Abstract
Lead-free ceramics Ba(Zr xTi1-x)O3 (x = 0.02-0.2) were prepared using a solid-state reaction technique. The structural and electrical properties were systemically investigated. Crystalline structures and microstructures were analyzed by X-ray diffraction and scanning electron microscope (SEM) at room temperature. All the samples possess pure perovskite structure. A small amount of Zr content has great effect on the microstructure of Ba(Zr xTi1-x)O3 ceramics. The homogeneous microstructure with grain size about 30µm is obtained for the sample at x = 0.05. The phase transitions merge together in one peak for the samples at x = 0.10 and the highest dielectric constant 15900 is obtained for the sample at x = 0.15. The Ba(Zr xTi1-x)O3 ceramics at x=0.05 exhibit excellent piezoelectric properties of high d33 = 208 pC/N, k p = 31.5% and Qm = 500.
Ceramics; Microstructure; Dielectric properties; Piezoelectric properties
Dielectric and piezoelectric properties of Ba(ZrxTi1x)O3 lead-free ceramics
Wei Li* * Electronic address: liwei_727@yahoo.cn ; Zhijun Xu; Ruiqing Chu; Peng Fu; Guozhong Zang
College of Materials Science and Engineering, PlaceNameLiaocheng PlaceTypeUniversity, Liaocheng 252059, country-regionplaceChina
ABSTRACT
Lead-free ceramics Ba(ZrxTi1x)O3 (x = 0.02-0.2) were prepared using a solid-state reaction technique. The structural and electrical properties were systemically investigated. Crystalline structures and microstructures were analyzed by X-ray diffraction and scanning electron microscope (SEM) at room temperature. All the samples possess pure perovskite structure. A small amount of Zr content has great effect on the microstructure of Ba(ZrxTi1x)O3 ceramics. The homogeneous microstructure with grain size about 30µm is obtained for the sample at x = 0.05. The phase transitions merge together in one peak for the samples at x = 0.10 and the highest dielectric constant 15900 is obtained for the sample at x = 0.15. The Ba(ZrxTi1x)O3 ceramics at x=0.05 exhibit excellent piezoelectric properties of high d33 = 208 pC/N, kp = 31.5% and Qm = 500.
Keywords: Ceramics; Microstructure; Dielectric properties; Piezoelectric properties.
1. INTRODUCTION
Lead zirconate titanate (PZT) ceramics are the most widely used piezoelectric materials due to their superior piezoelectric properties close to the morphotropic phase boundary (MPB) between rhombohedral and tetragonal phases. Nevertheless, they are not environment friendly for lead oxide toxicity. With the recent growing demand of global environmental protection, many researchers have greatly focused on lead-free ceramics to replace the lead-based ceramics [1-3].
Barium titanate (BaTiO3) is one of the most widely studied lead-free piezoelectric material [4-10]. It is well known that BaTiO3, which is a typical ABO3 perovskite-type material, has five kinds of crystal systems: hexagonal, cubic, tetragonal, orthorhombic and rhombohedral, depending on the phase transition temperature: 1432 ºC, 130 ºC, 5 ºC and -90 ºC, respectively [5,6]. Donor-doping BaTiO3 solid solutions with ions (e.g., Ca2+, Sr2+, La3+, Zr4+ and Nb5+, etc.) have been and continue to be of interest for investigation, not only because of their various applications, but also for their interesting dielectric and ferroelectric behaviors [7-9]. In particular, compositionally modified BaZrxTi1xO3 (BZT) receives much attention due to the tunable structure and electrical properties to specific applications, because of Zr4+ is chemically more stable than Ti4+ [9]. The polymorphic phase transitions of BaZrxTi1xO3 (rhombohedral - orthorhombic T1, orthorhombic - tetragonal T2 and tetragonal - cubic Tc) move closer with increasing Zr content and merge near room temperature for the composition of x = 0.15. Further increase in Zr content, especially for x > 0.25, the samples show broad dielectric peaks with frequency dispersion, i.e., ferroelectric-relaxor behavior [10,11]. In the last few years, BZT ceramics have been used as a dielectric material in multi-layer ceramic capacitors (MLCC). The compositional and microstructure modification play important roles to meet the required dielectric constant and dielectric temperature characteristic, however, dependence of ferroelectric properties on Zr content has not been well understand [12,13]. In this work, the structure, dielectric properties and ferroelectric properties of the BaZrxTi1xO3 (x = 0.02-0.2) ceramics as a function of Zr content were systemically investigated.
