development of this important natural enemy should include studies on the below ground control of Ortheziidae scale insects since these insects also live in soil litter in humid habitats and feed on fungi, mosses and plant roots (Kondo et al., 2013Kondo, T.; Peronti, A.L.; Kozár, F.; Szita, É. 2013. The citrus orthezia, Praelongorthezia praelonga (Douglas) (Hemiptera: Ortheziidae), a potential invasive species. p. 301-319. In: CABI, Wallingford, UK. (CABI Invasive Species Series, 3).). Entomopathogenic endophytes are well-known among the fungi, especially from the order Hypocreales e.g., Acremonium, Beauveria, Clonostachys, Isaria, Lecanicillium, Verticillium (Vega, 2008Vega, F.E. 2008. Insect pathology and fungal endophytes. Journal of Invertebrate Pathology 98: 277-279. DOI: 10.1016/j.jip.2008.01.008
https://doi.org/10.1016/j.jip.2008.01.00...
; Vega et al., 2008Vega, F.E.; Posada, F.; Catherine Aime, M.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. 2008. Entomopathogenic fungal endophytes. Biological Control 46: 72-82. DOI: 10.1016/j.biocontrol.2008.01.008
https://doi.org/10.1016/j.biocontrol.200...
); however, few are aware that the common plant pathogenic genus Colletotrichum (Glomerellales) also contains endophytic entomopathogens. Clarifying the taxonomy of C. nymphaeae var. entomophilum may provide the impetus for further research on this overlooked entomopathogenic fungus. The assignment of a formal name along with morphological and molecular characterization provides a solid framework for facilitating the evaluation and approval of this important fungus for biological control.
Acknowledgments
This study is a part of the IMBICONT research project (Improved Biological Control for IPM in Fruits and Berries) (project number 1024151001) funded by the Innovation fund, Denmark.
References
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. 2016. Fungi with multifunctional lifestyles: endophytic insect pathogenic fungi. Plant Molecular Biology 90: 657-664.
- Batista, A.C.; Bezerra J.L. 1966. On the parasitism of Colletotrichum gloeosporioides and other fungi in Orthezia praelonga Douglas. Brotéria 35: 68-74 (in Portuguese, with abstract in English)
- Bultman, T.L.; Faeth, S.H. 2002. Endophytic fungi and interactions among host plants, herbivores, and natural enemies. p. 89-123. In: Tscharntke, T.; Hawkins, B.A., eds. Multitrophic level interactions. Cambridge University Press, Cambridge, England.
- Carbone, I.; Kohn, L.M. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553-556. DOI: 10.2307/3761358
» https://doi.org/10.2307/3761358 - Crouch, J.; O'Connell, R.; Gan, P.; Buiate, E.; Torres, M.F.; Beirn, L.; Shirasu, K.; Vaillancourt, L. 2014. The genomics of Colletotrichum p. 69-102. In: Dean, R.A.; Lichens-Park, A.; Kole, C., eds. Genomics of plant-associated fungi: monocot pathogens. Springer, Heidelberg, Germany. DOI: 10.1007/978-3-662-44053-7_3
» https://doi.org/10.1007/978-3-662-44053-7_3 - Crous, P.W.; Verkley, G.J.M.; Groenewald, J.Z.; Samson, R.A. 2009. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands. (Fungal Biodiversity 269. CBS Laboratory Manual Series 1).
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37-113. DOI: 10.3114/sim0010
» https://doi.org/10.3114/sim0010 - Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G.; Peng, G. 2011. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum Plos Genetics 7: e1001264. DOI: 10.1371/journal.pgen.1001264
» https://doi.org/10.1371/journal.pgen.1001264 - Garcia-Roa, F.A. 1995. Management of Orthezia praelonga, A Citrus Pest = Manejo de Orthezia praelonga, una Plaga de los Cítricos. CO-BAC, Santafé de Bogotá, Bogotá, Colombia (in Spanish).
- Gardes, M.; Bruns, T.D. 1993. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113-118. DOI: 10.1111/j.1365-294X.1993.tb00005.x
» https://doi.org/10.1111/j.1365-294X.1993.tb00005.x - Hanlin, R.T. 1998. Combined Keys to Illustrated Genera of Ascomycetes. APS Press, St. Paul, MN, USA.
- Hemmi, T.; Kawase, Y. 1954. On a new anthracnose of water lily caused by Gloeosporium nymphaeae sp. Bulletin of Naniwa University 4: 1-6.
- Kondo, T.; Peronti, A.L.; Kozár, F.; Szita, É. 2013. The citrus orthezia, Praelongorthezia praelonga (Douglas) (Hemiptera: Ortheziidae), a potential invasive species. p. 301-319. In: CABI, Wallingford, UK. (CABI Invasive Species Series, 3).
