Abstract
Cobalt ferrite composite thin films, CoFe2O4/SiO2, were prepared by sol-gel process, using tetraethylorthosilicate (TEOS) as a precursor for silica, and metallic nitrates as precursors for ferrite. The obtained dip-coated films thermally treated at 550, 750, and 950 ºC were transparent, homogeneous and adherent. Their magnetic properties were measured from 2.5 K to 300 K using a SQUID magnetometer. Superparamagnetic behaviour was observed at room temperature and below a blocking temperature appears with coercivity values increasing with annealing temperature.
Magnetic thin films; Sol-gel process; Magnetic nanocomposite
SURFACES, INTERFACES, AND THIN FILMS
Magnetic nanocomposite thin films prepared by sol-gel process
Nelcy D. S. MohallemI; Luciana M. SearaI; Miguel A. NovakII; Elis H. C. P. SinneckerII
ILaboratório de Materiais Nanoestruturados, DQ, UFMG, 31270-901, Belo Horizonte - MG, Brazil
IIInstituto de Física, UFRJ Rio de Janeiro, RJ, Brazil
ABSTRACT
Cobalt ferrite composite thin films, CoFe2O4/SiO2, were prepared by sol-gel process, using tetraethylorthosilicate (TEOS) as a precursor for silica, and metallic nitrates as precursors for ferrite. The obtained dip-coated films thermally treated at 550, 750, and 950 ºC were transparent, homogeneous and adherent. Their magnetic properties were measured from 2.5 K to 300 K using a SQUID magnetometer. Superparamagnetic behaviour was observed at room temperature and below a blocking temperature appears with coercivity values increasing with annealing temperature.
Keywords: Magnetic thin films; Sol-gel process; Magnetic nanocomposite
I. INTRODUCTION
Nanocomposite materials formed by metallic or oxide particles dispersed in polymer, ceramic or vitreous matrices [1] have important application in areas such as catalysis and electronics [2]. An interesting class of nanocomposite materials is formed by nanometer sized magnetic particles dispersed in insulating matrix [3]. These nanocrystalline particles have a high surface/volume ratio, leading to magnetic properties different from those of bulk materials. Such properties are also highly dependent on the particle size distribution as well as on the aggregation of particles when compared to different production methods.
Sol-gel process has proved to be an efficient method to prepare ultra-fine particles dispersed in different matrices and, particularly, to produce thin film. Through this method, a good control of the sample morphology, texture, structure, and chemical composition can be attained by carefully monitoring the preparation parameters [4-8]. The use of an inorganic matrix allows narrow dispersion of particle size, and homogeneous distribution.
In this report, we show the morphological, structural and magnetic studies of CoFe2O4/SiO2 thin films prepared by sol- gel process as a function of different thermal treatment temperature.
II. EXPERIMENTAL
CoFe2O4/SiO2 nanocomposite thin films were prepared by sol- gel process using a precursor solution obtained from the mixture of tetraethylorthosilicate (TEOS; Merck), ethyl alcohol, water, Co(NO3)2.6H2O and Fe(NO3)2.9H2O (Carlo Erba). The films were prepared with 30 wt.% of ferrite dispersed in the silica matrix. The precursor solution was stirred for 1h for homogenization and rested until the viscosity reached the best value condition (2-5 cp) for the dip-coating process. The films were prepared onto clean quartz substrates using withdrawal speed of 0.6 mm/s. After the coating process, the samples were dried in air at ambient temperature ( ~ 25ºC) for 24h, dried at 80ºC for 1h and finally treated at 550, 750, and 950ºC for 10 minutes.
Thicknesses were measured by profilometry with a Alphastep 100 profilometer, Tencor Instruments, with an estimated 6% uncertainty. The transparency of the films was determined by optical transmission spectra measured with an ultraviolet and visible spectrometer (U3010, Hitachi).
Film porosity and topography were investigated by atomic force microscopy (LNLS) using a Nanoscope III from Digital Instruments, equipped with an extended modulus for phase imaging. The images were generated through the intermittent contact mode, using a silicon probe tip with 5 nm of curvature radius.
The evolution of the crystallinity structure and nanoparticule size with the thermal treatment temperature were observed by low angle X-ray diffractometry (incidence angle of 5º) at the D12A-XRD1 beam of the Laboratorio Nacional de Luz Sincrotron using radiation of 1.5424 Å.
Magnetization measurements of the composite films as function of field and temperature were performed using a Cryogenic SX-600 SQUID magnetometer between 2 and 300K, up to 6T.