2. EXPERIMENTAL
BaZrxTi1xO3 ceramics of x=0.02, 0.05, 0.07, 0.10, 0.15 and 0.20 were prepared by conventional solid-state reaction technique, respectively. Raw materials of BaCO3 (99.0%), ZrO2 (99.0%) and TiO2 (99.5%) were mixed with addition of alcohol, then dried and calcined at 1200 ºC for 4 h. Thereafter, they were remixed and pressed into 12mm-diam pellets and sintered at 1400 ºC for 5 h in air. The sample crystallization behavior was examined using an X-ray diffraction meter using a Cu Kα radiation (λ = 1.54178 Å ) (XRD, D8 Advance, Bruker Inc., Germany). The dielectric properties were measured by the precision impedance analyzer (4294 Agilent Inc., America) controlled by a computer at 100kHz with the testing temperature ranged from room temperature to 200 ºC. Ferroelectric hysteresis loops were measured at room temperature using an aix-ACCT TF2000FE-HV ferroelectric test unit (aix-ACCT Inc., Germany). The piezoelectric constant d33 was measured using a tester quasi-static d33 meter (YE2730 SINOCERA, China). The mechanical quality factor Qm and the planar electromechanical coupling factor kp were calculated following IEEE standards by using the impedance analyzer.
3. RESULTS AND DISCUSSION
Figure 1 shows the XRD patterns of the Ba(ZrxTi1x)O3 ceramics. As can be seen, all the samples show pure perovskite structure, suggesting that Zr diffuse into the BaTiO3 lattice to form a solid solution. Moreover, it is clearly seen that the diffraction peaks (220) at 66º shift significantly to low angle with increasing Zr content. Although the microscopic mechanism underlying this observation is currently unclear, the obvious shift and evolution of the splitting (202)/(220) peaks with increasing Zr content imply that Zr doping not only induces the lattice distortion but also changes the phase composition of Ba(ZrxTi1x)O3 ceramics. Due to the fact that the ionic radius of Zr4+ (0.86 Å ) is larger than that of Ti4+ (0.75 Å ), thus, the substitution of Ti4+ with Zr4+ could increase the lattice parameter of ceramics [14,15]. Fig. 2 shows the SEM micrographs of Ba(ZrxTi1x)O3 ceramics (x=0.02, 0.05 and 0.20). The microstructure of Ba(Zr0.02Ti0.98)O3 ceramics is inhomogeneous and some distinct pores exist in the grain boundary. For the sample at x=0.05, the microstructure is homogeneous and little pores exist in the grain boundary, while the grain size is about 30µm. It is well known that clear grain boundary and uniformly distributed grain size could enhance the mechanical strength of piezoelectric ceramics and be advantageous to the electric properties [16]. For the sample at x=0.20, the microstructure is inhomogeneous and some of the grain size become singularly large (50µm).
The dielectric constants as a function of temperature for the Ba(ZrxTi1x)O3 system measured at frequency of 100 kHz are shown in Fig. 3. As can be seen, two obvious phase transitions above 20ºC corresponding to the orthorhombic-tetragonal and tetragonal-cubic, respectively, are observed for the samples of x=0.02-0.07. The Tc shifts to lower temperature while T2 shifts to higher temperature with the increase of Zr content. This is the well-known pinching effect in these compositions [10]. With further increase of Zr content, at x = 0.10, the three phase transitions merge together in one broad peak. This result is different from the previous studies, i.e., the three phase transitions merge together at x=0.15 [13]. On the other hand, the dielectric constants of Ba(ZrxTi1x)O3 ceramics increase with increasing Zr content. The highest dielectric constant (15900) is obtained for the sample at x = 0.15.