- MacKenzie, S.J.; Peres, N.A.; Barquero, M.P.; Arauz, L.F.; Timmer, L.W. 2009. Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 99: 620-631. DOI: 10.1094/phyto-99-5-0620
» https://doi.org/10.1094/phyto-99-5-0620 - Manamgoda, D.; Udayanga, D.; Cai, L.; Chukeatirote, E.; Hyde, K. 2013. Endophytic Colletotrichum from tropical grasses with a new species C. endophytica Fungal Diversity 61:107-115. DOI: 10.1007/s13225-013-0256-3
» https://doi.org/10.1007/s13225-013-0256-3 - Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. 2008. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353-374.
- Marcelino, J.A.; Gouli, S.; Parker, B.L.; Skinner, M.; Schwarzberg, L.; Giordano, R. 2009. Host plant associations of an entomopathogenic variety of the fungus, Colletotrichum acutatum, recovered from the elongate hemlock scale, Fiorinia externa Journal of Insect Science 9: 25. DOI: 10.1673/031.009.2501
» https://doi.org/10.1673/031.009.2501 - Mascarin, G.M.; Guarín-Molina, J.H.; Arthurs, S.P.; Humber, R.A.; Andrade, R.M.; Demétrio, C.G.B.; Delalibera, I. 2016. Seasonal prevalence of the insect pathogenic fungus Colletotrichum nymphaeae in Brazilian citrus groves under different chemical pesticide regimes. Fungal Ecology 22: 43-45.
- Nirenberg, H. 1976. Studies on morphological and biological differentiation in the Liseola section of the genus Fusarium = Untersuchungen Über die morphologische und biologische differenzierung in der Fusarium-section Liseola. Mitteilungen, Biologische Bundesanstalt fur Land und Forstwirtschaft 169: 1-117 (in German).
- O'Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; Altmüller, J. 2012. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44: 1060-1065.
- Photita, W.; Taylor, P.W.J.; Ford, R.; Hyde, K.D.; Lumyong, S. 2005. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Diversity 18: 117-133.
- Ramos, A.S.; Lemos, R.N.; Costa, V.A.; Peronti, A.L.; Silva, E.A.; Mondego, J.M.; Moreira, A.A. 2018. Hymenopteran parasitoids associated with scale insects (Hemiptera: Coccoidea) in tropical fruit trees in the eastern Amazon, Brazil. Florida Entomologist 101: 273-278.
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. 2002. Thermotolerance generated by plant/fungal symbiosis. Science 298: 1581. DOI: 10.1126/science.1078055
» https://doi.org/10.1126/science.1078055 - Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; White Jr., J.F. 2007. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Molecular Ecology 16: 1701-1711.
- Tamura, K.; Peterson, D.; Peterson, N.; Stetcher, G.; Nei, M.; Kumar, S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731-2739.
- Teixeira, M.A.; Bettiol, W.; Cesnik, R. 2001. Pathogenicity of Colletotrichum gloeosporioides, Orthezia praelonga pathogenicity agent, to citrus leaves, flowers and fruits = Patogenicidade de Colletotrichum gloeosporioides, agente de patogenicidade da Orthezia praelonga, para folhas, flores e frutos cítricos. Summa Phytopathologica 27: 352-357 (in Portuguese).
- Teixeira, M.A.; Bettiol, W.; Cesnik, R.; Vieira, R.F. 2004. Pathogenicity of the fungus Colletotrichum gloeosporioides, pathogen of Orthezia praelonga, to several fruits and to pumpkin seedlings. Revista Brasileira de Fruticultura 26: 356-358 (in Portuguese, with abstract in English).
- Thiers, B. 2018. Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. Available at: http://sweetgum.nybg.org/science/ih/ [Accessed Nov 7, 2018]
» http://sweetgum.nybg.org/science/ih/ - van der Aa, H.A. 1978. A leaf spot of Nymphaea alba in the Netherlands. Netherlands Journal of Plant Pathology 84: 109-115.
- Vega, F.E. 2008. Insect pathology and fungal endophytes. Journal of Invertebrate Pathology 98: 277-279. DOI: 10.1016/j.jip.2008.01.008
» https://doi.org/10.1016/j.jip.2008.01.008 - Vega, F.E.; Posada, F.; Catherine Aime, M.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. 2008. Entomopathogenic fungal endophytes. Biological Control 46: 72-82. DOI: 10.1016/j.biocontrol.2008.01.008
» https://doi.org/10.1016/j.biocontrol.2008.01.008 - White, T.J.; Bruns, T.; Lee, S.; Taylor. J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. p. 315-322. In: Innis, M.A.; Gelfand, D.H.; Sninksy, J.J.; White, T.J., eds. PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA, USA.
Edited by
Publication Dates
-
Publication in this collection
20 Dec 2019 -
Date of issue
2020
History
-
Received
20 Aug 2018 -
Accepted
30 Apr 2019