III. RESULTS AND DISCUSSION
The films obtained were transparent, adherent, and free of cracks. All samples presented layers with thickness (500 ± 40) nm when treated at 550 ºC and (400 ± 30) nm when treated at 950 ºC. The optical transmission of the films in the visible region increases with increasing treatment temperature showing maximum transmittance at 900nm of 84% for the film treated at 550 ºC and 91% for the one treated at 950 ºC. At 500 nm the transparency decreases due to the film absorption in this range.
The X-ray peaks of the composite film (shown in Fig. 1) are in accordance with those of a typical crystalline Co ferrite, with spinel structure without a preferred orientation. The peak intensities increase with increasing the thermal treatment temperature. No peaks associated to quartz, crystobalite or intermediary products were found. The crystallite sizes of the ferrite dispersed in the matrix were estimated from Debye-Scherrer's formula and were found to be between (5±2) and (20±5) nm for samples heated between 550 and 950 ºC.
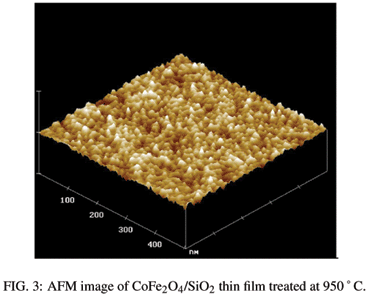
AFM analysis showed characteristic images of the films treated at 550 ºC (Figure 2) with porous surfaces typical of xerogel structures. With the increase in the thermal treatment temperature particles with nearly spherical shape (see Fig. 3) became apparent. It is not possible to distinguish the ferrite particles from the silica matrix, but we believe that the silica encapsulated the ferrite nanoparticles. The particle size increased with the increase in the heat treatment temperature while the rugosity decreased.
Magnetization measurements done in the plane direction of the films showed typical superparamagnetic behavior at room temperature for the samples treated at 750 ºC (see Fig. 4) and 950 ºC, and almost no signal for the sample treated at 550 ºC. This indicates that the ferrite nanoparticles are formed above this temperature. At low temperature hysteresis develops below 100K becoming quite wide at 10K with coercivity of 1.2T. This behavior can be associated to the blocking of the superparamagnetic particle dynamics. In fact, zero field cooled (ZFC) as well as field cooled (FC) magnetization measurement were made as a function of temperature showing a maximum at 67K for the sample treated at 750 ºC (see Fig. 5). This maximum increased to 210K for the sample treated at 950 ºC (not shown), which is in agreement with the coarsening of the magnetic nanoparticle sizes.
IV. CONCLUSION
Transparent, homogeneous and adherent thin films formed by crystalline nanoparticle cobalt ferrite dispersed in silica matrix were successfully produced by sol-gel process. Thermal treatments were carried out at temperatures as high as 950ºC, without cracking, allowed to modify the surface morphology. The thickness of the films varied from 500 to 400 nm with higher treating temperatures. The atomic force microscopy showed that the films presented a porous homogeneous topography, changing to nanoparticulate morphology with heat treatment above 550 ºC, presenting Co ferrite particle sizes in the order of 20 nm at 950 ºC. The particle sizes increase with higher temperature heat treatment. Superparamagnetic behavior was observed at room temperature as well as hysteresis due to blocking of the magnetic dynamics at low temperature. We believe that this system allows the control growth of silica coated magnetic nanoparticles with different sizes and distances among them. Further studies in these systems are in progress.
Acknowledgements
This work was supported by CNPq and LNLS (AFM and low angle XRD analyses).
Received on 8 December, 2005
- [1] G. Ennas, A. Mei, A. Musinu, G. Piccaluga, G. Pinna, and S. Solinas, J. of Non Cryst. Solids 232-234, 587 (1998).
- [2] C. Estournčs, T. Lutz, J. Happich, T. Quaranta, P. Wissler, and J. L. Guille, J. Magn. Magn. Mater. 173, 83 (1997).
- [3] L. Armelao, G. Granozzi, E. Tondello, P. Colombo, G. Principi, P. P. Lottici, and G. Antonioli, J. Non-Cryst. Solids 192&193, 435 (1995).
- [4] L. Zhang, G. C. Papaefthymiou, R. F. Ziolo, and J. Y. Ying, Nanostructured Materials 9, 185 (1997).
- [5] C. Yan, F. Cheng, C. Liao, J. Kuang, Z. Xu, L. Chen, H. Zhao, Z. Liu, Y. Wang, T. Zhu, and G. He, J. Magn. Magn. Mater. 192, 396 (1999).
- [6] C. J. Brinker (Ed.), The Physics and Chemistry of Sol-Gel Processing, Academic Press, San Diego, 1990.
- [7] N. D. S. Mohallem, L. M. Seara, App. Surf. Sci. 214, 143 (2003).
Publication Dates
-
Publication in this collection
29 Nov 2006 -
Date of issue
Sept 2006
History
-
Received
08 Dec 2005