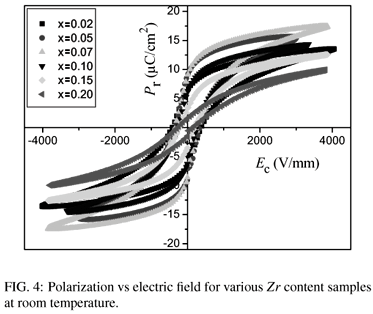
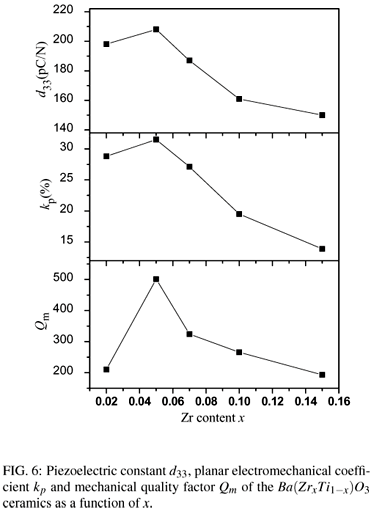
The hysteresis loops of polarization versus electric field are shown in Fig. 4. The remnant polarization Pr and the coercive fields Ec as a function of composition are shown in Fig. 5. It can be seen that the coercive field of the Ba(ZrxTi1x)O3 ceramics at x = 0.02 is 390V/mm, while the value decreases continuously with the increase of Zr content. The coercive fields of the Ba(ZrxTi1x)O3 ceramics at x = 0.05, x = 0.07, x = 0.10, x = 0.15 and x = 0.20 are 330V/mm, 260V/mm, 240V/mm, 180V/mm, and 140V/mm, respectively. With increasing Zr content, the remnant polarizations of the Ba(ZrxTi1x)O3 ceramics increase to a maximum value 9.0 µC/cm2 at x=0.05 and then decrease. Fig. 6 shows the piezoelectric coefficient d33, planar mode electromechanical coupling coefficient kp and mechanical quality factor Qm of Ba(ZrxTi1-x)O3 ceramics as a function of Zr content. At x=0.02, d33, kp and Qm are 198 pC/N, 28.8% and 210, respectively. With raising of x to 0.05, the d33, kp and Qm reach their maximum values of 208 pC/N, 31.5% and 500, respectively. The highest d33 value 208 pC/N of the Ba(ZrxTi1x)O3 ceramics could be attributed to the relative high Pr (9.0 µC/cm2) and low Ec(330V/mm) for the sample at x=0.05. The highest Qm (500) for the sample at x = 0.05, which is twice as large as other Ba(ZrxTi1x)O3 ceramic, is considered to be reasonably consistent with it's clear grain boundary and uniformly distributed grain size.
4. CONCLUSIONS
Lead-free Ba(ZrxTi1x)O3 (x = 0.02-0.2) ceramics prepared by solid-state reaction were systemically investigated. Results show that all the samples are pure perovskite structure. The phase transitions merge together for the samples at x = 0.10 and the highest dielectric constant 15900 is obtained for the sample at x = 0.15. The ceramics at x = 0.05 exhibite excellent piezoelectric properties of high d33 = 208 pC/N, kp = 31.5% and Qm=501.
Acknowledgments
This work was supported by the program for the National Natural Science Foundation of China (Grant No. 50602021 and Grant No. 50802038) and Research Foundation of Liaocheng University (No. X0810041).
[1] Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya, and M. Nakamura, Nature 432 84 (2004).
[2] E. Hollenstein, M. Davis, D. Damjanovic, and N. Setter, Appl. Phys. Lett. 87 182905 (2005).
[3] P. Kantha, K. Pengpat, P. Jarupoom, U. Intatha, G. Rujijanagul, and T. Tunkasiri, Curr. Appl. Phys. 9 460 (2009).
[4] Z. Yu, R.Y. Guo, and A. S. Bhalla, J. Appl. Phys. 88 410 (2000).
[5] X.S. Wang, L.L. Zhang, H.Liu, J.W. Zhai, X. Yao, Mater. Chem. Phys. 112 675 (2008).
[6] C.J. Xiao, C.Q. Jin, X.H. Wang, Mater. Chem. Phys. 111 209 (2008).
[7] P.Z. Zhang, M.R. Shen, L. Fang, F.G. Zheng, X.L. Wu, J.C. Shen, and H.T. Chen Appl. Phys. Lett. 92 222908 (2008).
[8] R.L. Brutchey, G.S. Cheng, Q. Gu, and D.E. Morse, Adv. Mater. 20 1029 (2008).
[9] T. Maiti, R. Guo, and A.S. Bhalla, Appl. Phys. Lett. 89 122909 (2006).
[10] P.S. Dobal, A. Dixit, and R.S. Katiyar, J. Appl. Phys. 89 8085 (2001).
[11] Z. Yu, R. Guo, A.S. Bhalla, J. Cryst. Growth 233 460 (2001).
[12] F. Zimmermann, M. Voigts,W. Menesklou, E. Ivers-Tiffe'e, J. Eur. Ceram. Soc. 24 1729 (2004).
[13] Z. Yu, C. Ang, R.Y. Guo, and A. S. Bhalla, J. Appl. Phys. 92 2655 (2002).
[14] B.L. Cheng, C.Wang, S.Y.Wang, H. Lu, Y.L. Zhou, Z.H. Chen, and G.Z. Yang, J. Eur. Ceram. Soc. 25 2295 (2005).
[15] P.Z. Zhang, M.R. Shen, L. Fang, F.G. Zheng, X.L. Wu, J.C. Shen, and H.T. Chen, Appl. Phys. Lett. 92 222908 (2008).
[16] Z.P. Yang, B. Liu, L.L. Wei, Y.T. Hou, Mater. Res. Bull. 43 81 (2008).
(Received on 19 May, 2010)
- [1] Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya, and M. Nakamura, Nature 432 84 (2004).
- [2] E. Hollenstein, M. Davis, D. Damjanovic, and N. Setter, Appl. Phys. Lett. 87 182905 (2005).
- [3] P. Kantha, K. Pengpat, P. Jarupoom, U. Intatha, G. Rujijanagul, and T. Tunkasiri, Curr. Appl. Phys. 9 460 (2009).
- [4] Z. Yu, R.Y. Guo, and A. S. Bhalla, J. Appl. Phys. 88 410 (2000).
- [5] X.S. Wang, L.L. Zhang, H.Liu, J.W. Zhai, X. Yao, Mater. Chem. Phys. 112 675 (2008).
- [6] C.J. Xiao, C.Q. Jin, X.H. Wang, Mater. Chem. Phys. 111 209 (2008).
- [7] P.Z. Zhang, M.R. Shen, L. Fang, F.G. Zheng, X.L. Wu, J.C. Shen, and H.T. Chen Appl. Phys. Lett. 92 222908 (2008).
- [8] R.L. Brutchey, G.S. Cheng, Q. Gu, and D.E. Morse, Adv. Mater. 20 1029 (2008).
- [9] T. Maiti, R. Guo, and A.S. Bhalla, Appl. Phys. Lett. 89 122909 (2006).
- [10] P.S. Dobal, A. Dixit, and R.S. Katiyar, J. Appl. Phys. 89 8085 (2001).
- [11] Z. Yu, R. Guo, A.S. Bhalla, J. Cryst. Growth 233 460 (2001).
- [12] F. Zimmermann, M. Voigts,W. Menesklou, E. Ivers-Tiffe'e, J. Eur. Ceram. Soc. 24 1729 (2004).
- [13] Z. Yu, C. Ang, R.Y. Guo, and A. S. Bhalla, J. Appl. Phys. 92 2655 (2002).
- [14] B.L. Cheng, C.Wang, S.Y.Wang, H. Lu, Y.L. Zhou, Z.H. Chen, and G.Z. Yang, J. Eur. Ceram. Soc. 25 2295 (2005).
- [15] P.Z. Zhang, M.R. Shen, L. Fang, F.G. Zheng, X.L. Wu, J.C. Shen, and H.T. Chen, Appl. Phys. Lett. 92 222908 (2008).
- [16] Z.P. Yang, B. Liu, L.L. Wei, Y.T. Hou, Mater. Res. Bull. 43 81 (2008).
Publication Dates
-
Publication in this collection
27 Sept 2010 -
Date of issue
Sept 2010
History
-
Received
19 May 2010